ABSTRACT
Introduction and objectives: In elderly and frail patients, there is limited evidence on the therapeutic management of left main coronary artery (LM) disease. The objective of this study was to evaluate mid-term clinical outcomes in older adults undergoing percutaneous coronary intervention (PCI) of LM.
Methods: We conducted a retrospective study including all older patients (≥ 75 years) undergoing LM-PCI at a high-volume center between 2017 and 2021. The primary endpoint was a composite of major adverse cardiovascular events (MACE). Patients were grouped according to the presence of frailty based on the FRAIL scale. Inverse probability of treatment weighting was used to account for clinical differences between the 2 groups.
Results: A total of 140 patients were included in the study (median age 80 [78-84]; 36% women). Of them, 49% met the criteria for frailty. After a median follow-up of 19 [5-35] months, 40 MACE (29%) were recorded. The all-cause death rate was 32%. There were no differences in the risk of MACE between frailty groups, but patients with frailty had an increased risk of all-cause mortality (HRadj, 1.95 [1.02-3.75]; P = .046).
Conclusions: LM-PCI in older adults with multiple associated comorbidities could be considered a feasible option in this special population. The rate of MACE at follow-up was acceptable. Frailty was associated with a worse prognosis in terms of all-cause mortality at follow-up.
Keywords: Coronary artery disease. Left main coronary artery. Percutaneous coronary intervention. Elderly. Frailty.
RESUMEN
Introducción y objetivos: La evidencia sobre el abordaje terapéutico de la enfermedad del tronco coronario izquierdo (TCI) en pacientes ancianos y frágiles es limitada. El objetivo de este estudio fue evaluar los resultados clínicos a medio plazo en ancianos que recibieron una intervención coronaria percutánea (ICP) del TCI.
Métodos: Estudio retrospectivo en el que se incluyeron todos los pacientes ancianos (≥ 75 años) tratados con ICP del TCI en un centro de alto volumen entre 2017 y 2021. El objetivo principal fue un compuesto de eventos adversos cardiovasculares mayores (MACE). Los pacientes fueron agrupados en función de su fragilidad según la escala FRAIL. Se utilizó la ponderación de probabilidad inversa de tratamiento para tener en cuenta las diferencias clínicas entre los 2 grupos.
Resultados: Se incluyeron 140 pacientes (mediana de edad: 80 años [78-84]; 36% mujeres), de los cuales el 49% cumplían los criterios de fragilidad. Tras una mediana de seguimiento de 19 meses (5-35) se registraron 40 MACE (29%). La tasa de mortalidad por todas las causas fue del 32%. No se observaron diferencias en el riesgo de MACE entre los grupos, aunque los pacientes frágiles presentaron una mayor mortalidad por todas las causas (HRa = 1,95 [1,02-3,75]; p = 0,046).
Conclusiones: La ICP del TCI en pacientes ancianos con comorbilidad podría considerarse una opción factible en esta población especial. La tasa de MACE en el seguimiento resulta aceptable. La fragilidad se asoció con un peor pronóstico en términos de mortalidad por todas las causas durante el seguimiento.
Palabras clave: Enfermedad arterial coronaria. Tronco coronario izquierdo. Intervención coronaria percutánea. Paciente anciano. Fragilidad.
Abbreviations CABG: coronary artery bypass grafting. LM: left main coronary artery. PCI: percutaneous coronary intervention.
INTRODUCTION
The left main coronary artery (LM) supplies 84% of the blood flow to the left ventricle in patients with right dominance,1 making LM disease the coronary lesion with the worst prognosis. The prevalence of this disease is not negligible, as it is found in 4.8% of coronary angiograms,2 highlighting the prognostic importance of these lesions. Conservative treatment is a rarely a feasible option due to the high rate of cardiac adverse events during short-term follow-up, with a mortality rate exceeding 50%.3
Coronary artery bypass grafting (CABG) has traditionally been the most widely accepted revascularization strategy.4 In recent years, there have been significant pharmacological and technological improvements in percutaneous revascularization techniques, such as drug-eluting stents and intracoronary diagnostic techniques.5 These improvements, together with comparative studies, have prompted discussion on the various alternatives.6 Presently, the choice of revascularization strategy should be based on the complexity of the coronary anatomy and surgical risk.7
However, evidence is limited in older adults who are scarcely represented in classic studies. Furthermore, in these patients, frailty is a frequent and unstudied characteristic that can influence their prognosis. In this special population, CABG is usually ruled out due to high-surgical risk. On the other hand, percutaneous coronary intervention (PCI) could be a potential therapeutic option, although with little evidence to date.8 Consequently, we postulated that PCI of the LM might be feasible and safe in older patients, with a low incidence of associated complications and an acceptable rate of major adverse cardiac events (MACE) during follow-up.
METHODS
Study design
We conducted a retrospective, single-center study of older patients diagnosed with LM disease who underwent PCI. The study aimed to evaluate mid-term clinical outcomes and examine the prognostic significance of frailty in these patients. The study protocol was approved by the local clinical research ethics committee according to institutional and good clinical practice guidelines. Recruitment took place from January 2017 to December 2021 at Hospital Universitario Reina Sofía (Cordoba, Spain). Patients were eligible if they were aged ≥ 75 years at the time of LM disease diagnosis, and PCI was chosen as the treatment after deliberation by heart team discussion, or due to instability requiring emergent revascularization. Exclusion criteria consisted of end-stage chronic diseases, patients under palliative care, contraindications to dual antiplatelet therapy, and incomplete follow-up data. Included patients were grouped according to frailty status, determined by the FRAIL scale, with patients scoring 3 or more points considered frail.9 Definitions are shown in the supplementary data.
