Abstract
Introduction and objectives: To describe the efficacy of the BIOSS LIM C dedicated sirolimus-eluting stent to treat coronary bifurcation lesions, and impact on the bifurcation angle and carina through quantitative coronary angiography.
Methods: Observational prospective study including 124 patients with bifurcation lesions treated with a BIOSS LIM C dedicated sirolimus-eluting stent excluding restenotic lesions and those without main vessel involvement.
Results: The stent was successfully deployed in 121 patients (97.6%) while in 18 (14.5%) double stenting was used. The quantitative coronary analysis has shown proper stent expansion with a mean residual stenosis of 18% in the proximal segment, nearly 0% in the distal segment, and 21% in the side branch. The angiographic results of double stenting showed higher mean diameters (2.12 ± 0.30 vs 1.60 ± 0.42; P < .001), and lower residual stenosis (18.36 ± 9.94 vs 28.49 ± 14.19%, P < .01). Distortion imposed on the bifurcation angulation was minimal with an absolute reduction of 5 degrees (52.8 ± 18.4 vs 47.5 ± 17.2; P = .001).
Conclusions: The dedicated BIOSS LIM C stent has had a very high success rate to treat coronary bifurcation lesions. Angiographic results are good with a remarkably low impact on the native bifurcation angulation, and excellent results from double stenting. We think this can be a very useful device to treat coronary bifurcation lesions with the advantage of easing out the bailout deployment of a second stent into the side branch.
Keywords: Dedicated stent. Bifurcation lesion. BIOSS LIM C sirolimus-eluting stent.
RESUMEN
Introducción y objetivos: El estudio EPIC03-BIOSS se llevó a cabo para describir la eficacia del stent farmacoactivo dedicado BIOSS LIM C en el tratamiento de las lesiones en bifurcación, así como la modificación inducida sobre la lesión en bifurcación por angiografía cuantitativa automatizada.
Métodos: Estudio observacional prospectivo en el que se incluyeron 124 pacientes con lesión en bifurcación tratados con stent farmacoactivo BIOSS LIM C, excluidas las lesiones por reestenosis y aquellas en las que no había afección del vaso principal.
Resultados: El stent se implantó con éxito en 121 pacientes (97,6%); en 18 (14,5%) se utilizó una técnica de 2 stents. El análisis por angiografía cuantitativa automatizada mostró una estenosis residual media del 18% en el segmento proximal, de prácticamente el 0% en el segmento distal y del 21% en la rama lateral. Los resultados angiográficos para la técnica de doble stent muestran unos diámetros (2,12 ± 0,30 frente a 1,60 ± 0,42 mm; p < 0,001) y estenosis residuales (18,36 ± 9,94 frente a 28,49 ± 14,19%; p < 0,01) significativamente mejores. La distorsión sobre la angulación nativa del vaso resultó mínima, con una reducción absoluta de unos 5° (52,8 ± 18,4 frente a 47,5 ± 17,2°; p = 0,001).
Conclusiones: El stent BIOSS LIM C consigue una elevada tasa de éxito para el tratamiento de las lesiones en bifurcación. Los resultados angiográficos son buenos, destacando la escasa distorsión sobre la angulación nativa del vaso y los excelentes resultados. Consideramos que puede ser un buen dispositivo para el tratamiento de las bifurcaciones, la ventaja de poder facilitar la implantación no prevista de un segundo stent.
Palabras clave: Stent dedicado. Lesión en bifurcación. Stent liberador de sirolimus BIOSS LIM C.
Abbreviations
MB: main branch; OCT: optical coherence tomography; POT: proximal optimization technique; QCA: quantitative coronary angiography: SB: side branch.
INTRODUCTION
Percutaneous treatment of coronary bifurcation lesions can represent up to 20% of all coronary lesions treated.1 The definition of bifurcation lesion given by the European Bifurcation Club2 includes those that effect a relevant side branch (SB) whether by its angiographic diameter or the myocardium at risk associated with such SB.
The pseudo-fractal anatomy of coronary bifurcation lesions3 involves a significant caliber difference between proximal and distal segments. The study of the structural limits of tubular stents has favored the development of treatment techniques4,5 designed to optimize implantation in an anatomy that is not completely cylindrical with good angiographic and clinical results.6-10 Also, a progressive escalated strategy has been agreed based on parameters of damage to the SB from the provisional stenting technique to the complex double stenting one.
Although specific platforms have been designed to treat coronary bifurcation lesions, these have been limited for expert operator use only. On the one hand, dedicated stents can be categorized into stents designed to treat the main vessel by securing proper access to the SB, (Nile Croco & Pax, Minvasys, France, the Multi-Link Frontier, Abbott Vascular Devices, United States or the TAXUS Petal, Boston Scientific, United States). On the other hand, stents designed to treat the SB first to later complete the main branch (MB) with a tubular stent (Tryton Side Branch stent, Tryton Medical, United States; Sideguard, Cappella Inc, United States). However, because of the discrete results reported or their complexity, they have not become entirely popular.
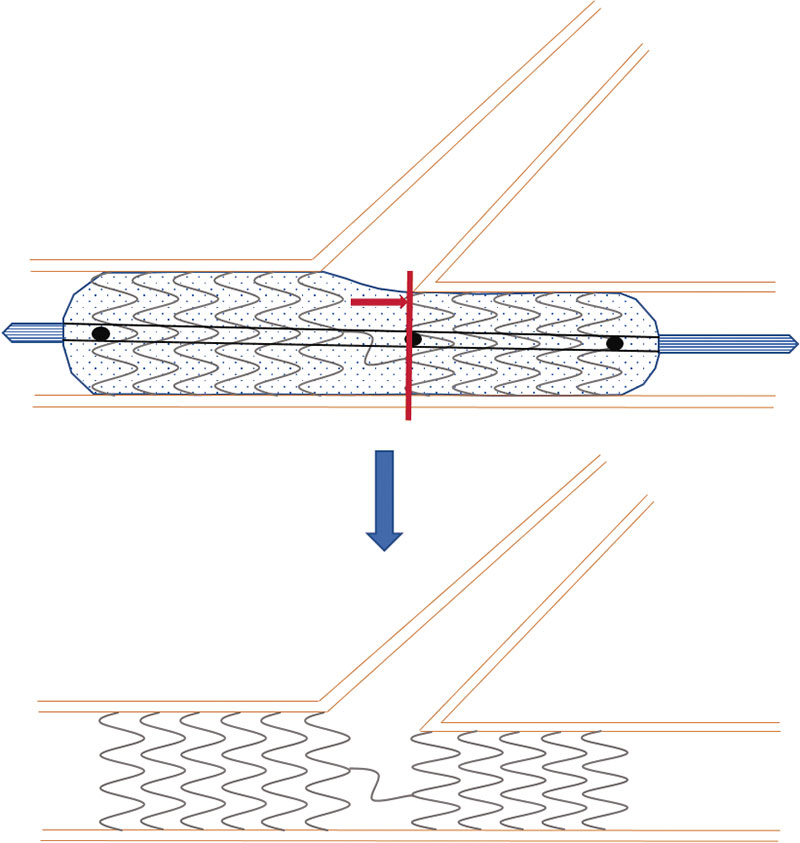
The BIOSS LIM C stent11 (Balton, Poland) is a 70 µm ultra-thin-strut chrome-cobalt platform with a sirolimus-eluting biodegradable polymer of polylactic acid. It is a dedicated stent for bifurcations that consists of 2 segments of different size linked by 2 long connective struts (figure 1). Goal is to keep the pseudo-fractal correlation between the proximal and distal portions of the SB and facilitate the technique to access the SB or the provisional stenting technique. Former studies have given good results with successful implantation rates of 100%, and target lesion revascularization rates from 6.8% to 9.8%.12-14
Figure 1. Image of the BIOSS stent design. Note the structure in 2 bodies with central space for the side branch.
