ABSTRACT
Introduction and objectives: Transcatheter aortic valve implantation (TAVI) is an increasingly used procedure to treat severe aortic stenosis (AS) that should be monitored in the real-world routine clinical practice. We assessed TAVI outcomes (SAPIEN 3) in terms of the patient’s health-related quality of life (HRQoL), clinical endpoints, and resource utilization considering a valid risk score.
Methods: This was an observational prospective study including all consecutive patients with severe AS treated with TAVI (Edwards SAPIEN 3, transfemoral access) conducted during the calendar year of 2018. A systematic assessment of the patients’ HRQoL (EQ-5D-5L, the 36-item Short Form Health Survey, and the Kansas City Cardiomyopathy Questionnaire), clinical endpoints, and resource utilization (length of stay at the hospital/intensive care unit setting) was implemented. Assessment was scheduled before the procedure (baseline), at discharge, and 1, 6, and 12 months after implantation. Multivariate regression models were applied to test outcomes while controlling the patients’ risk (eg, Society of Thoracic Surgeons risk score).
Results: A total of 76 patients (50% female) with a mean age of 82.05 ± 4.76 years, and 55% with intermediate-high risk were included. The rates of successful impantation and cardiac death were 97.37% and 2.63%, respectively, at 1 year. Significant reductions in mean and maximum gradients were achieved and maintained at follow-up. The mean length of stay at the hospital (5.2 6 ± 4.05) and intensive care unit setting (0.22 ± 0.64) was short. Significant improvements (all adjusted P < .05) were detected in the Kansas City Cardiomyopathy Questionnaire overall summary scores, EQ-5D-5L, and the 36-item Short Form (physical component summary).
Conclusions: This research highlights how positive clinical outcomes translated into significant improvements in relation to the patients’ HRQoL. Use of resources —generally low— was based on the Society of Thoracic Surgeons risk score. (SARU Study; code: 2017-01, Murcia, Spain).
Keywords: Aortic valve stenosis. Quality of life. Health resources. Length of stay. Clinical endpoint. Burden of illness.
RESUMEN
Introducción y objetivos: El uso del implante percutáneo de válvula aórtica (TAVI, transcatheter aortic valve implantation) está aumentando en el tratamiento de la estenosis aórtica grave. Por ello, el uso de TAVI en la vida real debe monitorizarse. Evaluamos los resultados del TAVI en términos de calidad de vida relacionada con la salud (CVRS), resultados clínicos y uso de recursos teniendo en cuenta un marcador de riesgo válido.
Métodos: Estudio observacional prospectivo incluyendo todos los pacientes consecutivos con estenosis aórtica grave tratados con TAVI (Edwards SAPIEN 3, acceso transfemoral) en 2018. Se evaluaron de forma sistemática la CVRS (EQ-5D-5L, Short Form-36 Health Survey, Kansas City Cardiomyopathy Questionnaire), los resultados clínicos y el uso de recursos (estancia en planta/unidad de cuidados intensivos). La evaluación se hizo antes de la intervención (basal), al alta y después de 1,6 y 12 meses del implante. Se aplicaron modelos de regresión multivariante para evaluar los resultados mientras se controlaba el riesgo del paciente (por ejemplo, escala de riesgo de la Society of Thoracic Surgeons).
Resultados: Se inc luyó a 76 pacientes (el 50% mujeres), con una edad media de 82,05 ± 4,76, y el 55% con riesgo intermedio-alto. Hubo un 97,37% de éxito del implante y la tasa de muerte de causa cardiovascular fue del 2,63% al año. Se consiguieron reducciones significativas en los gradientes medios y máximos, y se mantuvieron durante las visitas de seguimiento. Las estancias medias en planta (5,26 ± 4,05 días) y en la unidad de cuidados intensivos (0,22 ± 0,64 días) fueron bajas. Se detectaron mejoras significativas (todo ajustado p < 0,05) en el Kansas City Cardiomyopathy Questionnaire (puntuaciones generales), el EQ-5D-5L y el Short Form-36 (componente físico).
Conclusiones: Esta investigación destaca resultados clínicos positivos que se traducen en mejoras significativas en términos de calidad de vida de los pacientes. El uso de recursos, que fue en general bajo, también fue dependiente de la escala de riesgo de la Society of Thoracic Surgeons. (Estudio SARU, código: 2017-01, Murcia, España).
Palabras clave: Estenosis valvular aórtica. Calidad de vida. Recursos sanitarios. Estancia. Resultado clínico. Carga de la enfermedad.
Abbreviations
AS: Aortic stenosis. HRQoL: Health-related quality of life. HRU: Healthcare resource utilization. KCCQ: Kansas City Cardiomyopathy Questionnaire. STS: Society of Thoracic Surgeons. TAVI: transcatheter aortic valve implantation.