Outcomes
The main objective of the study was to describe mid-term clinical outcomes in older patients undergoing LM-PCI. We also aimed to compare clinical events according to the presence of frailty. The primary endpoint was a composite of MACE, defined as a composite of cardiovascular death (including death of uncertain cause), nonfatal myocardial infarction, the need for new revascularization, and stroke. Secondary outcomes were the individual components of MACE and all-cause mortality.
Angiographic analysis
Quantitative analysis of the coronary arteries was performed using the validated CAAS system (Pie Medica Imaging, the Netherlands). The basal anatomy of the LM bifurcation with the anterior descending artery and the circumflex artery was classified according to the Medina classification.10 The measurements analyzed included the reference diameter of the LM and its percentage of stenosis. The complexity of the coronary anatomy was studied using the SYNTAX scale.6
Statistical analysis
Categorical data are presented as counts (percentages), while continuous data are expressed as mean ± standard deviation or median [interquartile range]. Between-group comparisons were performed using the chi-square test or the Fisher exact test for categorical variables and the Student t-test or the Mann-Whitney U test for continuous variables. Kaplan-Meier curves and Cox regression models were used to analyze clinical events according to frailty. Inverse probability of treatment weighting (IPTW) was used to account for clinical differences between the 2 groups.11 Propensity scores were calculated using a logistic regression model that included the following covariates: age, sex, left ventricular ejection fraction, atrial fibrillation, chronic kidney disease, anemia, and chronic obstructive pulmonary disease. Standardized mean differences before and after weighting were used to evaluate the balance of the groups regarding the covariates. A difference of < 10% was considered to indicate a satisfactory balance. The distributions of the propensity scores before and after weighting were plotted to assess the degree of overlap between the 2 groups. Confidence intervals for the IPTW coefficients were obtained using robust sandwich-type variance estimators (figure 1 of the supplementary data).12 All tests were 2-tailed and significance was set at P < .05. Statistical analyses were performed using SPSS software (V 24; IBM Corp., United States) and R software (V4.0.3; R Foundation for Statistical Computing, Austria).
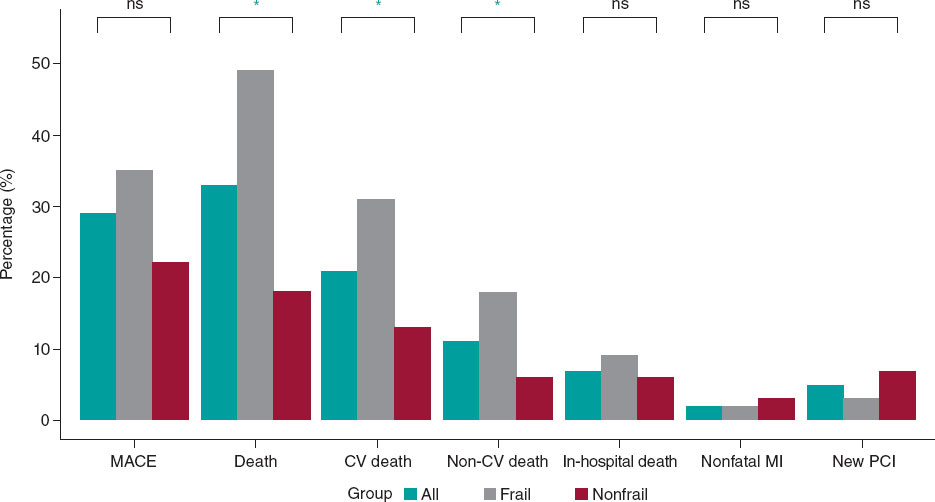
Figure 1. Main events to follow-up. CV, cardiovascular; MACE, mayor adverse cardiovascular events; MI, myocardial infarction; NS, nonsignificant; PCI, percutaneous coronary intervention. * P < .005.
RESULTS
During the study period, our hospital treated 437 patients with significant LM lesions percutaneously. Of them, a total of 140 patients met the inclusion criteria and were included in the analysis (figure 2 of the supplementary data).
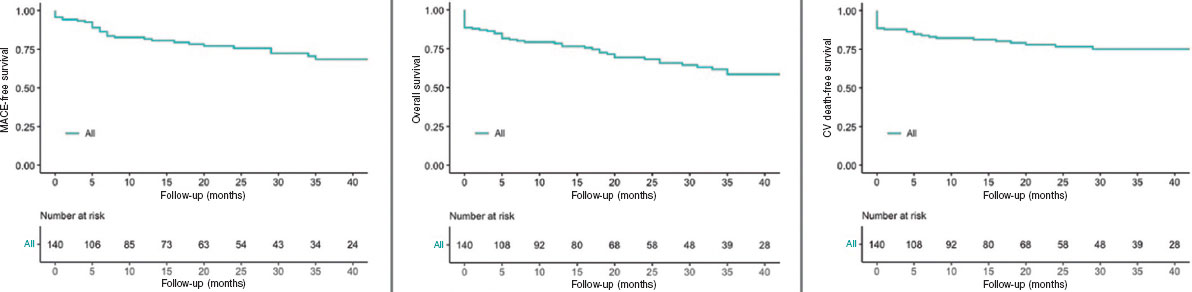
Figure 2. Kaplan-Meier Curves of the primary outcome and mortality. CV, cardiovascular; MACE, major adverse cardiovascular events.
