Cardialysis was founded in 1983 by visionary professionals from the Thoraxcenter at Erasmus University Medical Center (EMC) in Rotterdam, The Netherlands. This initiative emerged to address the need for a specialized research organization able to plan, execute, and report European cooperative clinical investigations in the field of cardiovascular research.1 The mission of Cardialysis is “to be at the heart of cardiovascular research” in the fullest sense of the phrase. To achieve this mission, the organization strives to collaborate with clinicians, trialists, research professionals, regulators, industry partners, and research organizations that share the same passion. Throughout the first 40 years, its well-established reputation has been based on dedication, diligence, and industry-leading standards. This has been made possible by attracting and retaining talented employees, as well as by cultivating long-standing relationships with investigators, clients, and partners.
Organizations similar to Cardialysis are typically classified as either academic research organizations (AROs) or contract research organizations (CROs). AROs, such as clinical trial units and epidemiology departments, are typically affiliated with universities or university hospitals, and usually design and manage investigator-initiated single-center or multicenter national clinical trials. These organizations are academically-driven and their traditional objectives are to facilitate research and education, publish innovative research in peer-reviewed journals, and support PhD programs. In contrast, CROs are private entities performing sponsor-driven clinical trials across all phases of clinical development. These organizations are business-driven, and expected to remain self-sustaining by establishing research contracts with the medical industry in a strict regulatory environment.
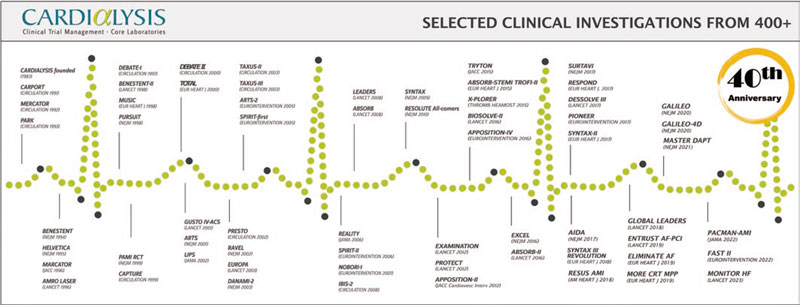
Similar to other world-leaders in cardiovascular research, Cardialysis has successfully adopted a hybrid ARO/CRO model, combining the best of the 2 models. First, the organization participates in innovative research that has played a pivotal role in the development and improvement of therapies in cardiology. This involvement has resulted in numerous high-impact publications and the completion of more than 100 theses through its support of in-house academic research. Figure 1 lists notable trials in which Cardialysis participated, including research in coronary artery disease, structural heart disease, heart failure, hypertension, and peripheral artery disease. Second, the organization proudly maintains a global network of renowned investigators who play key roles in the academic leadership of trials (eg, within steering committees), independent clinical events committees (CECs),2 independent data and safety monitoring boards,3 and imaging core lab supervision. Third, Cardialysis operates as a private, independent entity that serves both university hospitals worldwide, as well as leading pharmaceutical and medical device industry partners. Fourth, the organization maintains state-of-the-art infrastructure and follows standardized procedures, ensuring efficiency and quality. Finally, Cardialysis consistently adheres to applicable regulatory requirements for trial execution in all active regions, including Europe, the United States of America, and China.
Figure 1. Timeline of landmark trials conducted with Cardialysis.
THE CARDIALYSIS CORE LAB
Over the past half century, clinical research has had an enormous impact on clinical outcomes, which was eloquently summarized by Nabel and Braunwald.4 These advancements are inextricably linked to the development of therapeutic and diagnostic devices, especially in interventional cardiology. The development of coronary angioplasty involved progressive iterations in coronary stent technologies and continuous innovation in intracoronary imaging.5 Similarly, advancements in transcatheter therapies for aortic, mitral, and tricuspid conditions have paralleled the progress in imaging modalities and techniques.6 Independent and consistent assessments of imaging outcomes are an important component of dossier evaluations when commercialization approvals are sought for new devices.7
Cardialysis started as a central reading facility for electrocardiography-Holter monitoring. However, over time it has validated and implemented rigorous analysis methodologies for various imaging techniques within the coronary core lab. These include quantitative coronary angiography (n = 67 000+), intravascular ultrasound (n = 16 000+), near-infrared spectroscopy (n = 1500+), and optical coherence tomography (n = 5000+). Notably, Cardialysis also launched and maintains the globally used SYNTAX score (n = 10 000+) website.8 Due to a shift toward structural heart research, its echocardiography core lab has become its largest laboratory and serves large European and global investigations (n = 39 000+). Additionally, the organization operates magnetic resonance imaging (n = 1000+), cardiac computed tomography (n = 1000+), and electrocardiography-Holter core laboratories (n = 270 000+).