The objective of this study is to describe immediate angiographic results assessed through quantitative coronary angiography (QCA) in terms of deformation of the lesion native angulation and expansion, especially at the level of the polygon of confluence and at the origin of the side branch.
METHODS
Patients
This is a prospective, multicenter registry started by independent investigators that included patients with ischemic heart disease referred for percutaneous coronary revascularization of a bifurcated lesion considered as a lesion with distal branches with a minimum diameter of 2 mm.
Patients with lesions damaging the bifurcation on the SB only were excluded. Also, patients with restenosis, complete total coronary occlusions, a contraindication for dual antiplatelet therapy, cardiogenic shock, minors or patients who gave their express rejection to be included.
Study was conducted according to the Declaration of Helsinki and the study protocol was approved by the different ethics committees of participant centers. The specific written informed consent was obtained from all the patients included in the study.
Procedure
Stenting was performed following the implantation recommendations of every device (figure 2) by implanting and performing the final control in the angiographic view with better deployment of bifurcation. Anticoagulation with sodium heparin or low-molecular weight heparin was administered according to the usual standards of every cath lab. Specific treatment of each coronary bifurcation lesion was left to the operator’s criterion. Predilatation of both branches, the provisional stenting technique or the early double stenting technique were allowed whenever, at least, 1 dedicated stent from the study was used.
Figure 2. Scheme of the positioning of the BIOSS stent for correct implantation. Central marker needs to be adjusted to the carina of bifurcation, and the ostium of the distal main branch.
Procedural success was defined as the implantation of a BIOSS LIM C stent into the bifurcation lesion with residual stenosis on visual estimate < 30% in the MB and 50% in the SB.
Clinical follow-up
Telephone or on-site follow-up was conducted at 30 days and 12 months. Patients were surveyed on adverse cardiovascular events of death, myocardial infarction, stroke, stent thrombosis, need for new revascularization or bleeding.
Angiographic analysis
Angiographic analysis was conducted independently by an imaging lab (BARCICORE-Lab, Spain) using a specific dedicated software for bifurcations (QAngio XA 7.3, The Netherlands) with which all angiographic measurements were acquired including the measurements of bifurcation angulation. Two analysts selected the images before and after implantation without the intracoronary guidewire and in the same view (< 10º of difference). Angiographic analysis was conducted in end-diastole following the lab internal protocols.
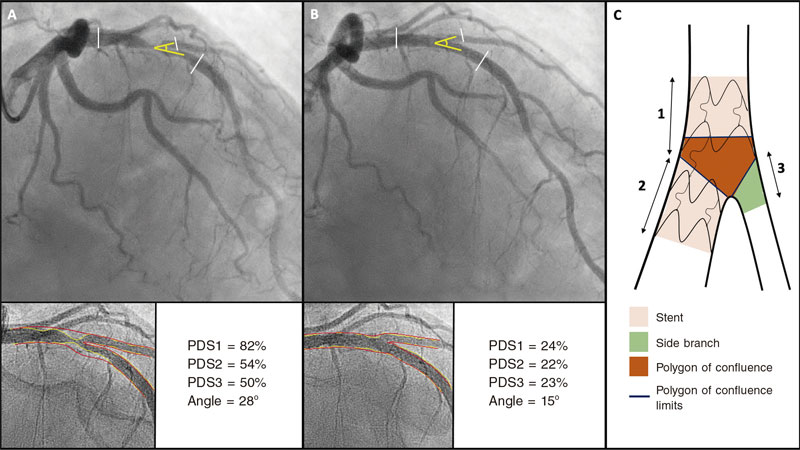
Software used allows us to measure the 3 segments of a bifurcation simultaneously (proximal MB, distal MB, and SB) and obtain individual results from all the segments including the polygon of confluence of bifurcation. All analyses were conducted taking the proximal and distal borders of the stent deployed as the reference framework. When the double stenting technique was used, the distal border of the stent implanted into the SB was used as the analysis distal limit. In case of single-stent implantation, only the proximal 5 mm of the SB were used. Figure 3 shows an example of angiographic analysis.
Figure 3. Quantitative coronary angiography showing the percentage diameter stenosis (PDS) at the proximal main vessel (1), distal main vessel (2), and 5 mm proximal to the side branch (3) before (A) and after the procedure (B). Also, it measures changes to the bifurcation angle between the distal branches. Image C shows the limits of each segment (1, 2, and 3), and the borders of the polygon of confluence.
Statistical analysis
All quantitative data are expressed as mean ± standard deviation (SD) while qualitative data are expressed as number (percentage). For the quantitative angiographic analysis (QCA) between angiographic values before and after implantation, Student t test was used for paired data (quantitative data) while the McNemar test (qualitative data) was used when appropriate. For comparison purposes between the cohorts treated with provisional stenting and double stenting, Student t test or the Mann-Whitney U test (quantitative data) were used. Also, the chi-square test or Fisher’s exact test (qualitative data) were used, when appropriate. P values ≤ .05 were considered statistically significant. Statistical analyses were conducted using the statistical software package SPSS version 20.