INTRODUCTION
Aortic stenosis (AS) is the most common cause of valvular heart disease1 with an estimated prevalence of 3%-5% in people ≥ 65 years to 7.4% in people > 85 years.2,3 Severe AS is the leading cause of valvular surgery among adults. AS typically has a variable but long latent period (asymptomatic) followed by a rapid progression stage after symptom onset (eg, dyspnea, angina or syncope), and has a poor prognosis if aortic valve replacement is not performed in a timely manner.4,5
Although open heart surgery has been the gold standard treatment for many years, aortic valve procedures have progressively become less invasive. Transcatheter aortic valve implantation (TAVI) has become the treatment of choice for inoperable patients with symptomatic, severe AS,6 and a valid alternative for high- and intermediate-surgical risk patients with improved clinical results regarding survival and functional capacity.7,8 Similarly, with new evidence from recent clinical trials, the indication for TAVI was extended to low-risk patients.9,10
According to current clinical guidelines,11 the multidisciplinary decision regarding procedures to solve AS requires an individualized and appropriate assessment of the candidates to optimize the benefits achieved in these patients (eg, regarding survival and symptom amelioration). To this end, significant factors impact the patients’ surgical risk (eg, surgeon-specific risk-adjusted composite according to the Society of Thoracic Surgeons [STS] score), the patient’s quality-adjusted life expectancy, baseline characteristics like frailty (eg, ≥ 2 score in the Katz scale), modifiable risk factors, and comorbidities (eg, chronic obstructive pulmonary disease, pulmonary hypertension, liver disease, previous stroke, anemia, and other systemic conditions). In Europe, recent guidelines recommend TAVI for patients > 75 years. Also, that all patients with AS between 70 and 75 years should be referred for TAVI assessment regardless of their surgical risk.12
Due to the wider indication for TAVI and the ageing demographic factor seen in Western countries,3 TAVI is increasingly used in the routine clinical practice across Europe. This underscores the importance of monitoring TAVI outcomes, particularly among elderly patients to better characterize performance in the real-world practice.
Therefore, our objective was to prospectively assess TAVI (SAPIEN 3, Edwards Lifesciences, United States) outcomes regarding the patient’s health-related quality of life (HRQoL), and the clinical outcomes considering their surgical risk. Also, as secondary endpoint, a description of healthcare resource utilization adjusted for surgical risk (STS score) was intended.
METHODS
Study design
This was a prospective, observational study of all consecutive patients with severe, symptomatic AS treated with elective TAVI via transfemoral access with SAPIEN 3 at the regional Hospital Universitario Virgen de la Arrixaca, a tertiary hospital and a regional referral center for cardiothoracic surgery and interventional cardiology located in Murcia, Spain. Patients received TAVI regardless of the study as part of the routine clinical practice. The recruitment stage was during the calendar year of 2018. In this study we present the observed results from the systematic djustent conducted 1 year after TAVI.
According to the ESC/EACTS guidelines,6 implantation decision was made by the heart team and all procedures followed the recommendations established by the manufacturer’s SAPIEN 3 valve instructions for use. All patients were followed for, at least, 12 months after TAVI and systematically assessed according to the hospital clinical protocol. Written patient information was provided to each participant, and the patient’s consent on data collection was signed before being included in the study that was conducted in full compliance with the recommendations guiding biomedical research in human subjects adopted by the 18th World Medical Assembly, Helsinki, Finland back in 1964. The study protocol was approved by the assigned ethics Committee (Murcia, SARU Study; code: 2017-01. Effective date, 02/06/2017).
Clinical assessment was conducted at baseline (preoperative), post-intervention (perioperative), and 30 days, 6 months, and 1 year after the procedure. The main objective clinical variables included echocardiographic measurements (eg, paravalvular and total aortic regurgitation, left ventricular ejection fraction, mean and maximum aortic valve gradient, effective orifice area), and major clinical events automatically available in the medical records (eg, all-cause and cardiovascular mortality, stroke, bleeding complications, myocardial infarction, new-onset atrial fibrillation, major vascular complications, permanent pacemaker implantation, rehospitalization and acute kidney injury). In addition, the patients’ New York Heart Association (NYHA) functional class IV was systematically registered. The patients’ risk profile was characterized based on the STS risk score which was validated for the in-hospital and 30-day mortality rates following surgical aortic valve replacement.13 Additionally, postoperative complications were defined based on a modified version of the Valve Academic Research Consortium criteria,14 and this score was routinely applied based on the clinical protocols of our referral hospital.
Finally, length of stay (LOS)–at the hospital and the intensive care unit (ICU) settings–associated with the TAVI procedure was automatically registered for each patient based on hospital medical records and described as a secondary endpoint in this research.
