Innovation in interventional cardiology
High-definition intravascular ultrasound
30/04/2019


New Valve Technology (Switzerland) recently received CE mark approval for the treatment indication of percutaneous implantation of the ALLEGRA aortic valve in a degenerated bioprosthesis.
The ALLEGRA valve has two main characteristics: firstly, it is a self-expanding supra-annular valve with flexible commissures, and secondly, it has a low frame height that minimizes potential occlusion of the coronary ostium.
This approval came after extensive in vitro research on the behaviour of the ALLEGRA valve when implanted in the more commonly-used bioprostheses,1 followed by some very promising clinical results from the VIVALL study.2
Internationally, close to 100 cases of ALLEGRA valve implantation in degenerated surgical bioprostheses have been performed with very favourable outcomes in terms of residual gradient, paravalvular leak, and pacemaker implantation. In the VIVALL study,2 a reduction in transvalvular gradient (invasive measurement) from 37.1 ± 13.3 mmHg to 11.6 ± 3.7 mmHg was observed, with a 30-day mortality of 0%, and no paravalvular leaks or pacemaker implantations; the effective orifice area increased from 1.18 ± 0.58 cm2 to 1.4 ± 0.52 cm2.
Currently, patients of this type are being recruited for the post-marketing clinical study FOLLOW; when complete, this data will expand the clinical evidence on this percutaneous valve.
REFERENCES
1. Sedaghat A, Sinning JM, Werner N et al. In vitro hydrodynamic and acute clinical performance of a novel self-expanding transcatheter heart valve in various surgical bioprostheses. EuroIntervention. 2018;13:2014-2017.
2. Schaefer U, Butter C, Landt M et al. Thirty-day outcomes of a novel transcatheter heart valve to treat degenerated surgical valves - The “VIVALL” multi-center, single-arm, pilot study. EuroIntervention. 2019;15:e757-e763.
Palabras clave: prótesis valvular percutánea. Keywords: percutaneous valve prothesis.
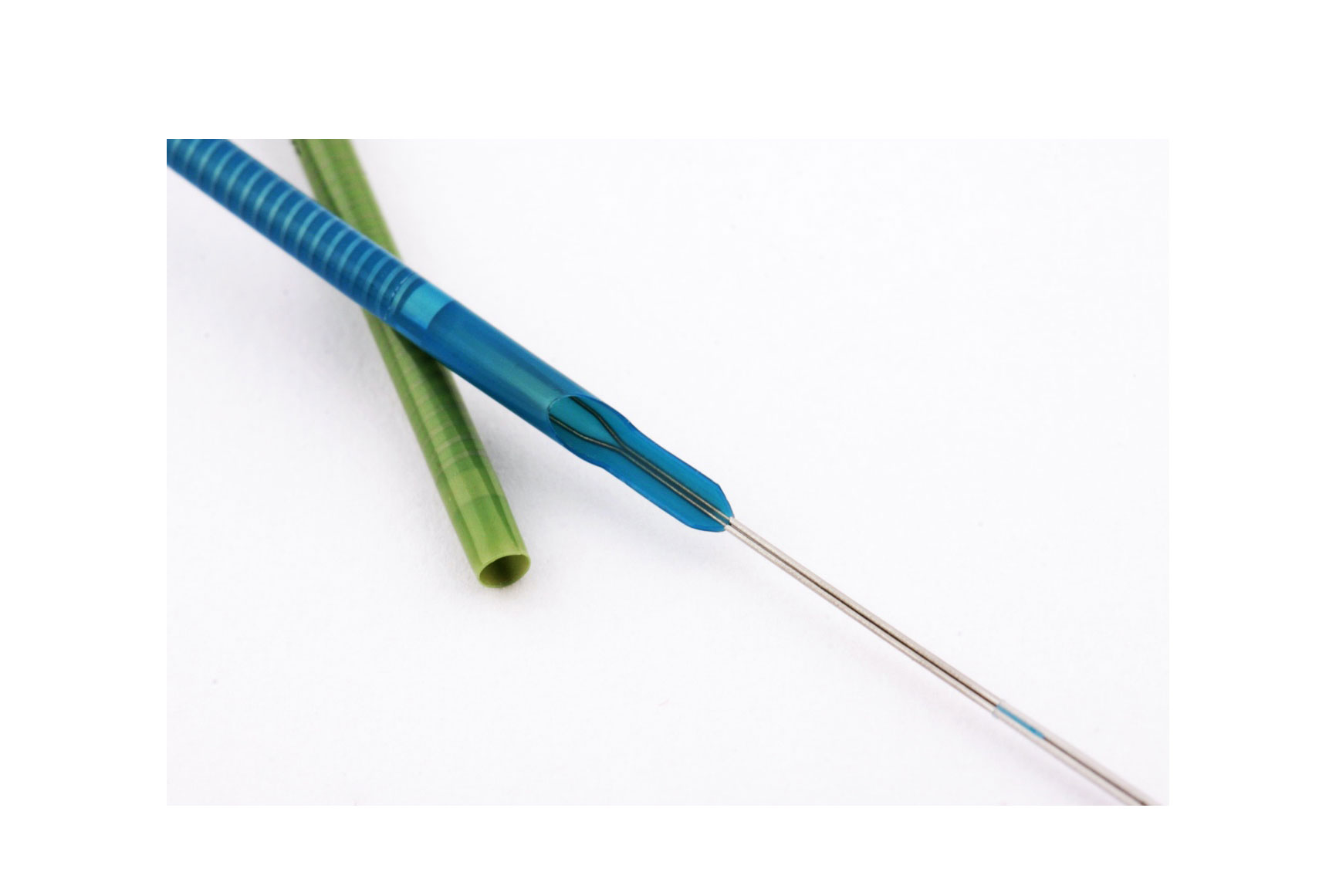
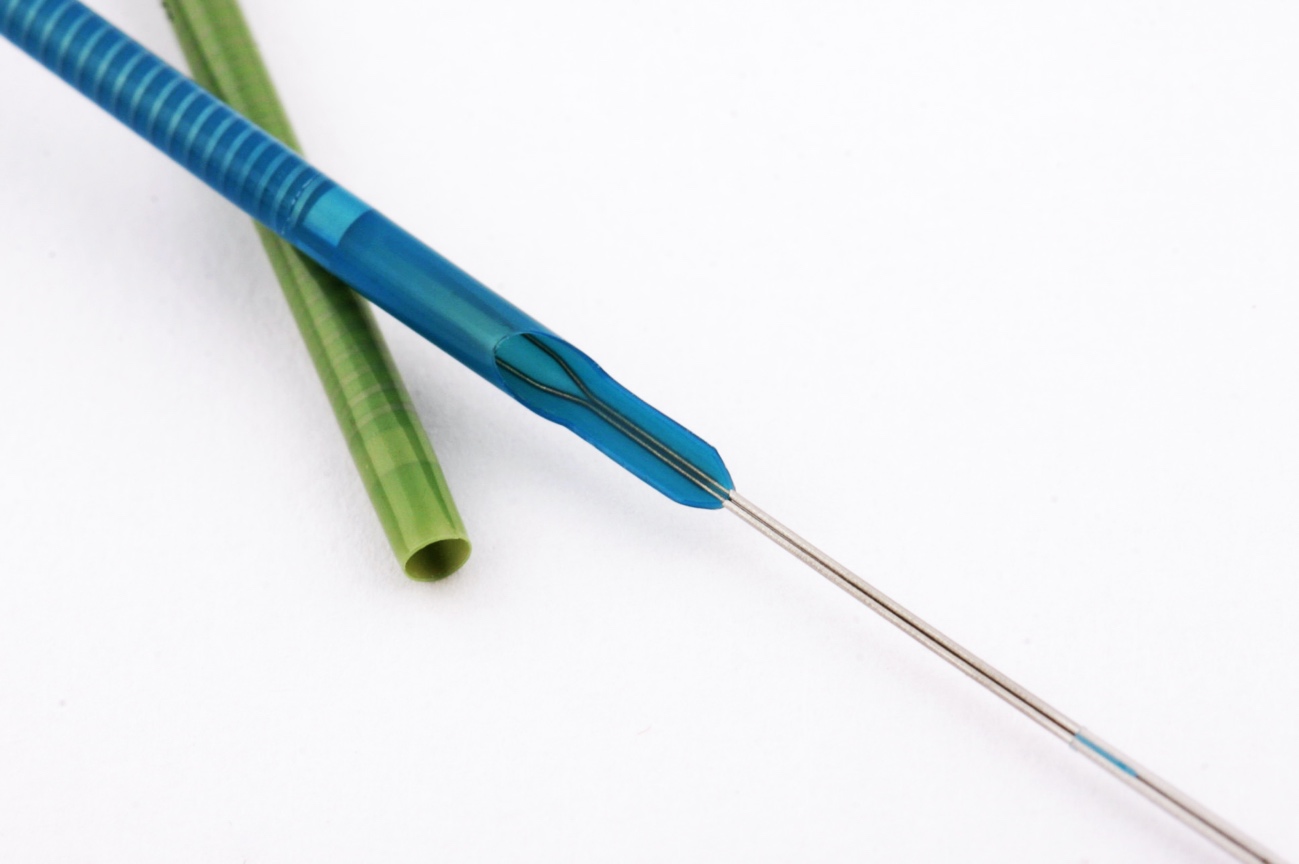
The guide extension catheter has become an essential device for treating stenotic lesions in complex coronary interventional procedures. When the guide catheter has limited support, reaching a target lesion for balloon dilatation and stent implantation can become mission impossible. Guide extension catheters, an extension of the guide catheter, facilitate safe device passage. It is amazing to see how they are able to reach lesions that balloons or stents would not even get near.