RESULTS
Baseline clinical data
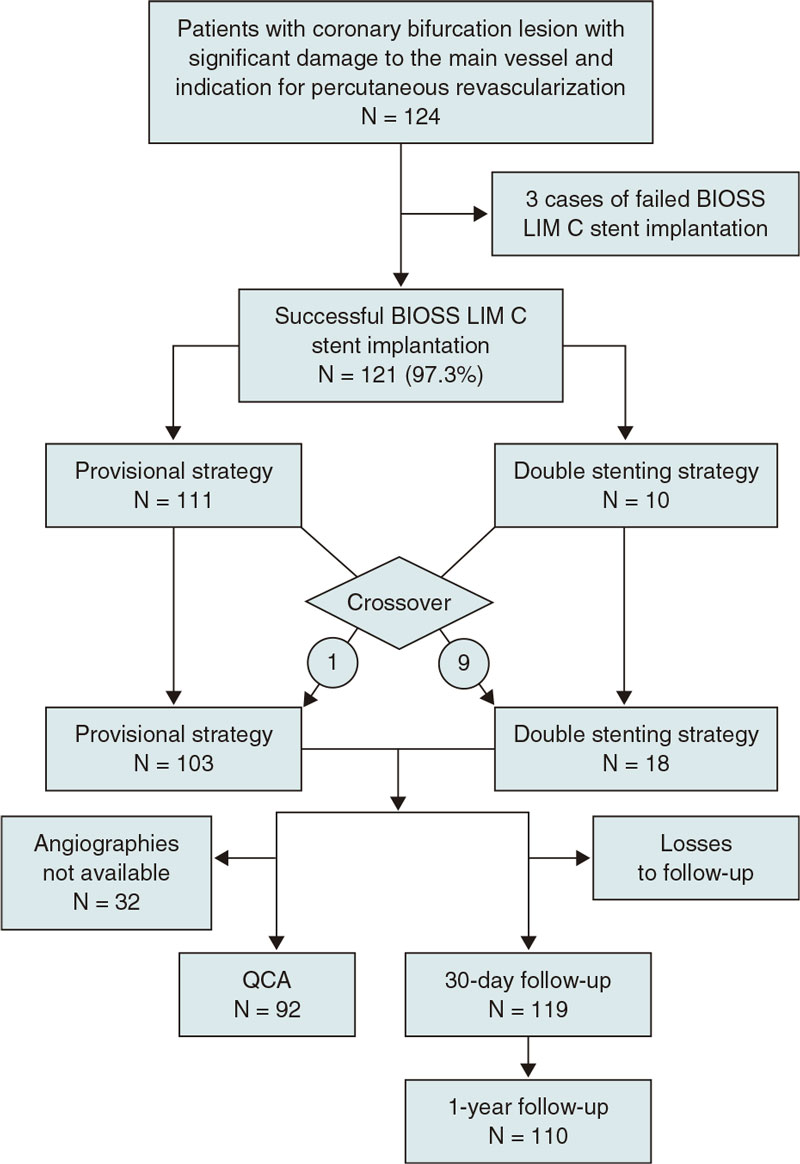
From August 2018 through February 2021 a total of 124 patients were included in the study (figure 4). The demographic data of patients are shown on table 1. We should mention the rates of patients with diabetes [26.9% (32/124)], and acute coronary syndrome [52,8% (66/124)], 12,8% (16/124) of whom had ongoing ST-segment elevation. No significant differences were reported in the baseline clinical characteristics between the single-stent and the double stenting cohorts.
Table 1. Description of population
| Total | |
|---|---|
| Baseline demographics | 124 |
| Feminine sex | 23 (18.47%) |
| Age | 65.48 (11.09) |
| Arterial hypertension | 79 (63.2%) |
| Dyslipidemia | 72 (57.6%) |
| Diabetes Mellitus | 32 (25.6%) |
| On insulin | 8 (6.4%) |
| Current smoker | 39 (31.2%) |
| Chronic kidney disease | 10 (8%) |
| Peripheral vasculopathy | 8 (6.4%) |
| Baseline treatment | |
| Acetylsalicylic acid | 81 (64.8%) |
| Clopidogrel | 34 (27.2%) |
| Ticagrelor | 18 (14.4%) |
| Prasugrel | 2 (0.6%) |
| Oral anticoagulant drugs | 9 (7.2%) |
| Vitamin K inhibitor | 1 (0.8%) |
| Direct-acting oral anticoagulants | 8 (6.4%) |
| Indication | |
| Stable angina | 37 (29.6%) |
| Silent ischemia | 12 (9.6%) |
| Ventricular dysfunction | 3 (2.4%) |
| STEACS | 6 (4.8%) |
| NSTEACS/unstable angina | 16 (12.8%) |
| NSTEACS/myocardial infarction | 34 (27.2%) |
| STEACS | 16 (12.8%) |
| AMI | 25 (20%) |
| Previous CABG | 4 (3.2%) |
| Previous PCI | 29 (23.2%) |
| Ejection fraction (%) | 54.31 (12.29) |
| Atrial fibrillation | 9 (7.2%) |
| Heart failure | 16 (12.8%) |
|
AMI, acute myocardial infarction; CABG, coronary artery bypass graft; NSTEACS, non-ST-segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; STEACS, ST-segment elevation acute coronary syndrome. |
|
Figure 4. Flowchart of the EPIC03-BIOSS study. QCA, quantitative coronary angiography.
Angiographic and procedural data
Angiographic and procedural data are shown on table 2 and table 3. Most procedures were performed via radial access (120, 96.8%) and the angiography revealed the presence of 3-vessel coronary artery disease in 19 patients (15.3%). In 10 cases (8%) the target lesion was found in the left main coronary artery. Regarding complexity, in 55 cases (44.3%) the lesions treated fell into the B2/C categories of the American Heart Association/American College of Cardiology while 32 lesions (25.8%) were categorized as moderate or severe.