Measurement of patient’s health-related quality of life
A comprehensive assessment was implemented by combining a patient-reported disease-specific tool that has a higher ability to capture changes in the patient’s health status during the observation period, and 2 generic tools to establish comparisons with findings from other procedures or diseases and with the Spanish normal population. Patients’ health-related quality of life was evaluated at baseline and during per protocol medical visits for health management (6 months and 1 year after TAVI).
Disease-specific tools
The Kansas City Cardiomyopathy Questionnaire (KCCQ)15 is a 23-item self-administered disease-specific questionnaire originally developed for patients with heart failure to monitor their reported symptoms and evaluate how and to what extent their heart failure impacts their quality of life (QoL) within a 2-week recall period. The KCCQ includes 6 distinct domains (physical function, symptoms, symptom stability, social limitation, self-efficacy, and quality of life) added to 2 summary scores: the clinical summary score (CSS) and the overall summary score (OSS). Summary scores can be transformed into 0–100 scales with higher scores being indicative of better levels of wellbeing to facilitate score interpretation. This tool has been recently revised and qualified for its use in heart failure by the United States Food and Drug Administration16 with minimal clinically important differences defined as 5-point changes in summary scales.17 Also, the KCCQ has a sound psychometrical performance when measuring functional status and HRQoL in patients with severe, symptomatic AS.18
Generic tools to measure health-related quality of life
The EQ-5D-519—a patient-reported measure—includes a descriptive system and the EQ visual analogue scale (EQ-5D-5L VAS). The former includes 5 different domains: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Responses to the EQ-5D-5L descriptive system were assigned preference-based (utility) weights from the Spanish population. The EQ-5D-5L VAS reflects the patient’s self-rated health on a vertical visual analogue scale (from 0, ‘the worst health you can imagine’ up to 100, ‘the best health you can imagine’).20,21
The Medical Outcomes Study Short Form-36 (SF-36)22 is one of the most widely used and evaluated generic HRQoL questionnaires. It includes 8 dimensions and 2 summary scores (physical component summary [PCS] and mental component summary [MCS]). In this study we used a standardization of summary scores based on age and gender using the Spanish population normative data.23
Statistical approach
First, an exploratory analysis was performed to characterise the analytic cohort presented in this manuscript. Descriptive statistics were used for continuous variables (eg, mean, standard error of measurement) and frequency tables or proportions for discrete variables. McNemar’s test for dependent samples was used to compare NYHA health states at follow-up. Regarding the objective of this study, multivariate models (linear general models with repeated measures at different time points —baseline, 6M, and 12M— as intra-subject factors) were computed to better assess the potential benefits of both regarding clinical endpoints and the patients’ HRQoL at follow-up (baseline, 6M, and 12M) while considering the patients’ comorbidities and risk profile at baseline. To this end, the STS predicted risk of mortality score was included because it is a weighted index of the patients’ risk robustly estimated using a Bayesian hierarchical model for both mortality and major complication events. This model considers 24 meaningful preoperative variables like age, sex, body surface area, atrial fibrillation, chronic heart failure, NYHA functional classification, chronic obstructive pulmonary disease, diabetes mellitus, need for insulin use, arterial hypertension, previous cardiac surgeries, concomitant mitral stenosis, unstable angina, previous percutaneous coronary intervention, and other variables. Based on the estimated STS score, patients were classified into 3 risk groups: high (≥ 8%), intermediate (≥ 4%), and low mortality risk (< 4%).13 Importantly, this score was also considered in the secondary endpoint associated with the description of the LOS (at both the hospital and ICU settings) related to TAVI procedures.
Regarding the size of the sample required to conduct the adjusted analyses described above, the estimated minimal sample size was set at 60 TAVI patients to compare within and between subject differences at 3 different time points of evaluation, effect size (f) was 0.25, statistical power (1-β), 0.9, and risk of type-I error (1-α), 0.95 assuming a weak correlation among repeated measures (0.3).
The software statistical package SPSS 27.0 for Windows (IBM Corp., United States) and the R software (The R Project for Statistical Computing, Institute for Statistics and Mathematics, Austria) were used for analysis.
RESULTS
A total of 76 consecutive patients, 50% female, with a mean age of 82.05 ± 4.76 years underwent elective TAVI during the study period comprising the analytical cohort. STS (surgical risk) score was 5.4 ± 3.41 while 42.5%, 43.8%, and 13.7% of the cases were classified as low-, intermediate-, and high-risk patients, respectively. A complete description of comorbidities is shown on Table 1. Previous coronary artery bypass graft was reported in 1 patient. A total of 6 cases (7.9%) were valve-in-valve procedures, and in 71 cases (93.4%) vascular access was via right femoral artery. Only 1 patient required general anaesthesia before TAVI (table 1 of the supplementary data). The patients’ functional status (NYHA classification) at baseline was remarkably impaired in most cases with 61.84%, and 19.74% of the patients having NYHA functional class III and IV, respectively.
