ABSTRACT
Introduction and objectives: Drug-eluting balloon (DEB) angioplasty is an effective technique to treat in-stent restenosis (ISR). Neointimal modification with cutting balloon (CB) or scoring balloon (SB) enhances the angiographic results of DEB, but with no benefits have been reported in the clinical endpoints at the mid-term. There is lack of information on the clinical long-term results of this strategy. We aim to compare very long-term results of CB before DEB vs standard DEB to treat real-world patients with ISR.
Methods: Retrospective cohort registry of DEB PCIs to treat ISR defined by the use of CB. The primary endpoint was clinically driven target lesion revascularization (TLR) at 5 years. The secondary endpoints were based on the ARC-2 criteria.
Results: From January 2010 to December 2015, 107 ISRs were treated with DEB in 91 patients. CBs were used in 51 lesions (46 patients). Both cohorts were well balanced regarding clinical, lesion, and procedural characteristics. Compared to standard DEBs, CBs showed lower, although statistically non-significant rates, of TLR at 5 years (9.8% vs 23.6%, OR, 0.36; 95% confidence interval 0.19 to 1.09 P = .05). The Kaplan-Meier cumulative incidence of time until TLR showed similar results (log-rank test P value = .05) with similar rates of TLR at 1 year (3.9% vs 7.1%, P = .68) as curve separation in the long-term. There were no differences in the secondary endpoints. No stent thrombosis was reported.
Conclusions: In a real-world setting, neointimal modification with CB before DEB vs standard DEB to treat ISR shows lower, although statistically non-significant rates of TLR at 5 years. This benefit has been confirmed in the long-term and is consistent with bare-metal and drug-eluting stents.
Keywords: Drug-eluting balloon. In-stent restenosis. Cutting/scoring balloon.
RESUMEN
Introducción y objetivos: El uso de balón farmacoactivo (BFA) es una estrategia efectiva en el tratamiento de la reestenosis de stents coronarios (RIS). La modificación neointimal con balón de corte (BC) o incisión junto con BFA se asocia a mejores resultados angiográficos, aunque sin impacto en eventos clínicos a medio plazo. Los resultados clínicos de esta estrategia a muy largo plazo en la vida real son desconocidos. Se evaluó la eficacia de BC junto con BFA frente a BFA estándar en un registro de pacientes de la vida real con RIS a muy largo plazo (5 años).
Métodos: Registro retrospectivo de 2 cohortes de pacientes con RIS tratados con BFA, definidas por el uso de BC. El evento primario fue la tasa de revascularización clínicamente indicada de la lesión tratada a 5 años. Se valoraron eventos secundarios según los criterios ARC-2.
Resultados: Entre enero de 2010 y diciembre de 2015 se usó BFA en 107 RIS en 91 pacientes. En 51 lesiones (46 pacientes) se utilizó BC. Ambas cohortes presentaron similares características clínicas y de procedimiento. Respecto al uso estándar de BFA, el BC consiguió una reducción numérica, pero no significativa, en la tasa de revascularización de la lesión tratada a 5 años (9,8% frente a 23,6%; odds ratio = 0,36; intervalo de confianza del 95%, 0,19-1,09; p = 0,05). El análisis de incidencia acumulada de Kaplan-Meier mostró resultados parecidos (log-rank, p = 0,05), con similar tasa de eventos a 1 año (3,9% frente a 7,1%; p = 0,68), y separación de las curvas con el tiempo. No se evidenciaron diferencias en los eventos secundarios. No hubo trombosis de stent en la cohorte.
Conclusiones: En una cohorte de la vida real, la modificación neointimal de la RIS con BC junto con BFA, en comparación con BFA estándar, logra una reducción numérica, pero no significativa, en la tasa de revascularización de la lesión tratada a 5 años. El beneficio de esta estrategia se evidencia a largo plazo y es consistente entre RIS de stent convencional y de stent farmacoactivo.
Palabras clave: Balon farmacoactivo. Reestenosis. Balon de corte.
Abreviaturas
BC: balón de corte o incisión. BFA: balón farmacoactivo. RIS: reestenosis de stent coronario. RLT: revascularización de la lesión tratada. SFA: stent farmacoactivo. SM: stent convencional.
INTRODUCTION
In-stent restenosis (ISR) is a common problem in the routine clinical practice regarding percutaneous coronary intervention (PCI), and its management is associated with high rates of target lesion revascularization (TLR).1 Together with the implantation of a new everolimus drug-eluting stent, the PCI with drug-coated balloon (DCB) is the strategy of choice to treat ISR after bare-metal stent (BMS) and drug-eluting stent (DES) implantation, and has a class I indication after confirmation that it can reduce the rate of TLR at the follow-up without having to implant a new layer of metal into the artery.2-5 Despite of this, TLR is still high in the long-term (up to 20% at 3 years),6-11 which is suggestive that new strategies may be needed to improve these results.