Baseline characteristics
The baseline clinical characteristics, clinical presentation and antithrombotic treatment administered are detailed in table 1. The median age of the patients was 80 [78-84] years and 36% (51 patients) were women. Most of the patients had a history of hypertension (84%, 118 patients) and 58% (81 patients) were diabetic. More than a third of the patient cohort had a previous personal history of ischemic heart disease (37%, 52 patients) and 33% (46 patients) had chronic kidney disease. Among noncardiovascular comorbidities, active cancer was present in 11 patients (8%) and prior blood transfusions had been required in 16 patients (11%). The mean EuroSCORE II was 3.07 [1.96-5.7] to assess surgical risk. Forty-eight patients (34%) had left ventricular systolic dysfunction at the time of revascularization.
Table 1. Patients’ baseline characteristics
| Characteristics | Total n = 140 | Nonfrail n = 72 (51) | Frail n = 68 (49) | P |
|---|---|---|---|---|
| Baseline clinical characteristics | ||||
| Age, years | 80 [78-84] | 80 [77-84] | 80 [78-84] | .090 |
| Female sex | 51 (36) | 18 (25) | 33 (49) | .004 |
| Hypertension | 118 (84) | 61 (85) | 57 (84) | .884 |
| Diabetes | 81 (58) | 36 (50) | 45 (66) | .053 |
| Hypercholesterolemia | 112 (80) | 56 (78) | 56 (82) | .999 |
| Smoking history | 7 (5) | 5 (7) | 2 (3) | .442 |
| Previous ischemic heart disease | 52 (37) | 31 (43) | 21 (31) | .136 |
| Chronic kidney disease | 46 (33) | 22 (33) | 24 (39) | .481 |
| Atrial fibrillation | 22 (16) | 7 (10) | 15 (22) | .041 |
| Peripheral artery disease | 20 (14) | 14 (20) | 6 (9) | .073 |
| COPD | 17 (12) | 6 (8) | 11 (16) | .156 |
| Previous stroke | 16 (11) | 10 (14) | 6 (9) | .073 |
| Valve disease | 15 (11) | 7 (7) | 10 (15) | .114 |
| Anemia | 29 (21) | 10 (14) | 19 (28) | .040 |
| Active cancer | 11 (8) | 7 (10) | 4 (6) | .399 |
| Liver disease | 4 (3) | 3 (4) | 1 (2) | .339 |
| Previous blood transfusions | 16 (11) | 5 (7) | 11 (16) | .086 |
| Recent surgery or trauma | 38 (27) | 19 (26) | 19 (28) | .836 |
| EuroScore II | 3.07 [1.96-5.7] | 2.76 [1.83-4.18] | 3.80 [2.04-7.85] | .010 |
| Glomerular filtration rate (mL/min) | 71.4 [48.4-87.3] | 76.71 [51.01-87.51] | 61.40 [41.40-81.85] | .072 |
| Creatinine (mg/dL) | 1.02 [0.87-1.30] | 1.00 [0.80-1.85] | 1.03 [0.90-1.50] | .109 |
| Hemoglobin (mg/dL) (mean, ±SD) | 12.6 (± 2) | 13.02 (± 2) | 12.16 (± 1.9) | .017 |
| Hematocrit | 38.6 [34.6-43.0] | 39.6 [36.0-44.7] | 36.6 [33.9-42.1] | .031 |
| Platelets (× 109/L) | 208 [171-246] | 211 [182-244] | 196 [160-250] | .340 |
| Hs-cTnI (ng/L) | 954 [40-7352] | 2250 [30-10 000] | 650 [40-5600] | .245 |
| LVEF | 60 [39-67] | 60 [45-68] | 58 [35-63] | .245 |
| LV systolic dysfunction | 48 (34) | 20 (32) | 28 (46) | .106 |
| Clinical presentation | ||||
| Acute coronary syndrome | 85 (61) | 45 (63) | 40 (59) | .656 |
| NSTEMI | 61 (44) | 28 (39) | 33 (49) | .250 |
| STEMI | 9 (6) | 6 (8) | 3 (4) | .495 |
| Unstable angina | 15 (11) | 11 (15) | 4 (6) | .101 |
| Chronic coronary syndrome | 55 (39) | 27 (38) | 28 (41) | .656 |
| Antiplatelet therapy | ||||
| Dual antiplatelet therapy | 104 (74) | 57 (79) | 47 (69) | .174 |
| Aspirin + clopidogrel | 61 (43) | 31 (43) | 30 (44) | .899 |
| Aspirin + ticagrelor | 43 (31) | 26 (36) | 17 (25) | .154 |
| Triple antiplatelet therapy | ||||
| Aspirin + clopidogrel + anticoagulant | 36 (26) | 15 (2) | 21 (31) | .174 |
COPD, chronic obstructive pulmonary disease; Hs-cTnI, high sensitivity cardiac troponin I; LV, left ventricle; LVEF, left ventricular ejection fraction; NSTEMI, non-ST-elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction. Data are expressed as No. (%), mean ± standard deviation or median [interquartile range]. | ||||
The most common clinical presentation was acute coronary syndrome (85 patients, 61% of cases). Among these, onset consisted of ST-segment elevation myocardial infarction (STEMI) in 9 patients (6%), non-ST-segment elevation myocardial infarction in 61 patients (44%), and unstable angina in 15 patients (10%). The remaining patients (55, 39%) presented with chronic coronary syndrome.