Table 2. Angiographic data on visual estimate
| Total | Provisional | Complex | P | |
|---|---|---|---|---|
| Radial access | 120 (96%) | 110 (97.3%) | 10 (90.9%) | .314 |
| LMCA lesion | 11 (8.8%) | 10 (8.8%) | 1 (9.1%) | .830 |
| Proximal LAD | 77 (61.6%) | 71 (62.8%) | 6 (54.5%) | .746 |
| Coronary artery disease | .524 | |||
| 1 vessel | 55 (44%) | 51 (46.4%) | 4 (36.4%) | |
| 2 vessels | 47 (37.6%) | 43 (39.1%) | 4 (36.4%) | |
| 3 vessels | 19 (15.2%) | 16 (14.5%) | 3 (27.3%) | |
| Right dominance | 109 (87.2%) | 99 (87.6%) | 10 (90.9%) | .773 |
| Damaged bifurcation | .693 | |||
| LMCA-LAD/LCX | 10 (8%) | 8 (7.3%) | 2 (18.2%) | |
| LAD/Diagonal | 71 (56.8%) | 53 (57.5%) | 4 (54.6%) | |
| LCX/OMA | 28 (22.4%) | 25 (22.2%) | 3 (27.3%) | |
| RCA/PL | 15 (12.0%) | 15 (13.2%) | 0 (0%) | |
| Medina classification | .001 | |||
| 100 | 8 (6.4%) | 8 (7.3%) | 0 (0%) | |
| 010 | 18 (14.4%) | 18 (15.9%) | 0 (0%) | |
| 001 | 0 (0%) | 0 (0%) | 0 (0%) | |
| 110 | 38 (30.4%) | 38 (33.6%) | 0 (0%) | |
| 101 | 5 (4%) | 5 (4.4%) | 0 (0%) | |
| 011 | 10 (8%) | 6 (5.3%) | 4 (36.4%) | |
| 111 | 44 (35.2%) | 38 (33.6%) | 6 (54.5%) | |
| True | 59 (47.2%) | 49 (43.3%) | 10 (100%) | |
| Calcification (moderate or severe) | 32 (25.6%) | 28 (24.8%) | 4 (36.4%) | .542 |
| Proximal tortuosity | 25 (20%) | 20 (17.7%) | 5 (45.5%) | .04 |
| Thrombus | 15 (20%) | 14 (12.4%) | 1 (0.9%) | 1.000 |
| Type of lesion | .362 | |||
| A | 3 (2.4%) | 3 (2.7%) | 0 (0%) | |
| B1 | 37 (29.6%) | 31 (27.4%) | 6 (54.5%) | |
| B2 | 45 (36%) | 43 (38.1%) | 2 (18.2%) | |
| C | 10 (8%) | 10 (8.8%) | 0 (0.0%) | |
|
LAD, left anterior descending coronary artery; LCX, left circumflex artery; LMCA, left main coronary artery; OMA, obtuse marginal artery; PL, posterolateral; RCA, right coronary artery. |
||||
Table 3. Procedural description and follow-up
| Total | Provisional | Complex | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Procedure | 124 | 106 (85.5%) | 18 (14.5%) | |||||||
| Early strategy | 124 | 113 (90.4%) | 11 (9.6%) | |||||||
| Guide catheter | .289 | |||||||||
| 6-Fr | 113 (90.4%) | 104 (92%) | 9 (81.8%) | |||||||
| 7-Fr | 10 (8%) | 8 (7.1%) | 2 (18.2%) | |||||||
| 8-Fr | 1 (0.8%) | 1 (0.9%) | 0 (0%) | |||||||
| SB predilatation | 100 (80%) | 90 (79.6%) | 10 (90.9%) | .690 | ||||||
| Rotational atherectomy | 0 (0%) | 0 (0%) | 0 | 1.000 | ||||||
| Stenting | 121 (97.6%) | 111 (98.2%) | 10 (90.9%) | .314 | ||||||
| Length of stent | 19.73 (3.23) | 19.57 (3.21) | 21.45 (3.04) | .064 | ||||||
| POT | 33 (26.6%) | 30 (26.5%) | 3 (27.3%) | .800 | ||||||
| SB dilatation | 47 (37.9%) | 43 (38.1%) | 4 (36.4%) | .449 | ||||||
| Kissing after stenting the MB | 21 (44.7%) | 21 (48.8%) | 0 (0%) | |||||||
| SB dilatation only | 26 (55.3%) | 22 (51.2%) | 4 (100%) | |||||||
| Additional stenting | 17 (13.7%) | 16 (14.2%) | 1 (9.1%) | |||||||
| Stent into the SB | 18 (14.5%) | 9 (8%) | 9 (81.8%) | .000 | ||||||
| Kissing after stenting the SB | 16 (88.9%) | 7 (77.8%) | 9 (100%) | |||||||
| Imaging modalities | 11 (8.9%) | 8 (7.1%) | 3 (27.3%) | .023 | ||||||
| IVUS | 9 (7.3%) | 7 (6.2%) | 2 (18.2%) | |||||||
| OCT | 2 (1.6%) | 1 (0.9%) | 1 (9.1%) | |||||||
| Complications | 1 (0.8%) | 1 (0.9%) | 0 | |||||||
| Occlusion of the SB | 1 (0.8%) | 1 (0.9%) | 0 (0%) | |||||||
| Success (lack of stenosis ≥ 50%) | 114 (91.2%) | 104 (92%) | 10 (90.9%) | .338 | ||||||
| TIMI-flow grade at MB | .638 | |||||||||
| 3 | 114 (91.9%) | 103 (91.2%) | 11 (100%) | |||||||
| 2 | 0 (0%) | 0 (0%) | 0 (0%) | |||||||
| 1 | 3 (2.4%) | 3 (2.7%) | 0 (0%) | |||||||
| TIMI-flow grade at SB | .638 | |||||||||
| 3 | 114 (91.9%) | 103 (91.2%) | 11 (100%) | |||||||
| 2 | 0 (0%) | 0 (0%) | 0 (0%) | |||||||
| 1 | 3 (2.4%) | 3 (2.7%) | 0 (0%) | |||||||
| MB stenosis | 3.27% (6.14) | 3.24% (6.25) | 3.57% (4.76) | .892 | ||||||
| SB stenosis | 14.74% (19.94) | 15.55% (20.45) | 4.29% (4.5) | .211 | ||||||
| ≥ 50% stenosis of MB | 0 (0%) | 0 (0%) | 0 (0%) | 1.000 | ||||||
| ≥ 50% stenosis of SB | 7 (7.1%) | 7 (7.7%) | 0 (0%) | .585 | ||||||
| ≥ 30% stenosis of SB | 22 (22.4%) | 22 (24.2%) | 0 (0%) | .158 | ||||||
| 12-month follow-up | 110 (88.7%) | 92 (86.8%) | 18 (100%) | |||||||
| Death | 0 (0%) | 0 (0%) | 0 (0%) | |||||||
| Stent related AMI | 2 (1.8%) | 1 (1.1%) | 1 (5.5%) | .223 | ||||||
| Re-PCI | 6 (5.4%) | 5 (5.4%) | 1 (5.5%) | .555 | ||||||
| Thrombosis | 1 (0.9%) | 0 (0%) | 1 (5.5%) | .115 | ||||||
|
AMI, acute myocardial infarction; IVUS, intravascular ultrasound; OCT optical coherence tomography; POT, proximal optimization therapy; Re-PCI, re-percutaneous coronary intervention; SB, side branch; MB, main branch; TIMI, Thrombolysis in Myocardial Infarction. |
||||||||||
When we analyzed the differences between the cohorts treated with the provisional stenting technique and the double stenting one (table 2 and table 3) we saw that in the latter the rate of true bifurcations, proximal tortuosity, and use of imaging modalities was significantly higher.