The cutting balloon (CB) consists of small blades or nitinol bands on its surface to optimize the predilatation of coronary lesions by performing controlled fractures of the atheromatous plaque. Compared to the plain old balloon angioplasty, its use for the management of ISR is associated with structural changes of the neointima and acute improvements of the lumen area,12 although no angiographic or clinical benefit has been reported in the mid-term.13,14
The efficacy of the DCB depends on the transfer of drug from the surface of the balloon to the tissue where it exerts it antiproliferative effect.15 Theoretically speaking, greater the neointimal disarrays are associated with more effective transfers and smaller issue thickness. As a matter of fact, preclinical studies have suggested a greater effect of DCB inhibiting neointimal growth.16 This greater disarray and reduction of the neointima can be achieved using a CB before the DCB.
Although this hypothesis has not been confirmed in animal models in the short-term,17 the strategy has shown better angiographic results in the mid-term (6 to 8 months) (significant reduction of binary restenosis), but no effect on TLR or clinical events at the 1-year follow-up.18 No long-term results have been published on the use of this strategy.
Our objective was to assess the very long-term results of the use of CB plus DCB to treat ISR.
METHODS
Retrospective registry of cohorts of real-world patients with, at least, 1 ISR treated with DCB at a single high-volume PCI center (> 800/year) and a 5-year follow-up. Two different cohorts were defined based on the use of CB prior to the PCI with DCB (C_DCB) or standard DCB (S_DCB). The C_DCB cohort was defined by the use of, at least, 1 cutting balloon (Flextome Cutting Balloon, Boston Scientific, United States) or 1 scoring balloon (ScoreFlex, OrbusNeich, China). The use of the CB was left to the operator’s discretion. The ISR was defined as an angiographic stenosis > 50% in 2 different orthogonal radiographic projections inside the stent or < 5 mm from its borders plus symptoms of angina or objective confirmation of myocardial ischemia or fractional flow reserve/positive instantaneous wave-free ratio. Lesions were treated with 2 types of drug-coated balloons based on their availability at the time: the SeQuent Please (B. Braun Surgical, Germany) or the Pantera Lux (Biotronik, Switzerland). Data on the long-term progression of patients with ISR treater with the SeQuent Please DCB in this cohort regardless of the use of CB were reported beforehand.19
Exclusion criteria were cardiogenic shock or cardiac arrest in the index event, the presence of ≥ 3 layers of metal in the lesion with ISR and a contraindication to dual antiplatelet therapy with acetylsalicylic acid and a P2Y12 inhibitor for, at least, a month.
The clinical and procedural characteristics were obtained from the center and the cath lab databases. The coronary study of the lesions was performed with the Xcelera system (Philips, The Netherlands) using the projection with the highest degree of stenosis. The Mehran classification of ISR was used to categorize the lesions.20 The strategy of the procedure including the use and type of CB was left to the operator’s criterion. DCB dilatation lasted for, at least, 60 seconds at nominal pressure. The PCI, management, and previous and later treatment of the patients was performed based on the routine clinical practice.
The study was conducted in observance of the criteria established at the Declaration of Helsinki and the International Council on Harmonization Good Clinical Practice guidelines (ICH-GCP). Also, it was authorized by Hospital Clínico Lozano Blesa (Zaragoza, Spain) management and ethics committee. No informed consents were needed given the retrospective nature of the study. A 5-year long follow-up period was arranged. Every follow-up was performed by checking the electronic database of the regional healthcare system where all the patient’s clinical events were thoroughly detailed. Data were anonymized through internal numerical identification at the cath lab.
All events were defined in a standard way according to the ARC-2 consensus.21 The primary endpoint was the need for TLR with a clinically indicated DCB at 5 years and estimated on the overall number of all target lesions. Clinically indicated TLR was defined as a new-onset ISR > 70% or > 50% of the target lesion in the presence of ischemic symptoms, a positive inducible ischemia on stress testing dependent on the vessel or fractional flow reserve values ≤ 0.80 or instantaneous wave-free ratio values ≤ 0.89.
Secondary endpoints were the presence or lack of target vessel revascularization, and target vessel myocardial infarction (according to the universal definition22), all-cause mortality, death due to cardiac causes (acute myocardial infarction, severe arrhythmia, heart failure, unwitnessed or unknown death) or cardiovascular death (cardiac or stroke induced or due to other cardiovascular processes), BARC type ≥ 3 bleeding, stroke (new neurologic deficit > 24 h duration) or a composite endpoint of target lesion failure (TLR + target vessel myocardial infarction + cardiovascular death), target vessel failure (target vessel revascularization + target vessel myocardial infarction + cardiovascular death) or patient-oriented composite endpoint (any revascularization + acute myocardial infarction + stroke + overall death). These endpoints were estimated on the overall number of patients. Definitive or probable stent thrombosis was also defined based on the ARC-2 criteria and estimated on the overall number of lesions.
