During the second half of 2019, cangrelor started selling in our country, a “new” antiplatelet drug with especial pharmacological properties that make it especially appealing for the management of certain clinical situations in the percutaneous coronary intervention (PCI) setting. The adjective “new” is in quotation marks because the clinical trial conducted proved its superiority compared to clopidogrel with ICP. The CHAMPION PHOENIX trial1 was published in 2013 and it was approved by regulatory authorities back in 2015. This delay has probably caused the scientific evidence to lose relevance and, in consequence this drug might not be widely accepted within the cardiology community. Also, the indication specified in the technical label is only based on the conditions of the clinical trial that prompted its approval—which is mandatory—not on real-world practices. This may be confusing when selecting those patients who may benefit from this drug.2
In short, cangrelor is an intravenous reversible, high-affinity antagonist of the platelet P2Y12 receptor of adenosine diphosphate to which it binds directly (without need for conversion into an active metabolite). In pharmacodynamic terms, the properties of cangrelor are: a) rapid onset of action (3 to 6 minutes); b) powerful dose-dependent effect (inhibition > 90% of the P2Y12 receptor signaling pathway) in relation to the dose used in the PCI; and c) rapid offset of action (shot half-life of 3 to 5 minutes) with recovery of the baseline platelet function in 60 to 90 minutes after infusion.3
Given its pharmacological properties, cangrelor may help solve some of the problems posed by other antiplatelet agents. For example, oral P2Y12 receptor inhibitors (iP2Y12), especially clopidogrel and to a lesser extent prasugrel and ticagrelor, have a delayed optimal platelet inhibition, which is even more significant in the ST-segment elevation myocardial infarction (STEMI) setting, where the bioavailability of oral drugs may be compromised (worse intestinal absorption, vomiting, use of opioids or situations like intubation, therapeutic hypothermia or cardiogenic shock).3,4 Also, despite their efficacy in reducing thrombotic events, the use of parenteral glycoprotein IIb/IIIa inhibitors, may be associated with a higher risk of bleeding.
The clinical development of cangrelor as a coadjuvant therapy in the PCI setting is based on the CHAMPION program, in which the first 2 studies conducted (CHAMPION PCI5 and CHAMPION PLATFORM6) were prematurely interrupted due to their futility, in part attributed to a restrictive definition of myocardial infarction.3,7 On the contrary, the CHAMPION PHOENIX clinical trial did prove the superiority of cangrelor vs clopidogrel reducing the main variable of efficacy (a composite of death, myocardial infarction, ischemia guided revascularization or stent thrombosis) after 48 hours in patients who underwent PCI to treat stable angina or any type of acute coronary syndrome (ACS) and who were not eligible for oral iP2Y12 pretreatment.1 We should mention that in a combined analysis of the 3 trials, cangrelor was associated with a slightly higher risk of bleeding mainly at the cost of minor bleeding;8 this good safety profile is probably due to the fact that the drug is administered over a very limited span of time and its effect rapidly goes away after infusion.