Table 1. Preoperative characteristics of TAVI patients (N = 76)
| Previous disease | n | % |
|---|---|---|
| Dyslipidemia | 51 | 67.11 |
| Arterial hypertension | 66 | 86.84 |
| Previous stroke | ||
| With effects | 1 | 1.32 |
| Without effects | 4 | 5.26 |
| TIA | 4 | 5.26 |
| Liver disease | 0 | 0.00 |
| Diabetes mellitus | ||
| Diet | 1 | 1.32 |
| Oral agents | 22 | 28.95 |
| Insulin | 17 | 22.37 |
| No treatment | 1 | 1.32 |
| CKD | 26 | 34.21 |
| Smoker | ||
| Active smoker | 2 | 2.63 |
| Non-smoker | 48 | 63.16 |
| Former smoker | 25 | 32.89 |
| Oncological disease | 8 | 10.53 |
| COPD | 9 | 11.84 |
|
COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; DM, diabetes mellitus; TIA, transient ischemic attack. |
||
Clinical outcomes
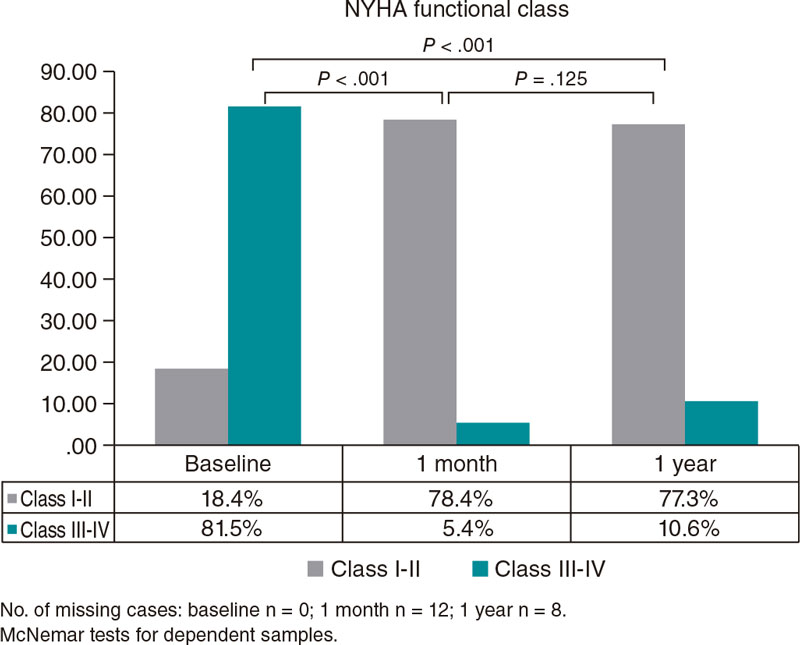
Successful implantation was achieved in 74 cases (97.37%), and 2 patients (2.63%) died within the first 30 days after the procedure (both cardiovascular causes). In this period, the observed rates of major complications or rehospitalizations were low (Table 2), and permanent pacemaker implantation was required for 5 patients. One year after TAVI, no additional cardiovascular deaths were reported. However, 7 patients died of other causes (Table 2). We should mention that the sample mortality rate was similar to that of a comparable general population (figure 1 of the supplementary data). According to echocardiographic measurements, significant benefits in mean and maximum gradients, and aortic regurgitation were achieved and maintained at follow-up (figure 2 of the supplementary data). Notably, among survivors, 78.4% of patients had a NYHA functional class I-II at 1 month, a benefit that was maintained at 1 year with 77.3% of patients in these functional levels.