During the procedure (table 3) the BIOSS LIM C stent was successfully implanted in 121 patients (97.6%). In the 3 cases when the stent was not implanted, the lesions showed moderate or severe calcification and proximal tortuosity. One case out of the 121 treated with stenting became complicated with SB occlusion following a dissection that could not be revascularized. In 30 (26.5%) out of the 113 cases (90.4%) where the early provisional stenting strategy was used, the POT (proximal optimization technique) was used. In 43 (38.1%) it was necessary to dilate the SB through kissing-balloon or simple dilatation (table 3). Finally, in 9 cases (7.2%) initially treated with the single-stent strategy, a second stent was needed in the SB. The double stenting strategy was initially adopted in 11 patients (9.6%). However, after SB dilatation and stent implantation into the MB the implantation of a second stent was deemed as unnecessary in 2 of them (18.2%). Therefore, 18 patients (14.5%) were eventually treated with the double stenting technique. The rates of predilatation, rotational atherectomy, use of POT, and successful implantation were similar in both cohorts.
Quantitative angiography of bifurcation
The angiographic images of 92 patients were available (table 4). Mean residual stenosis of 18% was seen in the proximal segment and nearly 0% in the MB distal segment. In the SB, the postoperative mean residual stenosis was 21% with significant residual stenosis in 5% of all patients treated with the provisional stenting technique.
Table 4. Quantitative coronary angiography of bifurcation in the entire cohort. Comparison between simple and double stent
| N = 92 lesions | Entire population (N = 92) | 1-stent technique (N = 75) | 2-stent technique (N = 17) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Pre | Post | P | Pre | Post | P | Pre | Post | P |
| Minimum lumen diameter, mm | 0.97 ± 0.48 | 1.70 ± 0.44 | < .001 | 0.99 ± 0.48 | 1.60 ± 0.42 | < .001 | 0.85 ± 0.47 | 2.12 ± 0.30 | < .001 |
| Maximum percentage diameter stenosis, % | 62.78 ± 17.70 | 26.62 ± 14.03 | < .001 | 61.63 ± 17.92 | 28.49 ± 14.19 | < .001 | 67.86 ± 16.25 | 18.36 ± 9.94 | < .001 |
| Carinal angle, degrees (º) | 52.8 ± 18.4 | 47.5 ± 17.2 | .001 | 52.3 ± 13.4 | 46.4 ± 18.1 | .002 | 55.3 ± 13.3 | 51.9 ± 12.5 | .161 |
| Proximal main branch | |||||||||
| Length, mm | 11.15 ± 5.28 | 10.86 ± 5.22 | .154 | 11.56 ± 5.59 | 11.21 ± 5.53 | .155 | 9.06 ± 2.50 | 8.97 ± 2.69 | .776 |
| Reference lumen diameter, mm | 3.83 ± 1.13 | 3.88 ± 0.80 | .674 | 3.65 ± 0.98 | 3.84 ± 0.71 | .089 | 4.75 ± 1.42 | 4.10 ± 1.12 | .066 |
| Minimum lumen diameter, mm | 1.63 ± 0.85 | 2.96 ± 0.62 | < .001 | 1.60 ± 0.77 | 2.85 ± 0.46 | < .001 | 1.78 ± 1.15 | 3.50 ± 0.93 | < .001 |
| Percentage diameter stenosis, % | 55.36 ± 20.81 | 18.09 ± 10.34 | < .001 | 54.12 ± 20.78 | 19.44 ± 10.01 | < .001 | 61.16 ± 20.58 | 11.77 ± 9.78 | < .001 |
| Binary stenosis (SD ≥ 50%), N (%) | 58 (63.0) | 0 | < .001 | 45 (60.0) | 0 | < .001 | 13 (76.5) | 0 | < .001 |
| Distal main branch (BIOSS) | |||||||||
| Length, mm | 10.35 ± 5.36 | 9.96 ± 5.46 | .190 | 10.61 ± 5.69 | 10.28 ± 5.76 | .105 | 9.13 ± 3.24 | 8.66 ± 3.43 | .082 |
| Reference lumen diameter, mm | 2.33 ± 0.45 | 2.34 ± 0.42 | .797 | 2.29 ± 0.45 | 2.32 ± 0.42 | .547 | 2.49 ± 0.39 | 2.42 ± 0.40 | .409 |
| Minimum lumen diameter, mm | 1.19 ± 0.56 | 2.28 ± 0.36 | < .001 | 1.24 ± 0.56 | 2.27 ± 0.37 | < .001 | 0.99 ± 0.53 | 2.34 ± 0.30 | < .001 |
| Percentage diameter stenosis, % | 48.43 ± 23.07 | 0.12 ± 15.00 | < .001 | 46.67 ± 22.98 | -0.62 ± 15.06 | < .001 | 60.61 ± 19.77 | 3.40 ± 14.74 | < .001 |
| Binary stenosis (SD ≥ 50%), N (%) | 43 (46.7) | 0 | < .001 | 31 (41.3) | 0 | < .001 | 12 (70.6) | 0 | < .001 |
| Side branch | |||||||||
| Length, mm | 6.32 ± 3.81 | 6.27 ± 3.47 | .689 | 5.20 ± 0.99 | 5.21 ± 0.89 | .905 | 11.58 ± 6.88 | 11.25 ± 6.08 | .549 |
| Reference lumen diameter, mm | 2.18 ± 0.48 | 2.22 ± 0.49 | .199 | 2.12 ± 0.46 | 2.16 ± 0.42 | .268 | 2.42 ± 0.51 | 2.49 ± 0.71 | .536 |
| Minimum lumen diameter, mm | 1.41 ± 0.64 | 1.75 ± 0.52 | < .001 | 1.46 ± 0.57 | 1.62 ± 0.43 | .022 | 1.19 ± 0.88 | 2.31 ± 0.50 | < .001 |
| Percentage diameter stenosis, % | 34.16 ± 27.52 | 20.94 ± 19.14 | < .001 | 30.06 ± 25.34 | 24.35 ± 17.13 | .070 | 52.24 ± 30.19 | 5.88 ± 20.77 | < .001 |
| Binary stenosis (SD ≥ 50%), N (%) | 22 (23.9) | 5 (5.4) | < .001 | 13 (17.3) | 5 (6.6) | .044 | 9 (52.9) | 0 | < .001 |
|
PDS, percentage diameter stenosis. |
|||||||||
Comparing patients treated with the single-stent technique or the double stenting technique (table 4) revealed that, at proximal segment level, expansion results are better with the double stenting technique with residual stenosis of 11% (vs 19% with the single-stent) although starting from greater baseline reference diameters (3.65 ± 0.98 vs 4.75 ± 1.42). The double stenting technique showed excellent results on the SB with a significantly greater minimum lumen diameter (2.31 ± 0.50 vs 1.62 ± 0.43, P = .01), and minimum residual stenosis of 5.9% (vs 24.3% with the provisional stenting technique) with special attention to the lack of stenosis > 50%. Results were similar in the distal segment.