A total of 104 patients (74%) were discharged with dual antiplatelet therapy. The main combination was aspirin and clopidogrel (61 patients, 43%). In 36 patients (26%), initial triple therapy (anticoagulation and dual antiplatelet therapy) was chosen due to concurrent conditions requiring chronic oral anticoagulation.
Based on the FRAIL scale, almost half of the patients (68 patients, 49%) met clinical criteria for frailty at the time of revascularization. The baseline characteristics of frail and nonfrail patients are shown in table 1. No statistically significant differences were found in terms of age, main cardiovascular risk factors or noncardiovascular comorbidities between the 2 groups. However, compared with nonfrail patients, those with frailty were more likely to be female (49% vs 25%; P = .004), to have atrial fibrillation (22% vs 10%; P = .041), a higher EuroSCORE level (3.80 vs 2.76; P = .010), and anemia (28% vs 14%; P = .040), and consequently a lower hematocrit and hemoglobin value (36.6% vs 39.6%; P = .031 and 12.16 mg/dL vs 13.02 mg/dL; P = .017, respectively).
Angiographic and procedural characteristics
Angiographic and procedural data are shown in table 2. The arterial access of choice was radial access (81% of procedures, 113 patients). A median SYNTAX score of 21 [15-29.5] was observed in 96 patients (68%) with multivessel disease, and 62 patients (44%) had a SYNTAX score > 22. The most common angiographic involvement of the LM was the distal segment (61%, 86 patients), while the most common plaque distribution according to the Medina classification was “1,1,1” (35 patients, 41% of LM bifurcation lesions). The strategy of choice for the treatment of the bifurcation was the provisional stent strategy (85% of LM bifurcation lesions, 73 patients), while the upfront 2-stent strategy was used in only 13 patients (15% of the LM bifurcation lesions). The mean diameter of the LM was 4.1 [± 3.5-4.5] mm with a mean angiographic stenosis of 62% (± 7). In 59 patients (42%), the procedure was guided using intravascular imaging techniques (58 patients using intracoronary ultrasound and 1 patient using coherence tomography). Coronary physiology was used in 5 patients (4%) to guide the need for revascularization or to check the result after percutaneous treatment. In 7 (5%) patients, mechanical support was required, either due to cardiogenic shock, or as a preventive measure in high-risk angioplasty (5 patients with an intra-aortic balloon pump and 2 with an Impella CP device [Abiomed, United States]). Intraprocedural complications occurred in 8 patients (6%), including a major complication in 4 patients (3 intraprocedural deaths and 1 cardiogenic shock), and a minor complication in 4 patients (1 coronary dissection with Thrombolysis in Myocardial Infarction (TIMI) grade 3 distal flow, 1 pseudoaneurysm, and 2 bleeding events from the femoral access resolved by stent implantation). The LM diameter was larger in patients with frailty than in those without (4 mm [4-4.5] vs 3.5 mm [3.5-4.5]; P = .023), a paradoxical finding since the percentage of women was higher in the group with frailty percentage of women. However, this information did not seem to be clinically relevant. No other clinically relevant differences were found between the 2 groups (table 2).
Table 2. Patients’ angiographic and procedural characteristics
| Characteristics | Total n = 140 | Nonfrail n = 72 (51) | Frail n = 68 (49) | P |
|---|---|---|---|---|
| Angiographic characteristics | ||||
| Multivessel disease | 96 (68) | 50 (69) | 46 (68) | .819 |
| SYNTAX score | 21 [15-29,5] | 21 [17-28.5] | 21.5 [14-30.6] | .752 |
| SYNTAX score > 22 | 62 (44) | 25 (39) | 31 (46) | .463 |
| LM diameter (mm) | 4 [3.5-4.5] | 3.5 [3.5-4.5] | 4 [4-4.5] | .023 |
| LM stenosis | 62 (± 7) | 64 (± 6) | 61 (± 5) | .342 |
| LM bifurcation | 86 (61) | 39 (54) | 47 (69) | .069 |
