Innovation in interventional cardiology
High-definition intravascular ultrasound
30/04/2019
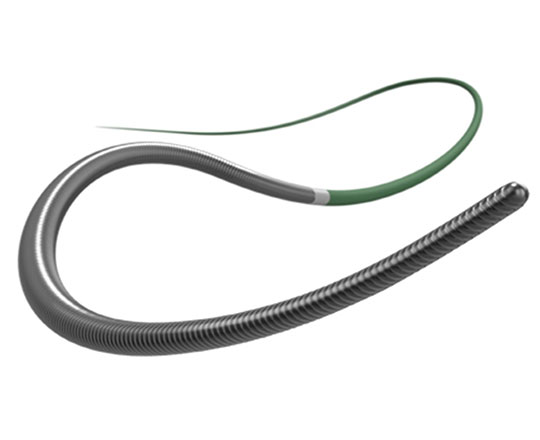

The Raider guidewire is a new coronary angioplasty guidewire for chronic total occlusion, brought to us by Teleflex Medical (USA). Given the fantastic technological advances in recent years, it would seem that continued innovation in guidewire design would be difficult, though not impossible.
This guidewire has a tapered stainless steel core surrounded by a 25 cm coil welded to the core at its proximal end. The distal 30 cm of the guidewire has a polymer coating, the tip is non-tapered, and the tip load is 4 g. It is available in 200 and 300 cm total length.
It is categorized as a medium-support guidewire. Its high flexibility does not limit its use as an added support to facilitate advancing a balloon or stent to more difficult locations.
The design, with a non-tapered tip, means a wider curve of attack can be pre-formed to enable access to more angulated locations. This feature also allows a larger knuckle-type curve to be created if the procedure requires it.
Its penetration power is excellent for increasing the strength of the guidewires used in total coronary occlusions. It is highly suitable for the stick-and-swap technique, in dissection procedures and in Stingray balloon re-entry.
What could it be compared to? A Pitol 200 (Abbott Vascular, USA). What does it add? Superior tactile control.
Keywords: Coronary angioplasty wire, complex percutaneous coronary intervention, microcatheters, chronic total occlusion.
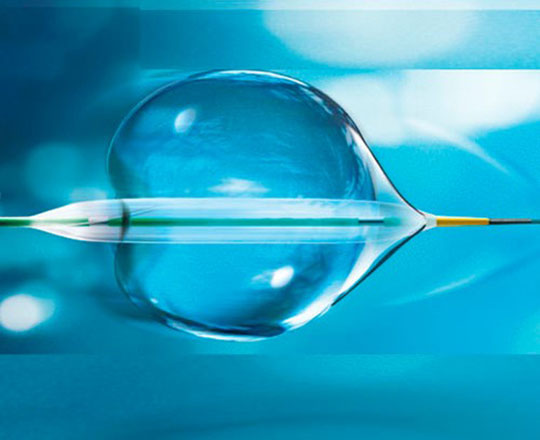

The POT balloon (BrosMed, China) is a dedicated noncompliant postdilatation balloon. Usually, noncompliant postdilatation balloons have shoulders measuring 3.2 mm, the shoulder being the distance from the balloon’s radiopaque marker to its tip. In this balloon, the extra short shoulder, measuring 0.6 mm, allows the device and the operator to remain within the stent, reducing the risk of proximal or distal vessel dissection when the stent is postdilated. It allows controlled postdilatation within the stent.
The balloon, with a diameter of 3 mm, has an entry profile of 0.015 to 0.020 mm and a crossing profile of 0.027 to 0.032 mm. These dimensions are excellent compared to what is already on the market. Worthy of note is the balloon’s low compliance, around 0.1 mm, with a rated burst pressure of 22 atm. The balloon measures between 2.25 and 5 mm in diameter and 6 to 15 mm in length. It is indeed a very useful device for bifurcations.
Keywords: Complex percutaneous coronary intervention, coronary bifurcation, stent postdilation, proximal optimization technique, stent, side-branch, malapposition.
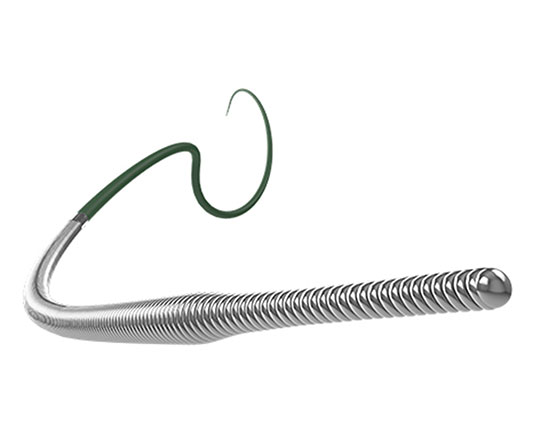