Quantitative angiography of the polygon of confluence
At the polygon of confluence (table 5) results show residual stenoses of 17.15% ± 10.96% at the polygon core, and 19.21% ± 20.56% for the ostium of the SB. When the provisional stenting and double stenting techniques were compared (table 5), data from the QCA show better minimum lumen diameters for the double stenting technique in the bifurcation core and the ostium of the SB, and almost identical for the ostium of the distal segment.
Table 5. Quantitative coronary angiography. Polygon of confluence. Comparative between simple and double stent
| N = 92 lesions | Provisional technique (N = 75) | Double stenting technique (N = 17) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | P | Pre | Post | P | Pre | Post | P | |
| Bifurcation core | |||||||||
| Reference lumen diameter, mm | 4.04 ± 1.13 | 3.97 ± 0.80 | .531 | 3.83 ± 0.97 | 3.88 ± 0.69 | .618 | 4.82 ± 1.34 | 4.35 ± 1.10 | .095 |
| Minimum lumen diameter, mm | 2.09 ± 0.94 | 3.28 ± 0.78 | < .001 | 1.87 ± 0.72 | 2.93 ± 0.49 | < .001 | 2.25 ± 0.98 | 3.63 ± 0.90 | < .001 |
| Percentage diameter stenosis, % | 48.49 ± 17.94 | 17.15 ± 10.96 | < .001 | 47.57 ± 18.23 | 18.76 ± 10.68 | < .001 | 52.39 ± 15.70 | 10.31 ± 9.63 | < .001 |
| Binary stenosis (SD ≥ 50%), N (%) | 46 (50.0) | 0 | < .001 | 35 (46.7) | 0 | < .001 | 11 (64.7) | 0 | .001 |
| Distal main branch ostium (BIOSS) | |||||||||
| Reference lumen diameter, mm | 2.34 ± 0.45 | 2.35 ± 0.43 | .842 | 2.29 ± 0.45 | 2.32 ± 0.42 | .544 | 2.55 ± 0.40 | 2.48 ± 0.43 | .406 |
| Minimum lumen diameter, mm | 1.38 ± 0.53 | 2.40 ± 0.37 | < .001 | 1.40 ± 0.50 | 2.39 ± 0.36 | < .001 | 1.32 ± 0.67 | 2.45 ± 0.41 | < .001 |
| Percentage diameter stenosis, % | 40.59 ± 21.36 | -3.67 ± 15.54 | < .001 | 38.61 ± 20.61 | -4.57 ± 15.76 | < .001 | 48.95 ± 23.07 | 0.13 ± 14.37 | < .001 |
| Binary stenosis (SD ≥ 50%), N (%) | 34 (37.0) | 0 | < .001 | 23 (30.7) | 0 | < .001 | 11 (64.7) | 0 | .001 |
| Side branch ostium | |||||||||
| Reference lumen diameter, mm | 2.21 ± 0.50 | 2.24 ± 0.51 | .280 | 2.14 ± 0.46 | 2.17 ± 0.41 | .375 | 2.51 ± 0.56 | 2.57 ± 0.72 | .553 |
| Minimum lumen diameter, mm | 1.56 ± 0.52 | 1.80 ± 0.55 | < .001 | 1.58 ± 0.47 | 1.65 ± 0.45 | .219 | 1.47 ± 0.71 | 2.40 ± 0.52 | < .001 |
| Percentage diameter stenosis, % | 28.21 ± 21.77 | 19.21 ± 20.56 | .004 | 24.91 ± 20.30 | 23.13 ± 17.97 | .501 | 42.19 ± 22.82 | 2.58 ± 22.99 | < .001 |
| Binary stenosis (SD ≥ 50%), N (%) | 16 (17.4) | 5 (5.4) | .012 | 9 (12.0) | 5 (6.7) | .267 | 7 (41.2) | 0 | .016 |
|
PDS, percentage diameter stenosis. |
|||||||||
Bifurcation angle (table 4) showed a slight change after stenting with a statistically significant reduction from 52.8 ± 18.4 º down to 47.5 ± 17.2º (P = .001). In the double stenting cohort, angulation modification was similar—in absolute terms—with a reduction of some 4º. However, this difference was not statistically significant (table 4). No significant correlation between the degree of angulation modification and the SB residual stenosis was reported (P = .86).
Clinical outcomes
Only 1 procedural complication was reported consisting of the occlusion of the SB in a patient treated with the provisional stenting technique that could not be solved. No major clinical events, cases of stent thrombosis or target lesion revascularization were reported at 30 days.
One year after implantation, 110 patients (88.7%) were contacted. A case of definitive device thrombosis (0.91%) was reported at 1-year follow-up especially in a case of double stenting treated with primary angioplasty. This case added to other 5 cases of new target lesion revascularization due to restenosis (4.54%) reveal a 12-month rate of target lesion failure of 5.45%. Two of these restenoses were found in the ostium of the SB and the remaining 3 were in the main vessel. No deaths and 3 infarctions (2.72%) were reported all of them associated with the device in relation to stent thrombosis and, in the other 2 cases, due to restenosis with minimum mobilization of troponin.
DISCUSSION
The study main findings are: a) the BIOSS LIM C dedicated stent has a high rate of success at 30 days in patients with complex coronary bifurcation lesions; b) such device is basically used with the provisional stenting strategy and is associated with a very reduced need for stenting in the SB; c) immediate angiographic outcomes show the proper behavior from the stent in the 3 bifurcation segments, as well as in the polygon of confluence where the contact surface between the stent and the artery is minimum.
Demographic data confirm that this is a non-selected population with a prevalence of risk factors, comorbidities, heart disease, and anatomical characteristics of the lesions we see in the routine clinical practice of any cath lab these days.