Data mining and analysis were performed using the SPSS 19.0. statistical software (IBM, United States). Quantitative variables were expressed as mean and standard deviation. Qualitative variables were expressed as relative percentage. The cumulative incidence of the endpoints at the follow-up was also estimated. The variables and the group endpoints studied were compared on a bivariate analysis using the chi-square test (or Fisher’s exact test, when appropriate) or the Student t test regarding the quantitative variables. Cox regression analysis was performed to estimate the primary endpoint predictors (including the variables associated with P values < .1). Survival was analyzed using the Kaplan-Meier method to build the cumulative incidence curve of time to the primary endpoint based on the strategy of treatment used. P values < .05 were considered statistically significant.
RESULTS
A total of 107 ISRs were treated with DCBs in 95 procedures performed on 91 patients from January 2010 through December 2015 (in 4 patients the PCI with DCB was repeated at the follow-up, in 1 case using a different DCB on the same previously treated lesion). A total of 51 lesions (42 patients) were treated with a PCI plus CB + DCB (C_DCB), and 56 lesions (49 patients) with standard DCB (S_DCB). A total of 53 lesions were treated with the SeQuent Please device, and 54 with the Pantera Lux. The cutting balloon and the scoring balloon were used in 36 and 15 lesions, respectively.
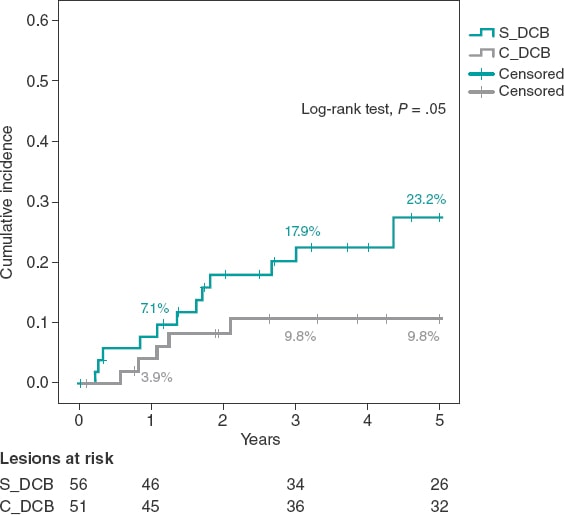
The study cohorts were similar regarding the clinical characteristics (table 1), and the lesion and procedural characteristics (table 2). Some of the differences reported in the C_DCB group where that radial access was more common, and the size of the stent and minimum lumen diameter were greater, although with a similar percent diameter stenosis of the lesion before and the after the PCI. Patients had a high prevalence of cardiovascular risk factors including diabetes in 35% of the cases. A total of 47 new coronary angiographies were performed at the follow-up. In 29 of these the target lesion had good results. The rate of new coronary angiography was similar in both groups (44.6% vs 41.2% in the C_DCB group. P = .71). A total of 18 TLRs were performed at the follow-up (16.8%) of which 17 were treated with a PCI (16 stent-in-stent), and 1 with coronary artery bypass graft. The rate of TLR was numerically lower in the C_DCB group at 1 (3.9% vs 7.1%; P = .68) and 3 years (9.8% vs 17.9%; P = .23). Compared to the S_DCB strategy, the use of the C_DCB reduced the 5-year rate of TLR although not statistically significant (9.8% vs 23.2%; OR, 0.36; 95% confidence interval [95%CI], 0.19-1.09; P = .05). The Kaplan-Meier analysis of the cumulative incidence curve revealed the differences seen at the 5-year follow-up (log-rank test, P = .05) with a similar 1-year event rate and curve separation consistent with the passing of the follow-up period (figure 1).