The CHAMPION PHOENIX trial has received 2 important criticisms that may condition its implementation in our routine clinical practice. The first is that cangrelor was never compared to prasugrel or ticagrelor (more powerful and effective than clopidogrel and drugs of choice in patients with ACS). The second is that iP2Y12 pretreatment before the PCI was considered an exclusion criterion. We should remember that, although there are serious doubts about its pretreatment benefit in the ACS setting, especially in the non-ST-segment elevation acute coronary syndrome setting,9 this strategy is widely used in our country. As a matter of fact, the European technical label of this drug specifically says that cangrelor is indicated in association with acetylsalicylic acid in patients “who undergo PCI and have not received an oral P2Y12 inhibitor before the PCI, and in whom oral treatment with P2Y12 inhibitors is not possible or desired”.2 Still, the pharmacological properties of cangrelor make it especially interesting in situations where not only the aforementioned pretreatment has not occurred, but also in circumstances where it is considered insufficient. Proof of this is the experience published from the Swedish national registry (SCAAR) during the first 2 years of drug use that found an almost exclusive use of cangrelor in patients with STEMI who underwent primary percutaneous coronary intervention; in this real-world study, cangrelor was combined with ticagrelor mainly, although the latter had already been administered in over 50% of the times in the prehospital setting, which is why in such cases the use of cangrelor would be considered an off-label indication.10
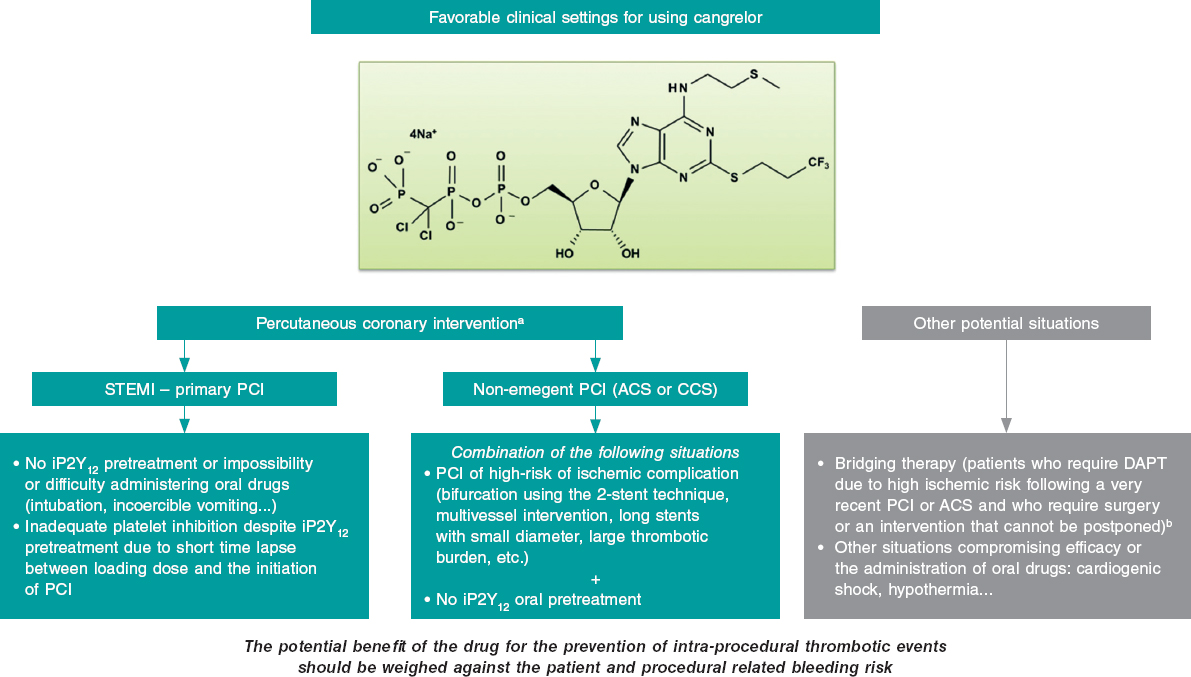
The clinical settings where, in our opinion, cangrelor can be useful both in the PCI setting (most of the cases) and in other situations are shown on figure 1. The most relevant setting would be the primary percutaneous coronary intervention in the management of STEMI, where its use is appropriate in the absence of proper pretreatment with iP2Y12 due to the impossibility or difficulty administering oral drugs (intubation or incoercible vomiting); it may also be considered if the loading dose of iP2Y12 is ineffective during the procedure (short span of time elapsed from its administration until the PCI is performed). Another acceptable situation (much less common than the latter) would be non-emergent high-risk PCIs (such as bifurcations using the dual stent technique, multivessel interventions, in the presence of great thrombotic load, etc.) in patients without iP2Y12 pretreatment. In any case, the drug potential benefit should always be weighed to prevent intraprocedural thrombotic events associated with the procedure and the patient’s bleeding risk. Also, we should remember that the use of cangrelor is ill-advised if glycoprotein IIb/IIIa inhibitors are going to be administered.
Figure 1. Potential favorable clinical settings for the use of cangrelor. ACS, acute coronary syndrome; CCS, chronic coronary syndrome; DAPT, dual antiplatelet therapy; iP2Y12, platelet P2Y12 receptor inhibitors; PCI, percutaneous coronary intervention; STEMI, ST-segment-elevation acute myocardial infarction.
a) The dose in the percutaneous coronary intervention setting should be 30 µg/kg in bolus followed by an infusion at rate of 4 µg/kg/min.
b) The dose studied in the bridging therapy is an infusion of 0.75 µg/kg/min.