The device had a high rate of implantation success that was consistent with the easiness of its design being successfully implanted in > 97% of the cases. Success rate is similar to that reported in most studies with tubular stents used in bifurcations, something unreported in previous series of dedicated stents (Axxess, Frontier, and Nile studies). For example, the Frontier stent15 had a rate of restenosis of nearly 29.9%. The Nile stent16,17 was successfully used in tortuous arteries and distal segments with acceptable results with a rate of target lesion revascularization of 8.4%. However, it required distribution of angiographic stenosis focused on the carina. The self-expandable Axxess stent18 showed favorable results with a 1-year rare of cardiovascular events and restenosis of 7.7% and 6.4%, respectively. Nonetheless, it only treated the polygon of confluence and the segment immediately proximal to the ostia of the branches. Also, it was limited to certain angles and lengths needing, on many occasions, the use of additional stents.
In 14.5% of the cases, the double stenting technique was used. This is a dedicated stent in such a way that, when crossing the SB, the lack of struts in the polygon of confluence facilitates its advance without requiring previous opening. Results from the study support just how easy it is to use it with the double stenting technique. In all the cases where it was used, a second stent was successfully implanted into the SB. In 6 (66%) out of the 9 cases where the double stenting strategy was planned, the SB stent was directly implanted without dilatation. This data, though very limited, could signal a possible advantage of the BIOSS LIM C dedicated stent to facilitate access of a second stent to the SB when necessary. On the other hand, angiographic results after implantation are particularly remarkable for the double stenting technique: both the minimum lumen diameters and the residual stenosis of the proximal segments and the SB are better compared to those seen in the cohort where the provisional stenting technique was used.
If procedural data are analyzed, something that calls our attention is the SB predilatation in 47 cases where the SB was not damaged significantly. The protocol did not define the obligation to predilate the SB. According to the investigator’s criterion in each case, the observation of a lesion that did not reach a 50% stenosis was still considered to pose risk of carina displacement.
Another aspect we should mention is the strikingly low use of the POT reported in 33 cases (26%), and similarly in both cohorts. On this regard, we should mention that the device is designed with a proximal segment of a greater caliber in such a way that with simple inflation this proximal postdilatation is already incorporated. The POLBOS I19 and II14 clinical trials showed that the use of POT improved the rate of target lesion revascularization. Also, a tendency towards less late lumen loss was reported. However, POT was used at a rate of 37%, which is similar to that of our cohort.
One of the main study endpoints was to assess the potential disadvantage of design in 2 stent segments. This design provides a space for the polygon of confluence—of 0.9 mm to 1.5 mm—between both segments linked by 2 connectors. In this space, the metal-to-artery ratio is significantly lower, which may contribute to a relatively systematic underexpansion.
Results of the provisional cohort on the polygon of confluence show that mean residual stenosis is 19% at the core of the polygon and 23% in the ostium of the SB. These data suggest a certain impact in the angiographic results of this relative lack of scaffold between both segments that we believe could be the cause for a certain degree of underexpansion at the polygon of confluence.
Another study primary endpoint was to see the degree of damage in the bifurcation native angulation. Godino et al.20 analyzed changes to the bifurcation angle after angioplasty using the 1 or 2-stent technique in a cohort of 215 patients. They described a mean reduction of around 10º of the angle in the left main coronary artery, and 7º in the remaining bifurcations with the double stenting technique. However, with the provisional stenting technique no significant differences were reported. In our study, the results seen in the overall population show a statistically significant change—although not very relevant numerically—of the bifurcation angle that went from 53º to 47º. Contrary to what was reported by Godino et al.20, in our study, in the 2-stent cohort, changes to the bifurcation angle were not significant with mean reductions of 3º. However, in the provisional stenting cohort, a significant reduction of the angle was seen of 6º. In any case, we consider that this just has simple statistical significance; it seems very unlikely that a 5º variation of the bifurcation angle can be seen through visual estimate, and much less that any clinical disadvantages can occur.
Overall, the follow-up results were good and consistent with what was described in former studies.14,19 There was a significant loss of cases for the QCA since 92 cases were available only. Another study limitation is that regarding the analysis of the double stenting technique. Although the QCA data suggest good results for the double stenting technique, the study was not designed to draw comparisons between the provisional and the double stenting techniques. Also, the information collected is limited, and the number of patients treated with the double stenting technique is not enough to reach any conclusions.
An additional limitation we would like to mention is the low use of imaging modalities. Probably in coronary bifurcation lesions its systematic use can be beneficial.
In conclusion, we believe that this study conducted at several centers with different operators reveals how relatively easy it is to use the BIOSS LIM C stent to treat coronary bifurcation lesions. We’re pretty sure this is significant enough to simplify the double stenting approach where the scarce distortion overlapping the bifurcation native angulation called our attention. On the other hand, the weak spot would be the feeble metal scaffold that remains in the polygon of confluence probably due to a certain degree of underexpansion. However, the good clinical outcomes reported at 1-year follow-up are indicative that such design is not really a problem.
Limitations
This was a prospective observational study, which means that any comparisons among the routine techniques used to treat coronary bifurcation lesions can be limited. The informed consent from all the patients was not obtained. Although information from 88% of the cohort was obtained, losses to follow-up were slightly higher than they should have been.
CONCLUSIONS
The BIOSS LIM C dedicated stent works well to treat coronary bifurcation lesions. Angiographically, the stent has a space at the polygon of confluence to facilitate access to the SB. This is associated with a lower metal-to-artery ratio conditioning residual stenosis of around 20%. However, such residual stenosis does not necessarily trigger more events at 1 year and, at the end, this carinal design allows easy access to the SB in case a second stent would be needed and with excellent results. Finally, the stent-induced distortion on the angle of the carina is limited, around 5º.
FUNDING
The study was funded with a graft from Fundación EPIC, which was unconditionally funded by the LOGSA group.
AUTHORS’ CONTRIBUTIONS
As the study lead co-investigators, B. García del Blanco, and A. Pérez de Prado drafted the protocol, managed funds, directed the project, recruited the patients, wrote part of the article, and made their contributions to the overall drafting of the article. J. Gómez-Lara conducted the angiographic analysis at the core lab, the statistical analysis, drafted part of the article, and made his contributions to the article overall draft. I. In his capacity of sub-investigator, Otaegui Irurueta recruited patients by performing procedures and protocol follow-ups, entered the data required in the data curation notebook, depurated and finalized the data entered in the database, was involved in statistical analysis, drafted part of the article, participated in the article overall draft by compiling all the sections drafted from the remaining authors, and responded to the corrections requested by reviewers. M.A. Carmona Ramírez dealt with all regulatory actions needed to start the study and include the different participant centers both with the Spanish Agency of Medicines and Medical Devices and the different centers and ethics committees. As study sub-investigators, the remaining authors recruited patients, performed the procedures and follow-ups according to protocol, filled out the data curation notebook, and responded to all the questions asked.