Table 1. Baseline characteristic of the patients
| S_DCB | C_DCB | P | |
| N = 49 patients/ 56 lesions | N = 42 patients/ 51 lesions | ||
| Age | 68.9 ± 11.3 | 67.7 ± 10 | .58 |
| Male | 85.7% (35) | 83.3% (35) | .75 |
| Arterial hypertension | 26.8% (14) | 23.8% (10) | .6 |
| Dyslipidemia | 46.9% (23) | 28.6% (12) | .7 |
| Smoking | 61.2% (30) | 57.1% (24) | .69 |
| Diabetes | 37.5% (21) | 35.3% (18) | .81 |
| AF in oral anticoagulants | 22.4% (11) | 19% (8) | .38 |
| Previous myocardial infarction | 55.1% (27) | 50% (21) | .62 |
| Previous coronary artery bypass graft | 6.1% (3) | 4.8% (2) | 1 |
| CKD (GFR < 60mL/min) | 32.7% (16) | 33.3% (14) | .94 |
| LVEF (%) | 54 ± 10 | 55 ± 9 | .51 |
|
AF, atrial fibrillation; CKD, chronic kidney disease; GFR, glomerular filtration rate; LVEF, left ventricular ejection fraction. |
|||
Table 2. Lesion and procedural characteristics
| S_DCB | C_DCB | P | S_DCB | C_DCB | P | |||
|---|---|---|---|---|---|---|---|---|
| N = 49 patients/ 56 lesions | N = 42 patients/ 51 lesions | N = 49 patients/ 56 lesions | N = 42 patients/ 51 lesions | |||||
| Procedural characteristics | Lesion characteristics | |||||||
| Clinical signs | .87 | Location of ISR | .35 | |||||
| Stable angina | 55.4% (31) | 56.9% (29) | LAD | 53.6% (30) | 45.1% (23) | |||
| Unstable angina/NSTEACS | 41.1% (23) | 41.2% (21) | LCX | 23.2% (13) | 15.7% (8) | |||
| STEACS | 3.6% (2) | 2% (1) | RCA | 16.1% (9) | 31.4% (16) | |||
| Radial access | 55.4% (31) | 78.4% (40) | .01 | LMCA | 5.4% (3) | 3.9% (2) | ||
| DCB caliber (mm) | 3.03 ± 0.37 | 3.15 ± 0.42 | .13 | Coronary artery bypass graft | 1.8% (1) | 3.9% (2) | ||
| DCB length (mm) | 20.2 ± 5.8 | 19.5 ± 4.7 | .53 | Mehran's angiographic classification of ISR pattern | .42 | |||
| DCB inflation pressure (atm) | 14 ± 3 | 14 ± 3 | .81 | IA | 1.8% (1) | 3.9% (2) | ||
| CB caliber (mm) | N/A | 2.93 ± 0.45 | IB | 3.6% (2) | 0% (0) | |||
| CB length (mm) | N/A | 8 ± 3 | IC | 41.1% (23) | 49% (25) | |||
| CB inflation pressure (atm) | N/A | 14 ± 3 | ID | 1.8% (1) | 3.9% (2) | |||
| NCB | 53.6% (30) | 70.6% (36) | .07 | II | 21.4% (12) | 27.5% (14) | ||
| NCB caliber (mm) | 3.12 ± 0.42 | 3.28 ± 0.43 | .14 | III | 21.4% (12) | 11.8% (6) | ||
| NCB length (mm) | 13.2 ± 3.1 | 12.6 ± 3.8 | .65 | IV | 8.9% (5) | 3.9% (2) | ||
| NCB inflation pressure (atm) | 18 ± 4 | 18 ± 3 | .74 | ISR based on type of stenting | .4 | |||
| Intracoronary imaging | 8.9% (5) | 5.9% (3) | .55 | BMS | 53.6% (30) | 37.3% (19) | ||
| Multivessel disease | 62.7% (32) | 47.7% (21) | .14 | DES | 33.9% (19) | 45.1% (23) | ||
| Complete revascularization | 82.4% (42) | 93.2% (41) | .13 | DES in BMS | 8.9% (5) | 11.8% (6) | ||
| P2Y12 inhibitor | .64 | DES in DES | 3.6% (2) | 5.9% (3) | ||||
| Clopidogrel | 88.2% (45) | 81.6% (36) | Time from implantation | 4.1 ± 4.8 | 3.8 ± 5 | .69 | ||
| Prasugrel | 3.9% (2) | 4.5% (2) | Bifurcation | 32.1% (18) | 23.5% (12) | .32 | ||
| Ticagrelor | 7.8% (4) | 13.6% (6) | Stent caliber (mm) | 2.96 ± 0.43 | 3.1 ± 0.56 | .02 | ||
| Duration of dual antiplatelet therapy | .27 | Stent length (mm) | 22.4 ± 6.5 | 22.8 ± 7.1 | .75 | |||
| 1 month | 3.9% (2) | 2.3% (1) | Reference diameter (mm) | 2.98 ± 0.48 | 3.12 ± 0.53 | .16 | ||
| 3 months | 21.6% (11) | 9.1% (4) | Minimum lumen diameter (mm) | 0.73 ± 0.51 | 0.68 ± 0.5 | .67 | ||
| 6 months | 21.6% (11) | 34.1% (15) | Length (mm) | 13.2 ± 5.6 | 11.7 ± 5.3 | .18 | ||
| 12 months | 52.9% (27) | 54.5% (24) | Stenosis (%) | 72 ± 18 | 75 ± 16 | .3 | ||
| Minimum lumen diameter post-PCI (mm) | 2.43 ± 0.46 | 2.77 ± 0.62 | .002 | |||||
| Acute lumen gain (mm) | 1.7 ± 0.64 | 2.08 ± 0.83 | .01 | |||||
| Stenosis post-PCI (%) | 14 ± 5 | 14 ± 6 | .45 | |||||
| Final TIMI grade 3 flow | 98.2% (55) | 100% (51) | 1 | |||||
|
BMS, bare-metal stent; CB, cutting balloon; DCB, drug-coated balloon; DES, drug-eluting stent; ISR, in-stent restenosis; LAD, left anterior descending coronary artery; LCX, left circumflex artery; LMCA, left main coronary artery; NCB, non-compliant balloon; NSTEACS, non-ST-segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; RCA, right coronary artery; STEACS, ST-segment elevation acute coronary syndrome; TIMI, Thrombolysis in Myocardial Infarction. |
||||||||
Figure 1. Kaplan-Meier analysis of the 5-year cumulative incidence of target lesion revascularization. DCB, drug-coated balloon.