Table 2. Clinical outcomes seen at 30 days and 1 year (N = 76)
| Variable | n | % |
|---|---|---|
| Successful implantation | 74 | 97.37 |
| Death (30d) | 2 | 2.63 |
| Cardiovascular death (30d) | 2 | 2.63 |
| AMI (30d) | 0 | 0.00 |
| Stroke (30d) | 3 | 3.95 |
| Major bleeding (30d) | 4 | 5.26 |
| All-cause rehospitalization (30d) | 4 | 5.26 |
| Permanent pacemaker implantation (30d) | 5 | 6.60 |
| CV rehospitalization (30d) | 2 | 2.63 |
| All deaths (1y*) | 9 | 11.84 |
| All cardiovascular deaths (1y) | 2 | 2.63 |
|
* All deaths reported a 1 year: Multiple myeloma: n = 1; sepsis (respiratory tract infection): n = 1; sepsis (renal disease): n = 1; pulmonary disease: n = 1; hepatocellular carcinoma: n = 1; stroke: n = 1; cardiac tamponade: n = 1 (cardiovascular death within 30d); thyroid cancer: n = 1; sudden cardiac death: n = 1 (cardiovascular death within 30d). |
||
HRQoL assessment
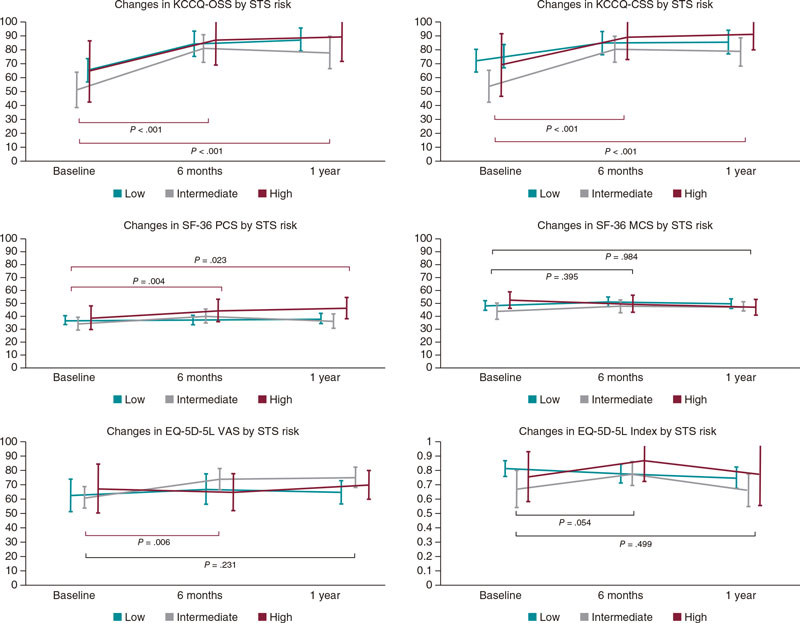
The patients’ HRQoL across the observational period according to STS risk score at baseline is shown on figure 1. In both summary scores of the KCCQ (OSS and CSS), statistically and clinically significant improvements after TAVI were seen. In OSS cases, the mean differences reported between baseline and 6 months ranged between 18.94 points (low risk) and 29.97 points (intermediate risk) indicative of a meaningful benefit (all P < .001). Similarly, the mean differences regarding the CSS ranged between 13.03 points and 27.3 points (low and intermediate, respectively). In addition, in both scales the observed benefit was maintained at follow-up with no differences being reported at 6 months and 1 year (P > .8 in both summary scales). Remarkably, these improvements were repeated across the 3 risk groups (figure 2).
Figure 1. New York Heart Association (NYHA) functional class at follow-up.
Figure 2. Changes in patient’s health-related quality of life at follow-up (n = 55, out of 67 survivors at 1 year; 82.1% of the sample). No differences in mean/median baseline values were seen in any of the health-related quality of life (HRQoL) measures taken between the patients who completed all the measurements and those with missing values at study period. EQ-5D-5 VAS, EQ visual analogue scale; KCCQ, Kansas City Cardiomyopathy Questionnaire; KCCQ CSS, KCCQ clinical summary score; KCCQ OSS, KCCQ overall summary score; SF-36 MCS, Medical Outcomes Study Short Form-36 Mental Component Summary; SF-36 PCS, SF-36 Physical Component Summary.
Regarding generic questionnaires, a positive impact was also seen in EQ-5D-5L VAS scores and the SF-36 Physical Component Summary, postoperatively, in all patients (P < .006 and P < .004, respectively). However, the size of detected differences was smaller considering the respective scales. No statistically significant differences were seen in the mental component summary (P = .395). In addition, values in all groups were comparable to normative population based on age and sex since baseline (mean—95% confidence interval [95%CI]—at baseline: 47.44, 44.70, and 50.18; 6 months: 49.77, 47.36, and 52.17; and 1 year: 48.64, 46.04, and 50.83-; P = .52). Finally, EQ-5D-5L index values were fairly similar across all time points with a slight increase observed at 6 months (P = .054 compared to baseline) and a mild drop at 1 year, not reaching statistical significance compared to baseline values (mean—95%CI—at baseline: 0.77, 0.71, and 0.83; 6 months: 0.80, 0.75, and 0.85; and1 year: 0.74, 0.68, and 0.80; P = .499).