CARDIALYSIS CLINICAL TRIAL ACTIVITIES
The workload involved in a clinical investigation should not be underestimated. For example, the ongoing IVUS CHIP randomized clinical trial (NCT04854070) involves 40 sites in Europe and more than 200 professionals in the execution of the study, excluding the members of the Ethics Committees. Managing and coordinating more than 200 professionals across 7 countries for a minimum of 5 years requires high levels of availability, dedication, consistency, and well-established standardized procedures. Having an all-inclusive research organization performing ambitious trials increases efficiencies. Table 1 lists the activities performed at Cardialysis, which are those expected of any all-round professional research organization. Further information is available on the Cardialysis website.8
Table 1. List of activities performed at a cardiovascular research organization
| Trial design and protocol development |
| Steering committee set-up and coordination |
| Site feasibility and selection |
| Site start-up and regulatory submissions |
| Site contracting |
| Project management |
| Monitoring management |
| Site management and site monitoring |
| Safety reporting |
| Medical monitoring |
| Electronic case report forms development and hosting |
| Data management |
| Clinical events committee set-up and coordination |
| Data and safety monitoring board set-up and coordination |
| Publication committee set-up and coordination |
| Biostatistics: sample sizing, statistical analysis plan, and statistical reporting |
| Medical writing including patient narratives |
| Publication strategy |
| Quality assurance and regulatory compliance |
THE ACADEMIC RESEARCH CONSORTIUM
In 2006, with the increasing need for consistent endpoint definitions in coronary artery disease research, and specifically due to the challenges posed by the classification of stent thrombosis, leading AROs in the United States and Europe, including Cardialysis, collaborated to found the Academic Research Consortium (ARC).8 The primary mission of the ARC is to promote informed and collaborative dialogue among stakeholders, with the goal of developing consensus definitions and nomenclature for targeted areas of new medical device development, and to disseminate such definitions in the public domain.8 The first initiative focused on endpoint definitions and classifications for coronary intervention trials and was published in 2007.9 This consensus document was developed in consultation with regulatory agencies in both the United States and Europe, and became one of the most cited articles in interventional cardiology. To date, the ARC has successfully launched and completed 20 programs and is currently running 15 new initiatives based on an unprecedented level of global scientific collaboration.8
EUROPEAN CARDIOVASCULAR RESEARCH INSTITUTE
Europe is known for its stringent regulations on academic research. Importantly, the sponsor of a clinical investigation is the legal entity (or individual) that ensures that regulations are met and that monitors patient safety either directly or through a data and safety monitoring board. The sponsor holds full rights to the study data (ie, has sole ownership) and is responsible for verifying data integrity, ensuring that published results are consistent with the locked final analysis database. Although prominent academic centers possess the expertise to sponsor clinical trials, the size of certain studies requires external support for manageability.
In response to this need, the European Cardiovascular Research Institute (ECRI) was founded in 2012 by the Cardialysis group as an academic research platform capable of overseeing the execution of large, multicenter clinical trials and of fulfilling sponsor responsibilities. Since April 2023, the ECRI has become an independent foundation (Stichting) under Dutch law. This institute combines 3 elements of success: its outstanding academic leadership represented by its pro bono scientific advisory board (figure 2), a well-established network of investigators and research professionals, and the possibility to conduct clinical research activities in partnership with Cardialysis. Through this collaborative model, the ECRI-Cardialysis joint venture has successfully conducted some of the largest interventional cardiology trials, with the GLOBAL LEADERS trial being the most representative.10 This trial enrolled 15 968 patients at 130 sites across 18 countries. Another example is the MASTER DAPT trial,11 which enrolled 4579 high-bleeding risk patients at 140 sites in 30 countries.
Figure 2. European Cardiovascular Research Institute foundation and its scientific advisory board. ECRI, European Cardiovascular Research Institute.
The ECRI-Cardialysis partnership is currently conducting 3 landmark investigations on the use of coronary imaging and coronary physiology to guide percutaneous coronary interventions. Up-to-date details are available on the Cardialysis website.8
A LANDMARK FIGURE
Numerous clinicians and clinical research professionals have passed through the door of Cardialysis, with some staying for a lifetime and others for a short period. Undoubtedly, the most salient contributor to the organization’s innovations and successes has been Prof. Patrick W. Serruys. His mentor, Prof. Paul Hugenholtz, acknowledged as the father of the European Society of Cardiology, and one of the founding members of Cardialysis, recognized Prof. Serruys as a natural talent and luminary in clinical research. Prof. Serruys joined Cardialysis during its early days and since then played a decisive role in the organization. His innovations and contributions included the development of coronary imaging techniques (eg, he was coinventor of quantitative coronary angiography); the establishment of best practices in interventional clinical trials, including the design and execution of the BENESTENT study,12 which was the first of its kind and remains one of the most cited publications in interventional cardiology; research in bioresorbable scaffolds; and an illustrious career that garnered multiple distinctions and awards.13 Although Prof. Serruys left the organization in 2019, his scientific legacy remains highly esteemed.