The 5-year cumulative incidence of secondary endpoints is shown on table 3. The incidence rate of target vessel-related composite endpoints (target lesion failure and target vessel failure) was numerically lower in the C_DCB group although not statistically significant. No differences were found in the remaining secondary endpoints. The overall mortality rate at the follow-up was 31.8% (n = 19) being neoplasms the most common cause (n = 7). The incidence rates of stroke and patient-oriented composite endpoint were high (10.9% and 51.6%, respectively), which was consistent with an old cohort with high cardiovascular risk. No cases of definitive or probable stent thrombosis were seen at the follow-up.
Table 3. 5-year cumulative incidence of primary and secondary endpoints
| S_DCB | C_DCB | P | |
|---|---|---|---|
| N = 49 patients/ 56 lesions | N = 42 patients/ 51 lesions | ||
| Primary endpoint | |||
| TLR (clinically justified) | 23.2% (13/56) | 9.8% (5/51) | .05 |
| Secondary endpoints | |||
| Target vessel revascularization | 28.6% (16/56) | 17.6% (9/51) | .18 |
| Any revascularization | 28.6% (14/49) | 26.2% (11/42) | .8 |
| Target vessel myocardial infarction | 7.1% (4/56) | 5.9% (3/51) | .79 |
| Myocardial infarction | 18.3% (9/49) | 7.2% (3/42) | .19 |
| Death due to cardiac causes | 4.1% (2/49) | 4.8% (2/42) | 1 |
| Cardiovascular death | 16.3% (8/49) | 11.9% (5/42) | .54 |
| Overall mortality | 36.7% (18/49) | 26.2% (11/42) | .28 |
| Stroke | 10.2% (5/49) | 11.9% (5/42) | .55 |
| BARC type 3-5 bleeding | 7.1% (4/49) | 3.9% (2/42) | .68 |
| Target lesion failure | 37.5% (21/56) | 25.5% (13/51) | .18 |
| Target vessel failure | 41.1% (23/56) | 25.5% (13/51) | .08 |
| POCE | 53.1% (26/49) | 50% (21/42) | .77 |
|
BARC, Bleeding Academic Research Consortium; DCB, drug-coated balloon; POCE, patient-oriented composite endpoints; TLR, target lesion revascularization. |
|||
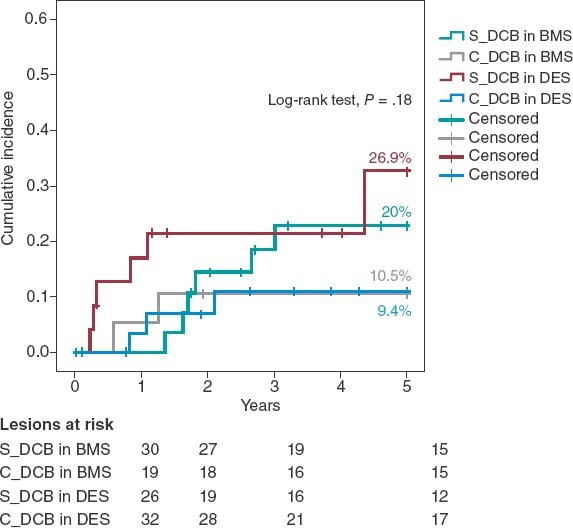
A Kaplan-Meier subanalysis based on ISR after BMS or DES implantation showed that the C_DCB strategy consistently reduced the 5-year rate of TLR in half with both types of stent although not statistically significant (figure 2).
Figure 2. Kaplan-Meier analysis of the 5-year cumulative incidence of target lesion revascularization based on whether the stent is made out of metal or is drug-eluting. BMS, bare-metal stent; DCB, drug-coated balloon, DES, drug-eluting stent.