| Medina (1,1,1) | 35 (41) | 20 (51) | 15 (32) | .690 |
| Medina (1,1,0) | 33 (39) | 10 (26) | 23 (49) | .027 |
| Medina (1,0,1) | 8 (9) | 3 (8) | 5 (11) | .724 |
| Medina (0,1,1) | 3 (3) | 2 (5) | 1 (2) | .588 |
| Medina (1,0,0) | 4 (5) | 1 (3) | 3 (6) | .623 |
| Medina (0,1,0) | 0 (0) | 0 (0) | 0 (0) | - |
| Medina (0,0,1) | 3 (3) | 3 (8) | 0 (0) | .089 |
| Intracoronary diagnostic technique | ||||
| Intravascular imaging | 59 (42) | 28 (39) | 31 (46) | .422 |
| IVUS | 58 (41) | 28 (39) | 30 (44) | .530 |
| OCT | 0 (0) | 0 (0) | 1 (2) | .486 |
| Intracoronary physiology test | 5 (4) | 4 (6) | 1 (2) | .367 |
| Procedure characteristics | ||||
| Radial access | 113 (81) | 60 (83) | 53 (78) | .253 |
| Contrast (mL) | 200 [160-255] | 215 [150-259] | 200 [160-250] | .553 |
| Temporary pacemakers | 6 (4) | 3 (4) | 3 (4) | 1.000 |
| LV assist devices | 7 (5) | 4 (6) | 3 (4) | 1.000 |
| Intra-aortic balloon pump | 5 (4) | 4 (6) | 1 (2) | .367 |
| Impella | 2 (1) | 0 (0) | 2 (3) | .239 |
| One-stent bifurcation technique | 73 (85) | 34 (87) | 39 (83) | .588 |
| Stent MB + kissing | 20 (27) | 12 (35) | 7 (18) | .077 |
| Two-stent bifurcation technique | 13 (15) | 5 (13) | 8 (17) | .636 |
| T stenting | 3 (23) | 2 (40) | 1 (12.5) | .498 |
| TAP | 2 (15) | 0 (0) | 2 (25) | .498 |
| Culotte | 5 (39) | 1 (20) | 4 (50) | .371 |
| DK-Crush | 2 (15) | 1 (20) | 1 (12.5) | 1.000 |
| SKS | 1 (8) | 1 (20) | 0 (0) | .413 |
| MB stent diameter (mm) | 3.5 [3-3.5] | 3.5 [3-3.5] | 3.5 [3-3.5] | .877 |
| MB stent length (mm) | 18 [15-18] | 18 [15-18] | 18 [15-18] | .896 |
| SB stent diameter (mm) | 3.5 [3-3.5] | 3.25 [2.8-3.5] | 3.5 [3-3.6] | .371 |
| SB stent length (mm) | 15 [12-18] | 15.5 [15-21] | 15 [11-18] | .342 |
| Complications | ||||
| Intraprocedural complications | 8 (6) | 6 (8) | 2 (3) | .157 |
| Major | 4 (3) | 3 (4) | 1 (2) | .356 |
| Minor | 4 (3) | 3 (4) | 1 (2) | .356 |
DK, double kissing; IVUS, intravascular ultrasound; LM, left main; LV, left ventricle; MB, main branch; OCT, optical coherence tomography; SB, side branch; SKS, simultaneous kissing stents. TAP, T and small protrusion. Data are expressed as No. (%), mean ± standard deviation or median [interquartile range]. | ||||
Clinical results at follow-up
After a median follow-up of 19 months [5-35], a total of 40 (29%) MACE were recorded: 3 (2%) patients had a nonfatal myocardial infarction, 7 (5%) patients required repeat revascularization (3 for restenosis of the LM, and 4 in a different vessel), and 30 patients (21%) died of cardiac and/or uncertain causes. No strokes were reported during follow-up. Sixteen patients (11%) died of noncardiac causes during follow-up.
Clinical outcomes are presented in figure 1 and figure 2. No independent predictor of MACE was identified. The independent predictors of all-cause mortality were left ventricular ejection fraction (hazard ratio [HR], 0.90 [0.96-0.99]; P = .014), chronic kidney disease (HR, 2.26 [1.16-4.42]; P = .017), and particularly the presence of frailty (HR, 2.42 [1.17-5.02]; P = .018) (table 1 of the supplementary data). The primary endpoint of MACE occurred in 24 (35%) patients in the frail group and in 16 (22%) patients in the nonfrail group (HR, 1.61 [0.79-3.28]; P = .193). Frail patients had an increased risk of cardiovascular mortality: 21 (31%) vs 9 (13%); HR, 2.64 (1.21-5.77); P = .015. All-cause mortality was also more frequent in the frail group: 33 (49%) vs 13 (18%); HR, 2.94 (1.55-5.59); P = .001). The events during follow-up are presented in table 2 of the supplementary data. After IPTW adjustment, only the difference in all-cause mortality remained significant (HR, 1.95 [1.02-3.75]; P = .046). Survival analysis of the weighted population is shown in figure 3.
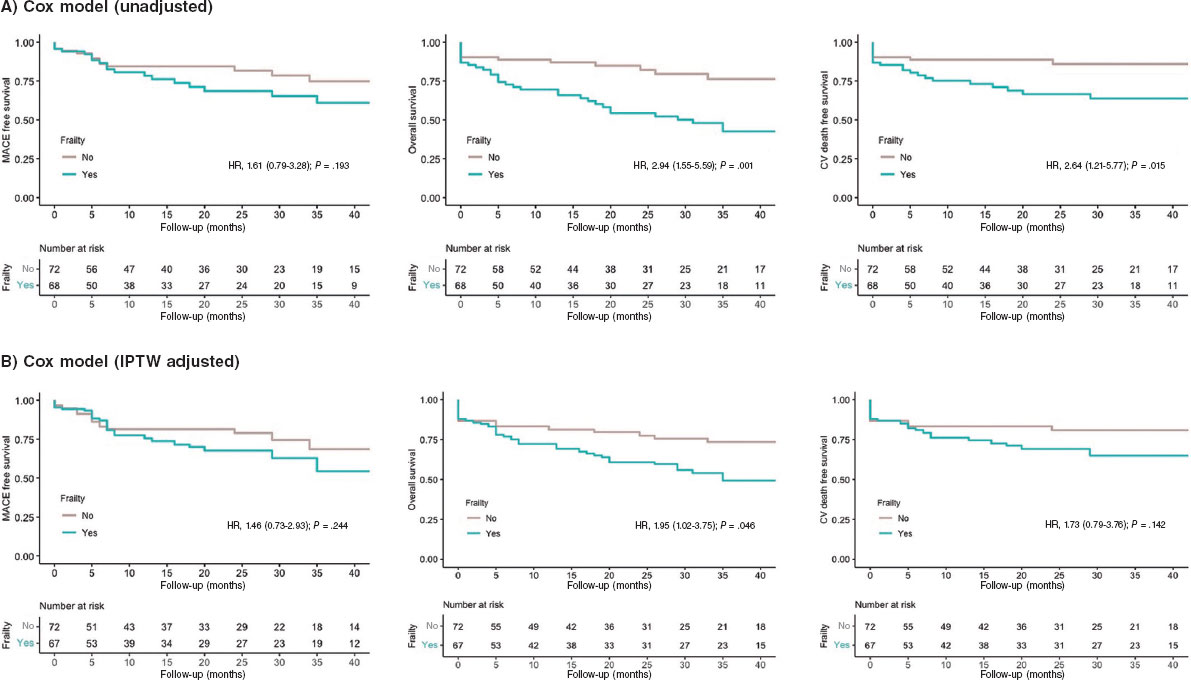
Figure 3. Kaplan-Meier Curves of the secondary outcomes. CV, cardiovascular; IPTW, inverse probability of treatment weighting; MACE, major adverse cardiovascular events.