Secondary endpoint: description of the procedure-related length of stay
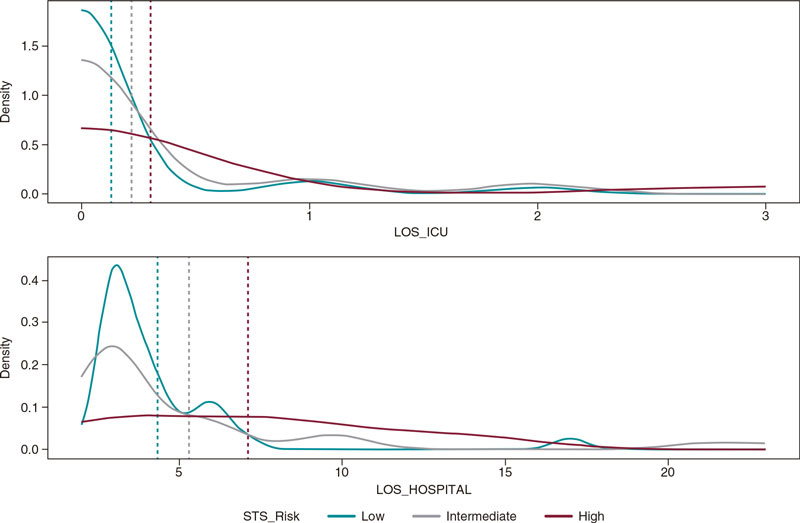
Mean LOS at the hospital setting was limited with a mean stay of 5.26 days (± 4.05), and only 10 patients (13.2%) required intensive care, 5 of whom (6.6% of the overall sample) remained at the ICU setting ≤ 1 day, 3 (3.9%) for 2 days, and 2 (2.6%) for 3 days. In figure 3, the ICU and hospital stays are shown based on the patients’ baseline risk (very low in all subgroups).
Figure 3. Length of stay (the hospital and the ICU settings) according to STS PROM score; n = 76 for LOS at the hospital setting, and n = 73 for LOS at the ICU setting. ICU, intensive care unit; LOS, length of stay; STS, Society of Thoracic Surgeons.
DISCUSSION
Ideally, severe, and symptomatic AS should be treated with a valve implanted via minimally invasive procedures while securing minimal perioperative and long-term risks, optimizing hemodynamic response, avoiding patients’ dependency on lifelong anticoagulation therapy, and maximizing their ability to do activities of daily living, and wellbeing. Insights from clinical trials indicate that the management of AS is moving in this direction.7,9 However it is still important to evaluate each innovation in real practice. This study provides new evidence on the performance of TAVI in elderly patients—mean age > 80 years—under real-world practice through a systematic prospective assessment of clinical outcomes, LOS, and patients’ reported outcomes 1 year after the procedure.
In our study, a noticeable positive clinical effect of TAVI was seen in all STS subgroups. Mean and maximum aortic gradients along with valve regurgitation improved significantly after the procedure, and major complications were kept at very reasonable rates. These outcomes were very similar to those recently published on SAPIEN 3.24 Also, regarding the NYHA scale, the percentage of patients with satisfactory functional status increased immediately after the procedure and was maintained at 1 year among survivors.
Importantly, these clinical benefits translated into meaningful improvements in the patients’ HRQoL. Particularly, the OSS of the KCCQ showed a mean change from baseline from 18.9 points in low-risk patients up to near 30 points in intermediate risk 6 months after treatment). Also, this benefit was preserved at 1 year with ranges between 21.9 points in low risk to 26.7 points in intermediate risk. These increments reported in the KCCQ were clearly over the minimal clinically important differences described in the medical literature for this tool.17 Actually, these results are especially important considering than a 10 points drop in KCCQ OSS scores turned out to be a prognostic factor for patients with AS associated with 34% more chances of dying at 12 months.18 Therefore, the HRQoL of older patients who survived 1 year improved significantly. Our results are similar to those from a former research that studied clinical outcomes from TAVI in clinical trials and registries including the SAPIEN 3. For instance, Baron et al.24—according to data from the SAPIEN 3 intermediate-risk registry—found changes at 1 year from TAVI in OS of 23.1 points (21.8-24.9; P > .001) among intermediate-risk patients. Their cohort had similar baseline characteristics and underwent TAVI with the same device (they did, however, include transfemoral and transapical access). In our study, we saw that this enhanced self-perceived health is also maintained in elderly patients classified as low- and high-risk patients.
Regarding generic tools, a positive trend was also detected in the EQ-5D-5L VAS and the PCS of the SF-36 (a summary component more focused on the overall functional performance of patients) with significant differences at 6 months and 1 year. We should mention that no differences were found at follow-up regarding the mental component summary. Also, the patients’ mental health was slightly lower compared to that reported in their reference population since baseline. Similarly, regarding the estimated utilities from the EQ-5D-5L, a global positive trend was seen at 6 months from baseline and a slight drop after 1 year. Nevertheless, all changes detected with this tool were minimal. This finding was surprising considering the great benefit demonstrated with both the OSS and the CSS of the KCCQ. However, as the EQ-5D-5L captures health-related quality of life more globally together with the mean age of the patients included, the slight decline seen could reflect general deterioration of health accumulated over the 1-year observational period (mean age of the sample > 80 years).