Aside from the C_DCB no association was found between the variables and the 5-year rate of TLR except for the location of ISR that was 100% in cases found in coronary artery bypass graft stents (3 cases) compared to 14.4% in cases found in the native coronary tree (P = .003). The 5-year rate of TLR was similar in diabetic patients (17.9% vs 16.2%; P = .81) in the ISR of DESs (17.2% vs 16.3%; P = .9) and in stents < 3 mm (12.9% vs 18.4%; P = .58) without any differences based on the type of DCB used (Sequent, 20.4% vs Pantera, 13.2%; P = .32). In the Cox regression analysis, the use of the C_DCB was not an independent predictor of TLR at 5 years being the ISR of a coronary artery bypass graft the only independent predictor (OR, 5.4; 95%CI, 1.5-19.8; P = .01).
DISCUSSION
As far as we know, the study presented here is the first one to confirm:
-
- The use of a CB in connection with a DCB in the ISR setting shows a tendency to reduce the rate of TLR.
-
- The benefit of this strategy is evident in the long-term.
-
- The benefit seems to be consistent in ISR after BMS and DES implantation.
-
- The strategy is safe and there are no traces of stent thrombosis when a CB is used.
Compared to the plain old balloon angioplasty for the management of ISR, the CB achieves greater lumen areas because it breaks down the elastic and fibrotic continuity of the neointima by reducing its integrity and resistence.12 However, this acute angiographic improvement is not associated with lower but high rates of TLR (18% to 29%) at the 1-year follow-up.13,14 Similarly, in our series, the use of the CB is associated with a significant increase of minimum lumen diameter and acute gain after the procedure (table 2) despite the fact that the caliber of non-compliant balloons and DCBs was similar between both groups. Although stent diameter was slightly larger in the C_DCB group, the final percent stenosis did not change significantly between both groups; still, this may be an important piece of information in our results since the size of the vessel has been described as an independent predictor of new restenosis.23
The use of the DCB to treat ISR is something common after several meta-analyses revealed that, together with DES implantation with in-stent everolimus, this strategy is the most effective one to avoid new revascularizations.2-4 Afterwards, in the RIBS IV (with DES) and RIBS V (with BMS) clinical trials Alfonso et al. proved the long-term superiority of DES implantation with in-stent everolimus.8,9,24 However, the philosophy of not adding a new metal layer (or delay it through time) and questions associated with its long-term safety10,11 have turned DCB implantation into a common practice to treat ISR. Added to the RIBS IV-V studies, other trials have reported on the long-term effectiveness of DCB (PEPCAD7 with BMS, and PEPCAD-DES6 and ISAR-DESIRE 310 with DES). Overall, in these 5 studies, a total of 94 TLRs were reported in 524 ISRs treated with DCB, which is a 3-year rate of TLR of 17.9%. These results are accurately reproduced in our S_DCB cohort with rates high enough to justify looking into ways to improve the efficacy of DCBs.
The efficacy of DCBs is based on a transfer of the drug to the neointima of ISR where it exerts its antiproliferative effect. The proper preparation of the lesion by reducing neointimal thickening and increasing the surface of contact with the balloon is the key to achieve successful DCB implantations.15 Preclinical studies suggest that greater neointimal disarrays can increase the release and retention of the drug into the tissue, thus increasing its effects.16 Considering the greater acute lumen gain and controlled disarray that the CB provides, results can improve if used together with the DCB. This hypothesis was put to the test, but not proven, in a preclinical trial. The reason was that the use of the CB was not associated with a lower neointimal volume or acute lumen loss. Nonetheless, this assessment was made was very early (28 days).17
The synergistic effects of CB plus DCB were also confirmed by Scheller et al.25 in the PATENT-C trial. They took a different angle and studied the addition of an antiproliferative drug (paclitaxel) to the scoring balloon that reduced the 1-year rate of TLR significantly (3% vs 32%; P = .004). This information is consistent with the 1-year rate of TLR of 3.9 seen in our C_DCB cohort. From a new and different angle too, while still observing the philosophy of not leaving any material behind in the long-term after the PCI, Alfonso et al. conducted the RIBS VI Scoring trial and analyzed the impact of a CB before bioresorbable scaffold implantation to treat ISR. However, the 1-year rate of TLR was not reduced (9.8 vs 11.1%).26