A new guide catheter extension has been released, called Boosting (QX Medical, USA), which has some new features that others do not have. The catheter is hydrophilic along its entire length, with a rounded tip to avoid a razor-blade effect on the atheroma plaque.
At the transition zone, a unique characteristic is worthy of note: the proximal part of the catheter is very wide, and its lumen accommodates the guide catheter without leaving a space between the two. This feature prevents flow between the guide catheter and the extension, so that, if fluid or contrast is given, it goes straight to the tip of the guide catheter extension. Also, if we want to advance a guidewire it can be introduced directly into the extension, without the risk of passing outside it. The portion that is used to push is made of two soldered wires with great flexibility and pushability that are integrated directly with the shaft of the catheter.
Palabras clave: Extensor de catéter guía. Keywords: Guide extension catheter.
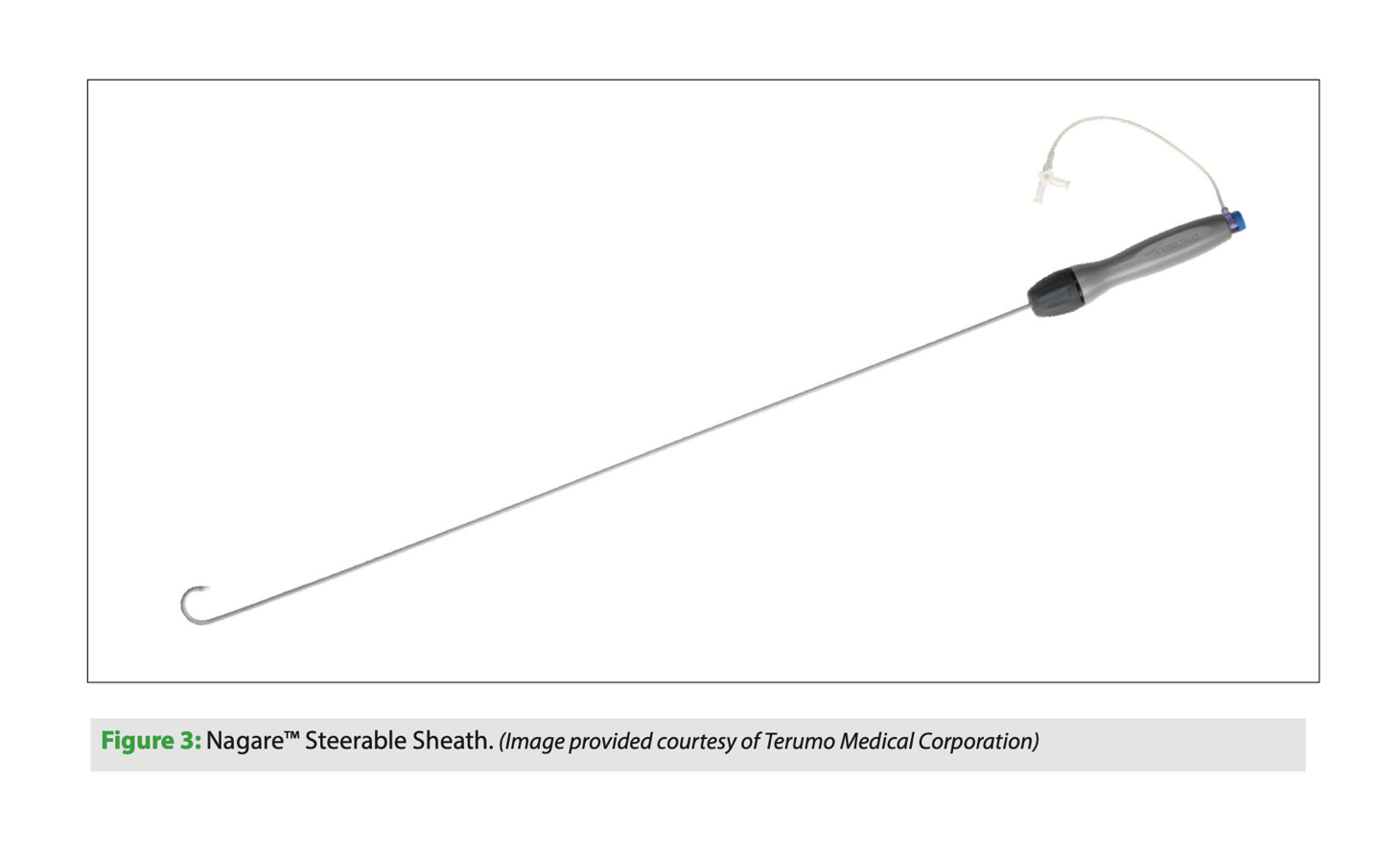
Nagare bidirectional steerable sheath

The Nagare bidirectional steerable sheath (Terumo, Japan) is a catheter that allows controlled navigation in all chambers of the heart. The sheath has bidirectional movement thanks to its TrueVector technology: the device is manufactured with a coaxial mesh in the shaft, unlike others that use pull wire systems to move the tip of the catheter. This mesh, which is Kevlar-coated, can move freely, and since it is pulled proximally along with the shaft, only the tip is deflected, with accuracy and stability. This prevents the classic whipping effect of other similar catheters in which deflection occurs along the length of the catheter rather than just the tip; this is an interesting improvement.
The Nagare bidirectional steerable sheath has an external sheath diameter of 12 Fr and an internal diameter of 8.8 Fr, with a usable catheter length of 73.7 cm. Its size allows other devices to be passed through it. It has a smooth transition to the dilator for a 0.032’’ guidewire. It can be deflected 90° or 180° on its longitudinal axis with a turning radius of 25 mm.
This device is indicated for controlled transseptal puncture. Structural interventional procedures that are performed on the left side of the heart often require a posterior puncture below the interatrial septum. A steerable sheath of this type allows better control of the puncture site.
Palabras clave: introductor deflectable, punción transeptal. Keywords: steereble sheath, transseptal puncture.