Other relevant aspects are the duration of infusion that can be reasonably maintained for at least 2 hours after the PCI. According to the drug technical label, it should be started before the procedure and the infusion lasting at least 2 hours or for as long as the pro- cedure lasts, which ever is longer. Still, this may not be enough in situations where the oral drugs early action is delayed. Also, special care is needed when transitioning to oral drugs; in general, the recommendation here is that ticagrelor can be administered at all times (before, during or after infusion) while thienopyridines can be prescribed after infusion (to avoid an interaction that would imply a time lapse without the proper antiplatelet therapy).3
Taking all this into consideration, cangrelor should be primarily used in the cath lab in situations of periprocedural high thrombotic risk when oral iP2Y12 pretreatment has not occurred, is ill-advised or insufficient. The availability of the drug will probably not change our antiplatelet strategy radically in the short term in the PCI setting. However, in some situations (especially primary percutaneous coronary interventions) its particular pharmacological profile will be very useful. Therefore, its use will probably grow in the interventional cardiology community as we become more familiar with it. In conclusion, cangrelor is an interesting addition to our therapeutic armamentarium in the PCI setting because it can individualize and, therefore, optimize our antithrombotic strategy.
CONFLICTS OF INTEREST
J.L. Ferreiro has declared he has received funds for his lectures for Eli Lilly Co., Daiichi Sankyo, Inc., AstraZeneca, Roche Diagnostics, Pfizer, Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, and Ferrer; also, he has received funds for his counselling for AstraZeneca, Eli Lilly Co., Ferrer, Boston Scientific, Pfizer, Boehringer Ingelheim, Daiichi Sankyo, Inc., Bristol-Myers Squibb; he has also receive research grants from AstraZeneca. J.A. Gómez-Hospital has declared he has received funds for his counselling for Abbott, Medtronic, Boston Scientific, Terumo, and IHT.
REFERENCES
1. Bhatt DL, Stone GW, Mahaffey KW, et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med. 2013;368: 1303-1313.
2. Kengrexal. Technical label or summary of product characteristics. Available online: https://cima.aemps.es/cima/pdfs/es/ft/115994001/FT_115994001.pdf. Accessed 18 May 2020.
3. Marcano AL, Ferreiro JL. Role of New Antiplatelet Drugs on Cardiovascular Disease: Update on Cangrelor. Curr Atheroscler Rep. 2016;18:66.
4. Ferreiro JL, Sánchez-Salado JC, Gracida M, et al. Impact of mild hypothermia on platelet responsiveness to aspirin and clopidogrel: an in vitro pharmacodynamic investigation. J Cardiovasc Transl Res. 2014;7:39-46.
5. Harrington RA, Stone GW, McNulty S, et al. Platelet inhibition with cangrelor in patients undergoing PCI. N Engl J Med. 2009;361: 2318-2329.
6. Bhatt DL, Lincoff AM, Gibson CM, et al. Intravenous platelet blockade with cangrelor during PCI. N Engl J Med. 2009;361:2330-2341.
7. White HD, Chew DP, Dauerman HL, et al. Reduced immediate ischemic events with cangrelor in PCI: a pooled analysis of the CHAMPION trials using the universal definition of myocardial infarction. Am Heart J. 2012;163:182-190.e4.
8. Steg PG, Bhatt DL, Hamm CW, et al. Effect of cangrelor on periprocedural outcomes in percutaneous coronary interventions: a pooled analysis of patient-level data. Lancet. 2013;382:1981-1992.
9. Ferreiro JL. Pre-Treatment With Oral P2Y12 Inhibitors in Non-ST-Segment Elevation Acute Coronary Syndromes: Does One Size Fit All? JACC Cardiovasc Interv. 2020;13:918-920.
10. Grimfjärd P, Lagerqvist B, Erlinge D, Varenhorst C, James S. Clinical use of cangrelor: nationwide experience from the Swedish Coronary Angiog raphy and Angioplasty Registry (SCAAR). Eur Heart J Cardiovasc Pharmacother. 2019;5:151-157.