DISCUSSION
The present study describes the feasibility of LM-PCI in a cohort of older patients. The main results were as follows: a) the rate of MACE at mid-term follow-up was 29%, mainly driven by cardiovascular and/or uncertain cause death; b) a high percentage of frailty was found in our population (49%); c) frail patients had a 2-fold increased risk of all-cause mortality during follow-up (HR, 1.95 [1.02-3.75]; P = .046) (figure 4).
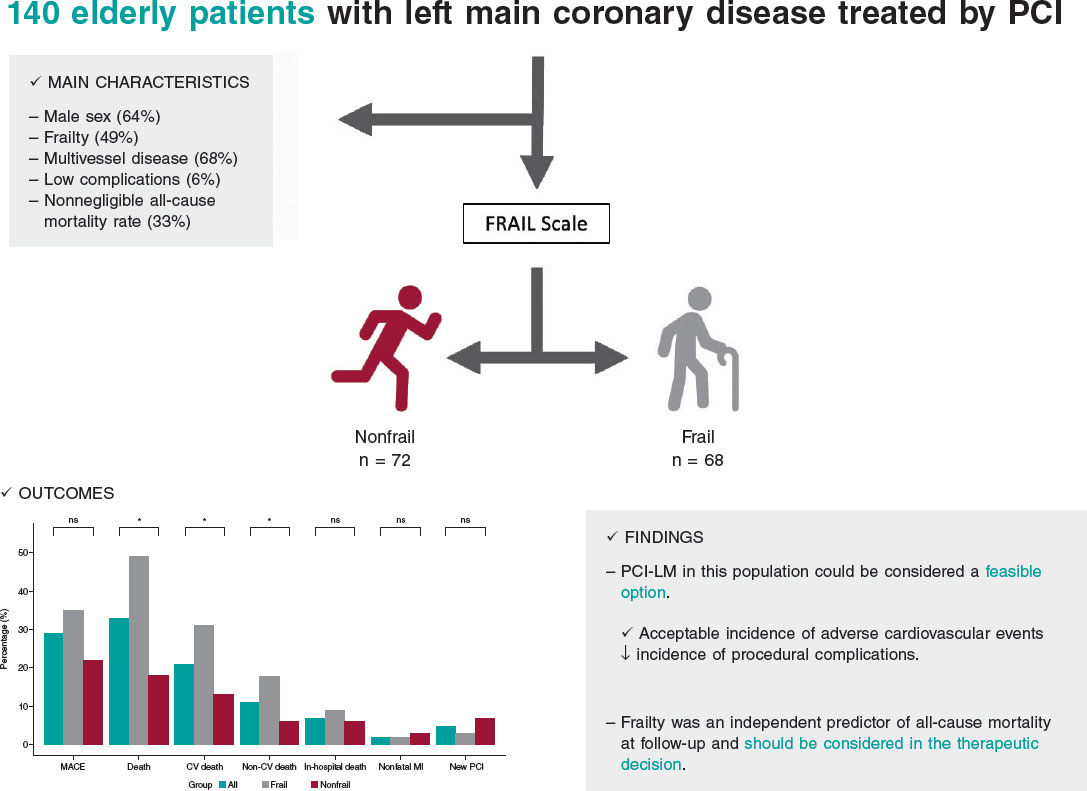
Figure 4. Central illustration. Results of percutaneous treatment of LM in elderly patients and impact of frailty. CV, cardiovascular; LM, left main coronary artery; MACE, major adverse cardiovascular events; MI, myocardial infarction; NS, non-significant; PCI, percutaneous coronary intervention.