The Warrior guidewire (Teleflex, USA) belongs to the group of high–penetration-power guidewires with a tip load of 14 g. Often, the interventional cardiologist must resort to using peripheral vascular interventional guidewires of 20 g to cross very hard coronary lesions. The Warrior has a long high-support segment designed to facilitate access with long stents (which can be rather inflexible) to difficult locations. It is a slim guidewire with a tapered tip measuring 0.009’’ and micro curves of 2 to 3 mm can be made at its tip (it is not pre-formed). The core is made of stainless steel and the most distal 20 cm is surrounded by coil. This coil has a diameter of 0.014’’ at the most proximal part and 0.009’’ at the most distal part, which provides great stability and capacity for torque response. It has a hydrophilic coating and is available in lengths of 200 and 300 cm.
It is exclusively a penetration guidewire and is used to gain access to highly-calcified proximal or distal capsules and carry out re-entry with R-CART or stick-and-go techniques when a Stingray balloon is used.
One aspect of great practical interest is the following: Teleflex has performed studies on the interaction between tip load and penetration power of their guidewires with different microcatheters, showing that the combination of the Turnpike microcatheter with the Warrior guidewire gives a penetration power of 33.2 g. This means you can avoid resorting to guidewires designed for other vascular territories.
If we had to compare this guidewire to other similar guidewires, we could compare it to the Confianza Pro 12 g (Asahi Intecc, Japan) or the Hornet 14 (Boston Scientific, USA).
Keywords: Coronary angioplasty wire, complex percutaneous coronary intervention, microcatheters, chronic total occlusion.


In 2021, this new Korean-made device has won the prestigious Red Dot Award, given to the best device for design and business projection.
The use of new technologies incorporating artificial intelligence algorithms is increasingly common in medical diagnostics.
Atrial fibrillation is the most common sustained arrhythmia, with a high incidence in older populations. New onset of atrial fibrillation in patients who have undergone coronary intervention confers a worse prognosis, with higher rates of adverse events and mortality. It is therefore important to detect it early.
This device, called CART-I (SkyLabs, South Korea), provides 24-hour heart-rhythm monitoring with 99.6% accuracy in the detection of atrial fibrillation, using a ring that is worn on the finger. It is made of surgical materials, and emits photoplethysmography signals that measure the heart rate, identify episodes of atrial fibrillation and generate an electrocardiogram in real time. The ring, which has a battery-life of 48 hours, is water resistant and sends the information to a mobile phone application where the recording can be viewed and sent to the patient’s doctor. The ring can store 1500 episodes of 10 seconds’ duration, but can also record 30 seconds of electrocardiogram at any time or perform a continuous analysis of recent hours.
The future of this technology is moving toward the analysis of other types of arrhythmias, continuous blood pressure monitoring, assessment of heart failure parameters, chronic obstructive pulmonary disease, analysis of stress levels, sleep quality, and assessment of obstructive sleep apnea syndrome.
Keywords: Atrial fibrillation, wireless technology.
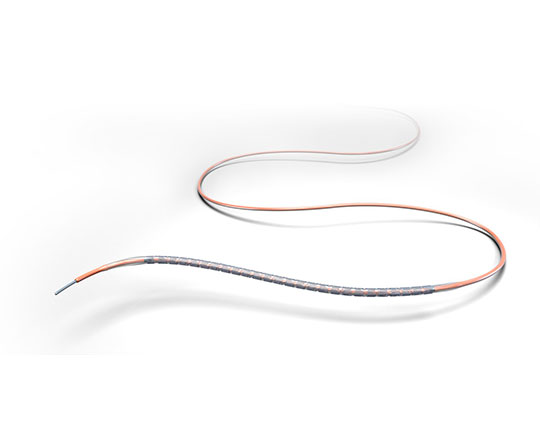

2021 continues with more new launches. In this case, it is the new Xience Skypoint stent (Abbott, USA), which has just been brought to market. This is a drug-eluting stent with improvements that provide excellent navigability, as well as greater post-dilation ranges to allow treatment any type of lesion and vessel.
Xience Skypoint has maintained many of the features of the Xience family. The stent design is of an everolimus-eluting cobalt-chromium multi-link platform; its fluoropolymer coating confers exceptional clinical outcomes in terms of stent thrombosis, and it also maintains the CE mark for 1 month of dual antiplatelet therapy in patients with high bleeding risk, offering the safest stent for all indications.
Xience Skypoint has a wider expansion range than the previous Xience generation, up to 5.75 mm diameter even with a length of 48 mm. This allows for greater expansion of the stent without longitudinal shortening, which improves precision in its positioning.
Other important changes in the device are the following: a more ergonomic connector port, with the largest stent dimensions, a slimmer catheter with a seamless one-piece shaft and a slimmer profile that guarantees high pushability with minimal force, facilitating its navigability in the treatment of more challenging lesions.
Keywords: Percutaneous coronary intervention, coronary stent.