Two randomized clinical trials have assessed the effect of CB implantation before DCB implantation to treat ISR. Aoki et al.27 found no angiographic differences at the 8-month follow-up in the ELEGANT trial. However, this was a comparative study vs a non-compliant balloon. Kufner et al.18 specifically tested the effects of CB implantation in the ISAR-DESIRE 4 trial. The primary endpoint was an angiographic result that confirmed that this strategy effectively reduced binary ISR at the 6 to 8-month follow-up. However, no differences were seen when the clinical events or TLR were assessed at the 1-year follow-up (16.2% vs 21.8%; P = .26). Qualitatively speaking, these results are consistent with what our series described because, although long-term benefits were reported, the 1-year rate of TLR did not change between our groups. No long-term data have ever been published so our cohort cannot be compared to corroborate the benefits described. Quantitatively speaking, we saw differences in the 1-year rate of TLR, much lower in our study (3.9% vs 7.1%). Three may be the reasons for this. In the first place, the scheduled angiographic assessment of the ISAR-DESIRE 4 trial because if we look at the Kaplan-Meier analysis of the TLR, in this study more clinical events were reported at the 6 to 8-month follow-up (when the angiographic assessment occurred). This is suggestive of a TLR guided by angiographic criteria (the so-called oculodilatory reflex) and not clinically justified as it was the case in our series. Secondly, the exclusive use of the scoring balloon vs the predominant use of the CB in our series since the CB achieves greater neointimal disarray and larger residual lumen diameters, thus increasing the efficacy of the DCB. Thirdly, the exclusive management of ISR after DES implantation vs ISR after any other type of stent implantation (BMS or DES) of our series since different authors have proposed the lower efficacy of the DCB to treat ISR after DES implantation.11,28 Based on this previous knowledge a subanalysis of the C_DCB strategy based on the type of stent used was conducted (figure 2). A consistent efficacy both in BMSs and DESs was seen with a similar 5-year rate of TLR in both subgroups (10.5% and 9.4% respectively)
Treating ISR with DCBs is a safe strategy associated with very low rates of stent thrombosis (around 1%) at the long-term follow-up.11 The role that a greater CB-induced neointimal tissue disarray plays in the appearance of thrombotic phenomena on the lesion is unknown. Consistent with the mid-term results of theISAR-DESIRE 4 trial, in our series, long-term target lesion thrombosis is null, which is a guarantee that the use of C_DCB is safe.
Limitations
Our study has several limitations. It is a retrospective, observational, and single-center study. Although the use of the DCB is the treatment of choice for the management of ISR in our center, it is possible that patients with more unfavorable ISR may have been excluded for having been treated with a DES. The use of intracoronary imaging was limited and the characterization of ISR could have given relevant information on the therapeutic strategy used and its long-term results. The size of the sample was not big enough to obtain powerful evidence. A larger sample size and longer follow-up is, therefore, guaranteed.
CONCLUSIONS
In a real-world cohort, changing the neointima of ISR with CB plus DCB vs standard DCB reduces the 5-year rate of TLR although not statistically significant. The benefit of this strategy is evident in the long-term and consistent between ISR after BMS and DES implantation.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
J.A. Linares Vicente: study design, data mining, and analysis and writing of the manuscript. J.R. Ruiz Arroyo: data and critical review of the manuscript. A. Lukic, B. Simó Sánchez, and O. Jiménez Meló: data mining. A. Riaño Ondiviela, P. Morlanes Gracia, and P. Revilla Martí: data mining.
CONFLICTS OF INTEREST
None reported.
WHAT IS KNOWN ABOUT THE TOPIC?
- The use of CB to treat ISR with DCB has been associated with better angiographic results although with no impact on the mid-term clinical events. The clinical outcomes of this long-term strategy are still unknown.
WHAT DOES THIS STUDY ADD?
- The use of CB plus DCB to treat ISR is associated with lower rates of TLR. The benefit of this strategy has been reported in the long-term. This benefit seems to be consistent with both ISR after BMS and DES implantation.
REFERENCES
1. Alfonso F, Byrne RA, Rivero F, Kastrati A. Current Treatment of In-Stent Restenosis. J Am Coll Cardiol. 2014;63:2659-2673.
2. Giacoppo D, Gargiulo G, Aruta P, Capranzano P, Tamburino C, Capodanno D. Treatment strategies for coronary in-stent restenosis: systematic review and hierarchical Bayesian network meta-analysis of 24 randomised trials and 4880 patients. BMJ. 2015;351:h5392.
3. Siontis GCM, Stefanini GG, Mavridis D, et al. Percutaneous coronary interventional strategies for treatment of in-stent restenosis: a network meta-analysis. Lancet. 2015;386:655-664.
4. Sethi A, Malhotra G, Singh S, Singh PP, Khosla S. Efficacy of Various Percutaneous Interventions for In-Stent Restenosis. Circ Cardiovasc Interv. 2015;8:e002778.
5. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
6. Rittger H, Waliszewski M, Brachmann J, et al. Long-Term Outcomes After Treatment With a Paclitaxel-Coated Balloon Versus Balloon Angioplasty. JACC Cardiovasc Interv. 2015;8:1695-700.
7. Unverdorben M, Vallbracht C, Cremers B, et al. Paclitaxel-coated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis: the three-year results of the PEPCAD II ISR study. EuroIntervention. 2015;11:926-934.
8. Alfonso F, Pérez-Vizcayno MJ, Cuesta J, et al. 3-Year Clinical Follow-Up of the RIBS IV Clinical Trial. JACC Cardiovasc Interv. 2018;11:981-991.