Currently, a new generation is shaping the present and future of the organization with unwavering commitment to innovation, collaborative research, and, above all, commitment to improved patient outcomes. The 40-year timeline of Cardialysis (figure 1) starts with the CARPORT trial led by Prof. Serruys from the Thoraxcenter, and concludes with the FAST II study led by Dr Joost Daemen14 and the MONITOR HF trial led by Dr Jasper Brugts,15 both also from the Thoraxcenter. While Cardialysis envisions expanding its European and global collaborations in the coming decades, its scientific partnership with EMC has remained strong and will continue to flourish.
THE FUTURE
Cardialysis pledges to continue contributing to the design, execution and reporting of clinical trials as an independent and scientifically-driven cardiovascular research organization and core laboratory. Its priorities are patient safety, data integrity, and the production of high-quality data, which have been achieved by committed employees, passionate investigators, and long-standing collaboration with its valued partners.
Additionally, Cardialysis remains committed to further advancing continued standardization of clinical trial definitions, design principles, and core laboratory methods, given that standardization has proven to be a catalyst in clinical research. Current innovations at Cardialysis focus on implementing artificial intelligence, streamlining clinical trial processes, harnessing the potential of real-world data, and mastering applicable regulatory science.
FUNDING
None.
CONFLICTS OF INTEREST
E. Spitzer is a board member and shareholder of Cardialysis and a board member of the ECRI.
ACKNOWLEDGMENTS
Our 40th anniversary is dedicated to our beloved colleague Eline Montauban van Swijndregt†.
REFERENCES
1. Organization, review, and administration of cooperative studies (Greenberg Report):a report from the Heart Special Project Committee to the National Advisory Heart Council, May 1967. Control Clin Trials. 1988;9:137-148.
2. Spitzer E, Fanaroff AC, Gibson CM, et al. Independence of clinical events committees:A consensus statement from clinical research organizations. Am Heart J. 2022;248:120-129.
3. United States Food and Drug Administration. Guidance for Clinical Trial Sponsors. Establishment and Operation of Clinical Trial Data Monitoring Committees. March 2006. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/establishment-and-operation-clinical-trial-data-monitoring-committees. Accessed 30 June 2023.
4. Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med. 2012;366:54-63.
5. van Zandvoort LJC, Ali Z, Kern M, van Mieghem NM, Mintz GS, Daemen J. Improving PCI Outcomes Using Postprocedural Physiology and Intravascular Imaging. JACC Cardiovasc Interv. 2021;14:2415-2430.
6. Casenghi M, Popolo Rubbio A, Menicanti L, Bedogni F, Testa L. Durability of Surgical and Transcatheter Aortic Bioprostheses:A Review of the Literature. Cardiovasc Revasc Med. 2022;42:161-170.
7. United States Food and Drug Administration. Clinical Trial Imaging Endpoint Process Standards. Guidance for Industry. April 2018. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-trial-imaging-endpoint-process-standards-guidance-industry. Accessed 30 June 2023.
8. Cardialysis. Available at: www.cardialysis.nl/library/links. Accessed 2 July 2023.
9. Cutlip DE, Windecker S, Mehran R, et al;and Academic Research C. Clinical end points in coronary stent trials:a case for standardized definitions. Circulation. 2007;115:2344-2351.
10. Vranckx P, Valgimigli M, Juni P, et al;and Investigators GL. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent:a multicentre, open-label, randomised superiority trial. Lancet. 2018;392:940-949.
11. Valgimigli M, Frigoli E, Heg D, et al;and Investigators MD. Dual Antiplatelet Therapy after PCI in Patients at High Bleeding Risk. N Engl J Med. 2021;385:1643-1655.
12. Serruys PW, de Jaegere P, Kiemeneij F, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med. 1994;331:489-495.
13. Serruys DP. Featuring:Dr Patrick Serruys. Eur Cardiol. 2018;13:80-82.
14. Masdjedi K, Tanaka N, Van Belle E, et al. Vessel fractional flow reserve (vFFR) for the assessment of stenosis severity:the FAST II study. EuroIntervention. 2022;17:1498-1505.
15. Brugts JJ, Radhoe SP, Clephas PRD, et al;investigators M-H. Remote haemodynamic monitoring of pulmonary artery pressures in patients with chronic heart failure (MONITOR-HF):a randomised clinical trial. Lancet. 2023;401:2113-2123.