The treatment of LM disease has traditionally been surgical, given the complexity involved and significant prognostic impact.13 However, the marked advances in interventional cardiology in recent decades have modified the approach.14,15 Contrasting evidence from clinical trials and meta-analyses shows that percutaneous treatment has similar results to surgical approaches in terms of mortality, acute myocardial infarction, and stroke at 5 years of follow-up.16 This shift has is reflected in the evolving recommendations in clinical practice guidelines, and the current European revascularization guidelines assign a grade of recommendation IA to both surgical and percutaneous strategies for the treatment of LM disease when the anatomy is not complex (SYNTAX < 22), and a class IIa recommendation for cases of intermediate complexity (SYNTAX 23-32).7
Nevertheless, the population analyzed in the study has specific clinical characteristics, and is not usually represented in large clinical trials (older patients and those with frailty and a high burden of associated comorbidities). These variables are not systematically included in surgical risk scores but are generally taken into account in routine clinical practice and often influence heart team decisions on the treatment strategy.17 Therefore, because this particular patient cohort is often excluded from research, there are no conclusive data on the benefit of percutaneous revascularization.
Our results are in line with those of previous registries in terms of MACE and all-cause mortality, as well as the association between age and a marked incidence of mortality due to noncardiac causes during follow-up. However, unlike earlier studies, we observed no differences in cardiovascular mortality, despite these patients having a more complex coronary anatomy than younger patients.18 In this regard, our study cohort had a median SYNTAX score of 21, and 44% of the patients had a score above 22. Like previous studies, this SYNTAX index score was not associated with a higher probability of cardiac events during follow-up in this special population.
In the present study, rates of acute myocardial infarction and new revascularization of the target lesion were lower than in other cohorts. Although it is difficult to make direct comparisons, we postulate that the use of new-generation drug-eluting stents and a higher proportion of revascularization guided by intracoronary diagnostic techniques may have influenced this finding. However, the use of intracoronary imaging techniques in our study was relatively low (42%) considering their benefit in patients with complex coronary lesions.19
In recent years, there has been growing interest in understanding the impact of comorbidities and frailty in older patients with cardiovascular disease.20,21 Several studies have compared invasive strategies with conservative approaches in older patients, demonstrating benefits for revascularization.22,23 However, the MOSCA-FRAIL trial compared both strategies in frail patients and observed that an invasive strategy did not confer additional benefit compared with conservative management of these patients, despite a fairly low percentage of LM disease.24 In our study, we observed a 2-fold increase in the risk of all-cause mortality in patients with frailty, suggesting the need to add systematic evaluation of frailty in older patients undergoing LM-PCI. Such assessment can aid in selecting the optimal therapeutic strategy, taking into account the likelihood of mortality during follow-up, irrespective of the application of an invasive strategy in coronary disease. These results, moreover, are consistent with other cardiovascular diseases with significant prevalence and mortality, such as heart failure.25
Study limitations
The present study has several limitations. First, it has the limitations inherent to its observational and retrospective design. Although the sample size is relatively small, it represents the largest study specifically focused on LM-PCI in older patients and analyses associated comorbidities and their impact on cardiovascular adverse events. Second, the absence of a control group receiving conservative treatment hinders the ability to draw more robust conclusions on the safety and efficacy of LM-PCI in these patients. In addition, the selection of cutoff points (age ≥ 75 years) to define this cohort of older patients was arbitrarily based on the exclusion criteria of the main clinical trials previously published. A high percentage of patients with frailty may not have undergone revascularization and would therefore have been excluded from the study. Regarding the prognostic significance of frailty, although we used IPTW to reduce confounding bias, we cannot rule out the possibility of residual confounding due to unmeasured covariables. Furthermore, there are no data on bleeding events during follow-up, which is an important concern given the impact of antiplatelet therapy in these patients. Finally, the percentage of intracoronary imaging use was lower than expected.
CONCLUSIONS
In real-life patients with advanced age and multiple associated comorbidities, percutaneous treatment of LM could be considered a feasible option, with an acceptable incidence of adverse cardiovascular events during follow-up and a low incidence of complications associated with the procedure. Frailty was an independent predictor of all-cause mortality during follow-up. When weighing the risks of LM-PCI in older patients, frailty should be taken into account in the therapeutic decision-making process.
FUNDING
None.
ETHICAL CONSIDERATIONS
The study protocol was approved by the Local Clinical Research Ethics Committee according to institutional and Good Clinical Practice guidelines. All patients signed the informed consent for publication. The authors confirm that sex and gender variables have been considered in accordance with the SAGER guidelines.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence was used in the preparation of the study.
AUTHORS’ CONTRIBUTIONS
I. Gallo, M. Alvarado and J. Perea contributed to data collection. R. González-Manzanares performed the statistical analysis. J. Suárez de Lezo and M. Romero contributed to the interpretation of the results. I. Gallo and F. Hidalgo wrote the manuscript. S. Ojeda and M. Pan reviewed the manuscript.
CONFLICTS OF INTEREST
S. Ojeda is associate editor of REC: Interventional Cardiology. The journal’s editorial procedure to ensure impartial processing of the manuscript has been followed. S. Ojeda has received consulting fees from Medtronic and Edwards and speaker fees from Philips, World Medical and Boston Scientific and is holder of a research grant (PI21/00949) from the Spanish Ministry of Science and Innovation (Instituto de Salud Carlos III). M. Pan has received speaker fees from Abbott, Boston Scientific, World Medical and Philips and holds a research grant (PI21/00949) from the Spanish Ministry of Science and Innovation (Instituto de Salud Carlos III). The remaining authors declare no conflicts of interest.
WHAT IS KNOWN ABOUT THE TOPIC?
- Coronary artery disease is closely related to age and the aging process.
- The prognosis of LM disease is uncertain and, due to due to advances in interventional cardiology in recent years, there is a need for further evidence on treatment options.