CONFLICTS OF INTEREST
A. Pérez de Prado is an associate editor of REC: Interventional Cardiology; the journal’s editorial procedure to ensure impartial handling of the manuscript has been followed. He has received research grants from the following research sponsors (Fundación EPIC): Abbott, Biosensors, Biotronik, Bristol-Myers-Squibb, Boston Scientific, Cardiva, iVascular, Shockwave Ltd, Terumo, Volcano Philips; also, fees for his theoretical or practical proctoring for Braun, Boston Scientific, and Terumo. The remaining authors declared no conflicts of interest whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- Dedicated stents facilitate better adaptation to fractal anatomy of coronary bifurcation lesions with less bifurcation angle distortion and access to the side branch. The BIOSS LIM C stent has shown favorable results in randomized clinical trials compared to second-generation tubular stents.
WHAT DOES THIS STUDY ADD?
- In this study, the angiographic pattern of coronary bifurcation lesions with the implantation of BIOSS LIM C dedicated stent is shown. Also, it shows the feasibility of its systematic use—with a high rate of success and scarce damage to bifurcation angulation—can have. Residual underexpansion at the polygon of confluence is acceptable for the provisional stenting technique despite a reduced metal-to-artery ratio, and excellent for the double stenting technique.
REFERENCES
1. Ojeda S, Romaguera R, Cruz-González I, Moreno R. Spanish Cardiac Catheterization and Coronary Intervention Registry. 29th Official Report of the Interventional Cardiology Association of the Spanish Society of Cardiology (1990-2019). Rev Esp Cardiol. 2020;73:927-936.
2. Burzotta F, Lassen JF, Lefèbre T, et al. Percutaneous coronary intervention for bifurcation coronary lesions: the 15th consensus document from the European Bifurcation Club. EuroIntervention. 2021;16:1307-1317.
3. Huo Y, Kassab GS. Scaling laws of coronary circulation in health and disease. J Biomech. 2016;49:2531-2539.
4. Foin N, Sen S, Allegria E, et al. Maximal expansion capacity with current DES platforms: a critical factor for stent selection in the treatment of left main bifurcations? EuroIntervention. 2013;8:1315-1325.
5. Finet G, Derimay F, Motreff P, et al. Comparative Analysis of Sequential Proximal Optimizing Technique Versus Kissing Balloon Inflation Technique in Provisional Bifurcation Stenting: Fractal Coronary Bifurcation Bench Test. JACC Cardiovasc Interv. 2015;8:1308-1317.
6. Maeng M, Hom NR, Erglis A, et al. Long-term results after simple versus complex stenting of coronary artery bifurcation lesions: Nordic Bifurcation Study 5-year follow-up results. J Am Coll Cardiol. 2013;62:30-34.
7. Hildick-Smith D, Behan MW, Lassen JF, et al. The EBC TWO Study (European Bifurcation Coronary TWO): A Randomized Comparison of Provisional T-Stenting Versus a Systematic 2 Stent Culotte Strategy in Large Caliber True Bifurcations. Circ Cardiovasc Interv. 2016;9:e003643.
8. Ferenc M, Gick M, Kienzle R-P, et al. Randomized trial on routine vs. provisional T-stenting in the treatment of de novo coronary bifurcation lesions. Eur Heart J. 2008;29:2859.
9. Ferenc M, Ayoub M, Büttner HJ, et al. Long-term outcomes of routine versus provisional T-stenting for de novo coronary bifurcation lesions: five-year results of the Bifurcations Bad Krozingen I study. EuroIntervention. 2015;11:856-859.
10. Erglis A, Kumsars I, Niemelä M, et al. Randomized comparison of coronary bifurcation stenting with the crush versus the culotte technique using sirolimus eluting stents: the Nordic stent technique study. Circ Cardiovasc Interv. 2009;2:27-34.
11. Gil RJ, Bil J, Kern A, Pawłowski T. First-in-man study of dedicated bifurcation cobalt-chromium sirolimus-eluting stent BIOSS LIM C® - Three-month results. Kardiol Pol. 2018;76:464-470.
12. Gil RJ, Bil J, Grundeken MJ, et al. Long-term effectiveness and safety of the sirolimus-eluting BiOSS LIM® dedicated bifurcation stent in the treatment of distal left main stenosis: an international registry. EuroIntervention. 2016;12:1246-1254.
13. Gil RJ, Bil J, Vassiliev D, Garcia LAI. First-in-man study of dedicated bifurcation sirolimus-eluting stent: 12-month results of BiOSS LIM® Registry. J Interv Cardiol. 2015;28:51-60.
14. Gil RJ, Bil J, Grundeken MJ, et al. Regular drug-eluting stents versus the dedicated coronary bifurcation sirolimus-eluting BiOSS LIM® stent: the randomised, multicentre, open-label, controlled POLBOS II trial. EuroIntervention. 2016;12:e1404-e1412.
15. Lefèvre T, Ormiston J, Guagliumi G, et al. The Frontier stent registry: safety and feasibility of a novel dedicated stent for the treatment of bifurcation coronary artery lesions. J Am Coll Cardiol. 2005;46:592-598.
16. Costa RA, Abizaid A, Abizaid AS, et al. Procedural and early clinical outcomes of patients with de novo coronary bifurcation lesions treated with the novel Nile PAX dedicated bifurcation polymer-free paclitaxel coated stents: results from the prospective, multicentre, non-randomised BIPAX clinical trial. EuroIntervention. 2012;7:1301-1309.
17. TCT-50: Complex Coronary Bifurcation Lesions Treated with the Novel Polymer-Free Dedicated Bifurcation Paclitaxel-Eluting Stent (Nile Pax): 9-Month Clinical and Angiographic Results of the Prospective, Multicenter BIPAX Clinical Trial. J Am Coll Cardiol. 2011;58:B15.
18. Verheye S, Agostoni P, Dubois CL, et al. 9-Month clinical, angiographic, and intravascular ultrasound results of a prospective evaluation of the Axxess self-expanding biolimus A9-eluting stent in coronary bifurcation lesions: the DIVERGE (Drug-Eluting Stent Intervention for Treating Side Branches Effectively) study. J Am Coll Cardiol. 2009;53:1031-1039.
19. Gil RJ, Bil J, Džavík V, et al. Regular Drug-Eluting Stent vs Dedicated Coronary Bifurcation BiOSS Expert Stent: Multicenter Open-Label Randomized Controlled POLBOS I Trial. Can J Cardiol. 2015;31:671-678.
20. Godino C, Al-Lamee R, C La Rosa C, et al. Coronary left main and non-left main bifurcation angles: how are the angles modified by different bifurcation stenting techniques? J Interv Cardiol. 2010;23:382-393.