9. Alfonso F, Pérez-Vizcayno MJ, García del Blanco B, et al. Long-Term Results of Everolimus-Eluting Stents Versus Drug-Eluting Balloons in Patients With Bare-Metal In-Stent Restenosis. JACC Cardiovasc Interv. 2016;9;1246-1255.
10. Kufner S, Cassese S, Valeskini M, et al. Long-Term Efficacy and Safety of Paclitaxel-Eluting Balloon for the Treatment of Drug-Eluting Stent Restenosis. JACC Cardiovasc Interv. 2015;8:877-884.
11. Giacoppo D, Alfonso F, Xu B, et al. Drug-Coated Balloon Angioplasty Versus Drug-Eluting Stent Implantation in Patients With Coronary Stent Restenosis. J Am Coll Cardiol. 2020;75:2664-2678.
12. Ahmed JM, Mintz GS, Castagna M, et al. Intravascular ultrasound assessment of the mechanism of lumen enlargement during cutting balloon angioplasty treatment of in-stent restenosis. Am J Cardiol. 2001;88:1032-1034.
13. Albiero R, Silber S, di Mario C, et al. Cutting balloon versus conventional balloon angioplasty for the treatment of in-stent restenosis. J Am Coll Cardiol. 2004;43:943-949.
14. Park S-J, Kim K-H, Oh I-Y, et al. Comparison of Plain Balloon and Cutting Balloon Angioplasty for the Treatment of Restenosis With Drug-Eluting Stents vs Bare Metal Stents. Circ J. 2010;74:1837-1845.
15. Byrne RA, Joner M, Alfonso F, Kastrati A. Drug-coated balloon therapy in coronary and peripheral artery disease. Nat Rev Cardiol. 2014;11:13-23.
16. Radke P, Joner M, Joost A, et al. Vascular effects of paclitaxel following drug-eluting balloon angioplasty in a porcine coronary model: the importance of excipients. EuroIntervention. 2011;7:730-737.
17. Kong J, Hou J, Ma L, et al. Cutting balloon combined with paclitaxel-eluting balloon for treatment of in-stent restenosis. ArchCardiovasc Dis. 2013;106:79-85.
18. Kufner S, Joner M, Schneider S, et al. Neointimal Modification With Scoring Balloon and Efficacy of Drug-Coated Balloon Therapy in Patients With Restenosis in Drug-Eluting Coronary Stents. JACC Cardiovasc Interv. 2017;10:1332-1340.
19. Linares Vicente JA, Ruiz Arroyo JR, Lukic A, et al. 5 year-effectiveness of paclitaxel drug-eluting balloon for coronary in-stent restenosis in a real-world registry. REC Interv Cardiol. 2019;2:92-98.
20. Mehran R, Dangas G, Abizaid AS, et al. Angiographic Patterns of In-Stent Restenosis. Circulation. 1999;100:1872-1878.
21. Garcia-Garcia HM, McFadden EP, Farb A, et al. Standardized End Point Definitions for Coronary Intervention Trials: The Academic Research Consortium-2 Consensus Document. Circulation. 2018;137:2635-2650.
22. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). Circulation. 2018;138:e618-e651.
23. Cassese S, Xu B, Habara S, et al. Incidence and predictors of reCurrent restenosis after drug-coated balloon Angioplasty for Restenosis of a drUg-eluting Stent: The ICARUS Cooperation. Rev Esp Cardiol. 2018;71:620-627.
24. Alfonso F, Pérez-Vizcayno MJ, García del Blanco B, et al. Comparison of the Efficacy of Everolimus-Eluting Stents Versus Drug-Eluting Balloons in Patients With In-Stent Restenosis (from the RIBS IV and V Randomized Clinical Trials). Am J Cardiol. 2016;117:546-554.
25. Scheller B, Fontaine T, Mangner N, et al. A novel drug-coated scoring balloon for the treatment of coronary in-stent restenosis: Results from the multi-center randomized controlled PATENT-C first in human trial. Catheter Cardiovasc Interv. 2016;88:51-59.
26. Alfonso F, Cuesta J, García del Blanco B, et al. Scoring balloon predilation before bioresorbable vascular scaffold implantation in patients with in-stent restenosis: the RIBS VI 'scoring' study. Coron Artery Dis. 2021;32:96-104.
27. Aoki J, Nakazawa G, Ando K, et al. Effect of combination of non-slip element balloon and drug-coating balloon for in-stent restenosis lesions (ELEGANT study). J Cardiol. 2019;74:436-442.
28. Habara S, Kadota K, Shimada T, et al. Late Restenosis After Paclitaxel-Coated Balloon Angioplasty Occurs in Patients With Drug-Eluting Stent Restenosis. J Am Coll Cardiol. 2015;66:14-22.