- Frailty is associated with a worse prognosis in various diseases.
WHAT DOES THIS STUDY ADD?
- LM-PCI in older adults is a feasible option in high-volume centers.
- Frailty is prevalent in older patients with LM disease and is associated with increased all-cause mortality.
REFERENCES
1. Collet C, Capodanno D, Onuma Y, et al. Left main coronary artery disease:pathophysiology, diagnosis, and treatment. Nat Rev Cardiol.2018;15:321-331.
2. Giannoglou GD, Antoniadis AP, Chatzizisis YS, et al. Prevalence of narrowing >or=50% of the left main coronary artery among 17,300 patients having coronary angiography. Am J Cardiol. 2006;98:1202-1205.
3. Ramadan R, Boden WE, Kinlay S. Management of Left Main Coronary Artery Disease. J Am Heart Assoc. 2018;7:008151.
4. Yusuf S, Zucker D, Peduzzi P, et al. Effect of coronary artery bypass graft surgery on survival:overview of 10-year results from randomized trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet. 1994;344:563-570.
5. Suárez de Lezo J, Medina A, Pan M, et al. Rapamycin-eluting stents for the treatment of unprotected left main coronary disease. Am Heart J. 2004;148:481-485.
6. Thuijs DJFM, Kappetein AP, Serruys PW, et al. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease:10-year follow-up of the multicentre randomised controlled SYNTAX trial.Lancet. 2019;394:1325-1334.
7. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
8. Ono M, Serruys PW, Hara H, et al. 10-Year Follow-Up After Revascularization in Elderly Patients With Complex Coronary Artery Disease. J Am Coll Cardiol. 2021;77:2761-2773.
9. Abellan van Kan G, Rolland Y, Bergman H, et al. The I.A.N.A. Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging. 2008;12:29-37.
10. Medina A, Suárez de Lezo J, Pan M. Una clasificación simple de las lesiones coronarias en bifurcación [A new classification of coronary bifurcation lesions]. Rev Esp Cardiol. 2006;59:183.
11. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW. the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661-3679.
12. Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW. survival analysis. Stat Med.2016;35:5642-5655.
13. Baydoun H, Jabbar A, Nakhle A, et al. Revascularization of Left Main Coronary Artery. Cardiovasc Revasc Med.2019;20:1014-1049.
14. Stone GW, Sabik JF, Serruys PW, et al. Everolimus-Eluting Stents or Bypass Surgery for Left Main Coronary Artery Disease. N Engl J Med. 2016;375:2223-2235.
15. Mäkikallio T, Holm NR, Lindsay M, et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE):a prospective, randomised, open-label, non-inferiority trial. Lancet. 2016;388:2743-2752.
16. Sabatine MS, Bergmark BA, Murphy SA, et al. Percutaneous coronary intervention with drug-eluting stents versus coronary artery bypass grafting in left main coronary artery disease:an individual patient data meta-analysis. Lancet. 2021;398:2247-2257.
17. Gach O, Louis O, Martinez C, et al. Predictors of early and late outcome of percutaneous coronary intervention in octogenarians. Acta Cardiol. 2003;58:289-294.
18. Gómez-Hospital JA, Gomez-Lara J, Rondan J, et al. Long-term follow-up after percutaneous treatment of the unprotected left main stenosis in high-risk patients not suitable for bypass surgery. Rev Esp Cardiol. 2012;65:530-537.
19. Lee JM, Choi KH, Song YB, et al. Intravascular Imaging-Guided or Angiography-Guided Complex PCI. N Engl J Med. 2023;388:1668-1679.
20. Díez-Villanueva P, Arizá-SoléA, Vidán MT et al. Recomendaciones de la Sección de Cardiología Geriátrica de la Sociedad Española de Cardiología para la valoración de la fragilidad en el anciano con cardiopatía. Rev Esp Cardiol. 2019;72:63–71.
21. Pernias V, García Acuña JM, Raposeiras-Roubín S, et al. Influencia de las comorbilidades en la decisión del tratamiento invasivo en ancianos con SCASEST. REC Interv Cardiol. 2021;3:15-20.
22. Kaura A, Sterne JAC, Trickey A, et al. Invasive versus non-invasive management of older patients with non-ST elevation myocardial infarction (SENIOR-NSTEMI):a cohort study based on routine clinical data. Lancet. 2020;396:623-634.
23. Tegn N, Abdelnoor M, Aaberge L, et al. Invasive versus conservative strategy in patients aged 80 years or older with non-ST-elevation myocardial infarction or unstable angina pectoris (After Eighty study):an open-label randomised controlled trial. Lancet.2016;387:1057-1065.
24. Sanchis J, Bueno H, Miñana G, et al. Effect of Routine Invasive vs Conservative Strategy in Older Adults With Frailty and Non-ST-Segment Elevation Acute Myocardial Infarction:A Randomized Clinical Trial. JAMA Intern Med. 2023;183:407-415.
25. Jiménez-Méndez C, Díez-Villanueva P, Bonanad C, et al. Frailty and prognosis of older patients with chronic heart failure. Rev Esp Cardiol. 2022;75:1011-1019.
* Corresponding author.
E-mail address: fjhl.87@gmail.com (F. Hidalgo).
@NachoGalloFer; @FranJHidalgo; @rafaelglezm; @MarcoA1788; @PereaJorge5; @cardiojsl; @OjedaOjeda18; @MPAOSS; @Cardio_HURS