Furthermore, consistent with recent experiences in centers of excellence regarding TAVI in Italy, the Netherlands, and the UK, where authors tested novel standardized clinical care pathways to optimize the process with early discharge while reducing complications and LOS,25 we saw a very limited hospital stay with only 13% of patients requiring ICU admission (6.5% of the patients needed 3 days at the ICU). In our center, following an individualized protocol for candidates eligible for TAVI, we saw very high-quality outcomes in elderly patients while minimizing the procedure-related LOS.
Limitations
Inherently to the nature of this observational study, our findings are subject to a few limitations. First, our findings come from the experience of a single center in a tertiary referral hospital very familiar with the procedure so the extrapolation of these results might be affected by the experience of the heart team. Importantly, sample size, especially in the high-risk group, was limited and subject to high dispersion of values and missing data at follow-up. Therefore, further research with a larger number of patients stratified by risk should confirm our findings. Despite this limitation, we should mention that our results observed in the intermediate-risk group are similar to those reported with larger samples allowing for extensive propensity score cohort adjustment.24
Also, in this analysis, we decided to use the STS score as a measure of patient-risk characterization because, even when we acknowledge that this was originally developed for surgical aortic valve replacement, it is a valid prognostic measure of mortality and occurrence of major complications in TAVI making up a comprehensive assessment of the patients’ health status.13,26 However, as it has been described in recent research,27,28 it would also be helpful to include a measure of frailty to complete thedjusttments. Unfortunately, this information was not routinely collected through a standard form as part of the standard clinical management when this study was conducted. Nevertheless, we should mention that in our center, the heart team involved in the decision-making process regarding the individualized management of the patients’ clinical condition, always considers frailty as a key parameter to better adjust the provision of care. Also, precisely due to this preoperative assessment most patients classified as low risk by the STS score (42.5%) were treated with TAVI instead of open surgery. Hence, frailty is a core aspect of this process, always among other important factors like patient preference, history of chest radiation, previous coronary artery bypass graft or porcelain aorta, and others. Although through the comprehensive analysis of clinical and patient-reported outcomes a consistent and positive tendency in outcomes has been shown, we should mention that our results come from a cohort of patients treated in 2018. Consequently, it would be interesting to conduct a new study in multiple centers to obtain data to compare the evolution of the current clinical practice outcomes to those from 2018. Therefore, further research is warranted to continue this monitorization of outcomes in larger samples of patients.
CONCLUSIONS
In conclusion, this research provides clinical and patient-reported evidence on the performance of TAVI in elderly patients with clinical benefits maintained 1 year after the intervention. Furthermore, the short hospital stay observed provides exploratory insights into the benefits of standardized protocols created to manage low- to high-risk patients safely and efficiently.
FUNDING
Edwards Lifesciences provided funds for the analysis of this study that was conducted and interpreted independently by clinicians and methodological experts.
AUTHORS’ CONTRIBUTIONS
E. Pinar, J. García de Lara, J. Hurtado, B. Martí-Sánchez, G. Leithold, and J. Cuervo were involved in the study idea and design, and in the analysis of the study data. All authors were involved in the interpretation of the results and in the critical revision of the paper regarding its intellectual content and agree on the final version of the manuscript to be published.
CONFLICTS OF INTEREST
J. Cuervo, who works for Axentiva Solutions, disclosed that Axentiva Solutions has received financial support in the form of consultancy payments from Edwards Lifesciences towards the design and analysis of the study, and for medical writing support. B. Martí-Sánchez, and P. González work for Edwards Lifesciences. E. Pinar, J. García de Lara, J. Hurtado, M. Robles, G. Leithold, and K. Rand declared no conflicts of interest whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- Severe AS is the leading cause of valvular surgery among adults.
- TAVI has become the treatment of choice for inoperable patients with symptomatic, severe AS, and a valid alternative for patients at high- and intermediate-surgical risk with improved clinical results regarding survival and functional capacity.
- There are factors that influence TAVI results like surgical risk, patient’s life expectancy, baseline characteristics, modifiable risk factors, and comorbidities.
WHAT DOES THIS STUDY ADD?
- Through a comprehensive assessment including clinical, functional, and quality of life variables, this study shows a positive performance of TAVI in elderly patients at follow up.
- Improvement in mean and maximum aortic gradients, and valve regurgitation.
- Higher percentage of patients with a satisfactory functional status according to the NYHA scale after the intervention.
- Clinical benefits also translated into HRQoL improvements, and effect that was seen among all risk groups.
- Overall, in this consecutive sample of patients, the TAVI-related LOS (hospital) was short.
REFERENCES
1. Iung B, Delgado V, Rosenhek R, et al. Contemporary Presentation and Management of Valvular Heart Disease: The EURObservational Research Programme Valvular Heart Disease II Survey. Circulation. 2019;140:1156-1169.
2. Ferreira-González I, Pinar-Sopena J, Ribera A, et al. Prevalence of calcific aortic valve disease in the elderly and associated risk factors: A population-based study in a Mediterranean area. Eur J Prev Cardiol. 2013;20:1022-1030.