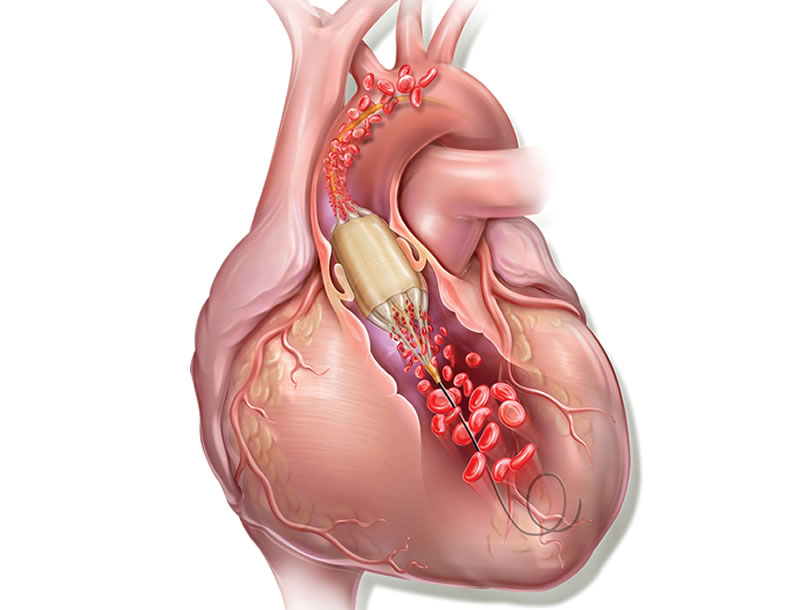
True Flow balloon valvuloplasty catheter

Aortic balloon valvuloplasty requires rapid cardiac pacing with a pacemaker to avoid the balloon moving while it is inflated and causing injury to the leaflets or even the annulus if it slips uncontrollably. In addition, during valvuloplasty, the inflated balloon (inflated for approximately 5 s) causes left ventricular outflow tract obstruction and ischemia, which in some patients may cause significant hemodynamic compromise.
True Flow (Bard, USA) is a valvuloplasty balloon catheter that allows continued blood flow while it is inflated. It has an outer fiber-based shell that prevents the balloon from sliding when it is opened onto the valve leaflets, so a pacemaker is not required. This shell is made of high-molecular weight polyurethane, polyester and aramid fibers (a structural component of Kevlar) and has minimal stretch and high resistance to rupture. The inside of the balloon has 8 small balloons around the edge of the main balloon, with an inner lumen between them where blood can flow. The device measures 3.5 cm long and is available in six sizes, with diameters from 18 mm to 26 mm. The smaller models are compatible with an 11 Fr sheath and the largest, with a 16 Fr sheath.
This type of balloon catheter allows valvuloplasty to be performed for 60 s or more without rapid pacing, with a 1/3 reduction in the mean aortic pressure while the balloon is inflated, which means a 50% reduction in the aortic gradient. This could be especially useful in patients with severe dysfunction.
Palabras clave: valvuloplastia aórtica, balón de valvuloplastia. Keywords: aortic valvuloplasty, valvuloplasty balloon.
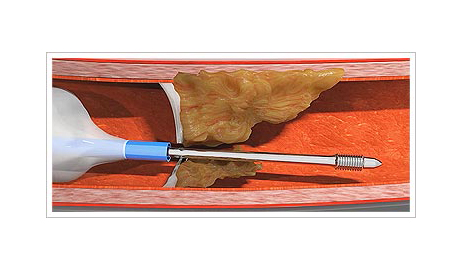
Capbuster

Several challenges may arise for the clinician performing an interventional procedure on coronary arteries with chronic total occlusion. One such challenge is that of an impenetrable plaque. Possible solutions to this problem include active and passive support techniques, as well as devices such as the Tornus (Asahi Intech, Japan) and the Turnpike Gold (Teleflex, USA). However, these devices require high passive support and they are not always effective.
The CapBuster (Capsos Medical, Ireland) is a balloon catheter combined with a special guide wire designed to perforate impenetrable plaques. The microcatheter balloon is inflated inside the coronary artery, proximal to the plaque. This balloon provides, within the coronary artery, the active support required to allow the guidewire to be advanced through the plaque. Another of its benefits is that, once inflated, the tip of the microcatheter centers in the coronary artery to ensure an excellent central, coaxial alignment with the plaque.
One the balloon is inflated, the guidewire is advanced as if it were a lag screw: it is turned clockwise to advance it and, one the plaque has been crossed, it is turned anticlockwise to remove it. Once the plaque has been penetrated, a standard guidewire for chronic total occlusion can be used as per the interventionist’s choice.
It is estimated that the CapBuster will obtain the CE mark in 2021. Until then, we will have to wait be this device that, in theory, will help solve these important problems.
Palabras clave: placa coronaria impenetrable, oclusión coronaria crónica. Keywords: uncrossable coronary plaque, chronic total occlusion.