3. Durko AP, Osnabrugge RL, Van Mieghem NM, et al. Annual number of candidates for transcatheter aortic valve implantation per country: current estimates and future projections. Eur Heart J. 2018;39:2635-2642.
4. Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of Aortic-Valve Sclerosis with Cardiovascular Mortality and Morbidity in the Elderly. N Engl J Med. 1999;341:142-147.
5. Otto CM, Burwash IG, Legget ME, et al. Prospective study of asymptomatic valvular aortic stenosis: Clinical, echocardiographic, and exercise predictors of outcome. Circulation. 1997;95:2262-2270.
6. Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Hear J. 2017;38:2739-2791.
7. Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet. 2016;387:2218-2225.
8. Herrmann HC, Thourani VH, Kodali SK, et al. One-Year Clinical Outcomes With SAPIEN 3 Transcatheter Aortic Valve Replacement in High-Risk and Inoperable Patients With Severe Aortic Stenosis. Circulation. 2016;134:130-140.
9. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019;380:1695-1705.
10. Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med. 2019;380:1706-1715.
11. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice. Circulation. 2021;143:e35-e71.
12. Kuck K-H, Bleiziffer S, Eggebrecht H, et al. Konsensuspapier der Deutschen Gesellschaft für Kardiologie (DGK) und der Deutschen Gesellschaft für Thorax-, Herz- und Gefäßchirurgie (DGTHG) zur kathetergestützten Aortenklappenimplantation (TAVI) 2020. Der Kardiol. 2020;14:182-204.
13. Kumar A, Sato K, Narayanswami J, et al. Current Society of Thoracic Surgeons Model Reclassifies Mortality Risk in Patients Undergoing Transcatheter Aortic Valve Replacement. Circ Cardiovasc Interv. 2018;11:e006664.
14. Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The valve academic research consortium-2 consensus document. J Am Coll Cardiol. 2012;60:1438-1454.
15. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245-1255.
16. US Food and Drug Administration -Center for Devices and Radiological Health. Medical Device Development Tool (MDDT) Qualification Decision Summary For Kansas City Cardiomyopathy Questionnaire (KCCQ). 2021. Available at https://www.fda.gov/medical-devices/science-and-research-medical-devices/medical-device-development-tools-mddt. Accessed 20 Nov 2022.
17. Butler J, Khan MS, Mori C, et al. Minimal clinically important difference in quality of life scores for patients with heart failure and reduced ejection fraction. Eur J Heart Fail. 2020;22:999-1005.
18. Arnold S V, Spertus JA, Lei Y, et al. Use of the Kansas City Cardiomyopathy Questionnaire for monitoring health status in patients with aortic stenosis. Circ Heart Fail. 2013;6:61-67.
19. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727-1736.
20. Hernandez G, Garin O, Pardo Y, et al. Validity of the EQ-5D-5L and reference norms for the Spanish population. Qual Life Res. 2018;27:2337-2348.
21. Ramos-Goñi JM, Pinto-Prades JL, Oppe M, Cabasés JM, Serrano-Aguilar P, Rivero-Arias O. Valuation and Modeling of EQ-5D-5L Health States Using a Hybrid Approach. Med Care. 2017;55:e51-e58.
22. Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483.
23. Vilagut G, Ferrer M, Rajmil L, et al. The Spanish version of the Short Form 36 Health Survey: a decade of experience and new developments. Gac Sanit. 2005;19:135-150.
24. Baron SJ, Thourani VH, Kodali S, et al. Effect of SAPIEN 3 Transcatheter Valve Implantation on Health Status in Patients With Severe Aortic Stenosis at Intermediate Surgical Risk: Results From the PARTNER S3i Trial. JACC Cardiovasc Interv. 2018;11:1188-1198.
25. Barbanti M, van Mourik MS, Spence MS, et al. Optimising patient discharge management after transfemoral transcatheter aortic valve implantation: the multicentre European FAST-TAVI trial. EuroIntervention. 2019;15:147-154.
26. Hemmann K, Sirotina M, De Rosa S, et al. The STS score is the strongest predictor of long-term survival following transcatheter aortic valve implantation, whereas access route (transapical versus transfemoral) has no predictive value beyond the periprocedural phase. Interact Cardiovasc Thorac Surg. 2013;17:359-364.
27. Green P, Arnold S V, Cohen DJ, et al. Relation of frailty to outcomes after transcatheter aortic valve replacement (from the PARTNER trial). Am J Cardiol. 2015;116:264-269.
28. Goudzwaard JA, de Ronde-Tillmans MJAG, El Faquir N, et al. The Erasmus Frailty Score is associated with delirium and 1-year mortality after Transcatheter Aortic Valve Implantation in older patients. The TAVI Care & Cure program. Int J Cardiol. 2019;276:48-52.