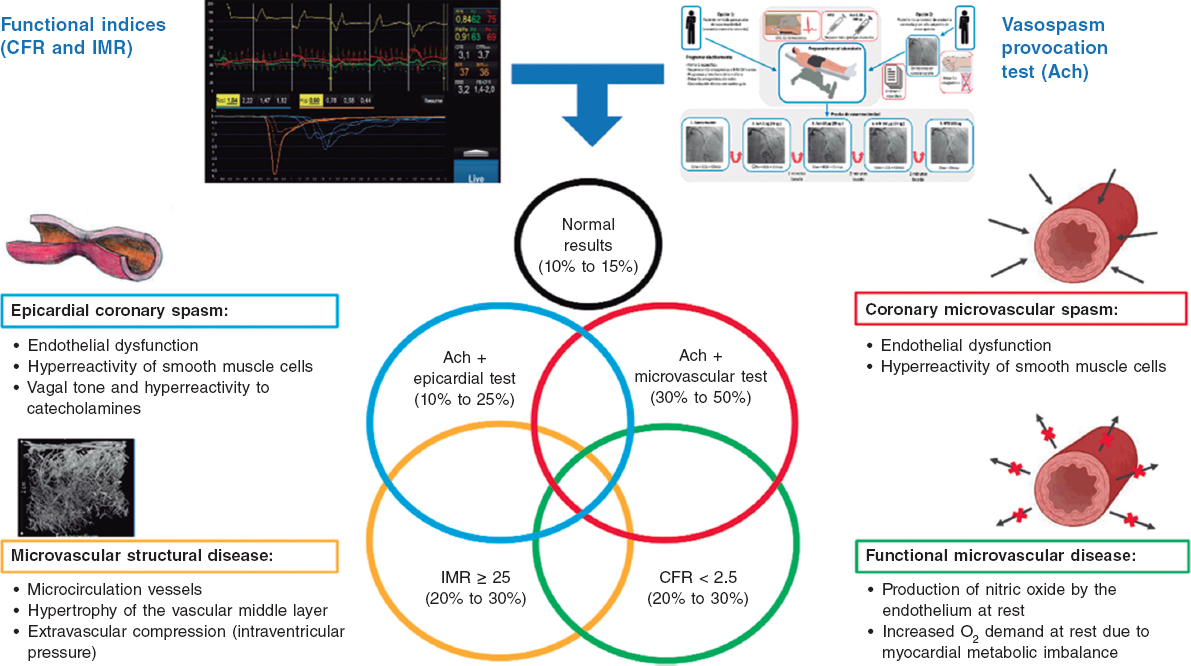
QUESTION: Do you think that there is, currently, any evidence behind the use of cerebral protection devices in transcatheter aortic valve implantation (TAVI)?
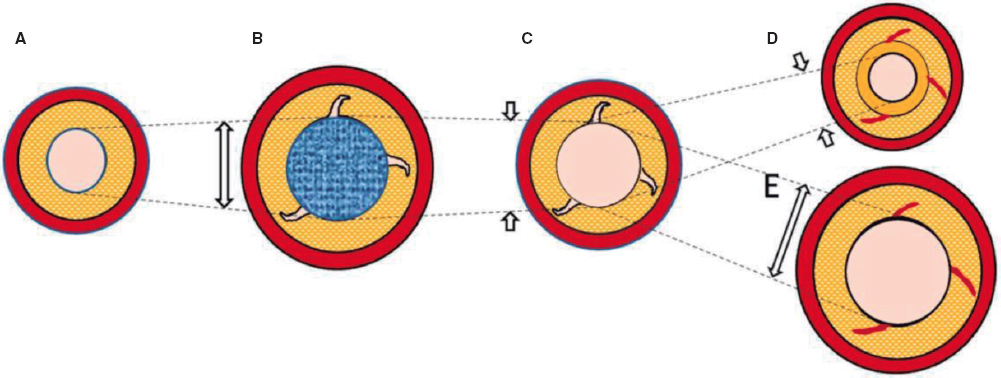
ANSWER: Former studies described that, during TAVI, loose debris like arterial wall fragments, thrombi, valve tissue, and foreign bodies often enters the circulation.1 These particles are the aftermath of the device making its way through the aorta towards the aortic annulus, the positioning and displacement of a calcified stenotic valve between the new valve stent and the aortic wall, and further manipulations to optimize results (postdilatation). To this date, numerous studies have been published on the safety and efficacy profile of these cerebral protection devices (CPD). Specifically, 4 randomized clinical trials have been published associated with the SENTINEL (Boston Scientific Corp., United States): MISTRAL-C,2 CLEAN TAVI,3 SENTINEL,4 and PROTECTED TAVR5 we can talk about later on. The MISTRAL-C trial used cerebral magnetic resonance imaging to demonstrate a significant reduction in the number of patients with multiple cerebral lesions (20% vs 0%; P = .03) and less cognitive impairment (4% vs 27%; P = .017). Similarly, the CLEAN TAVI trial also reported fewer novel lesions and of a smaller volume without any differences being reported in the number of clinical events in the group that used CPD. These studies demonstrated fragments being captured in almost in 100% of the cases. Several metanalyses6-13 also confirm these results regarding the number and volume of cerebral lesions described, and even some show lower rates of strokes in the DPC group.10,12,13 Therefore, we not only have the visual in situ demonstration of the particles being captured in the baskets following implantation, but also scientific evidence that CPD are effective capturing fragments released during TAVI that can land in cerebral circulation, thus lowering the number of cerebral lesions found on the magnetic resonance imaging during the procedure. However, whether capturing such particles with the device has a clear clinical benefit for its widespread use is still to be elucidated.
Q.: What do you make of the PROTECTED TAVR trial?
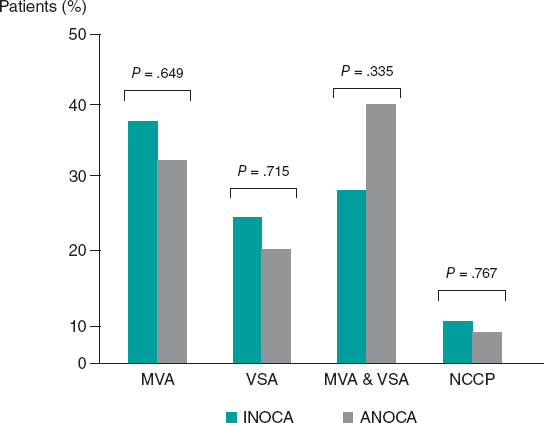
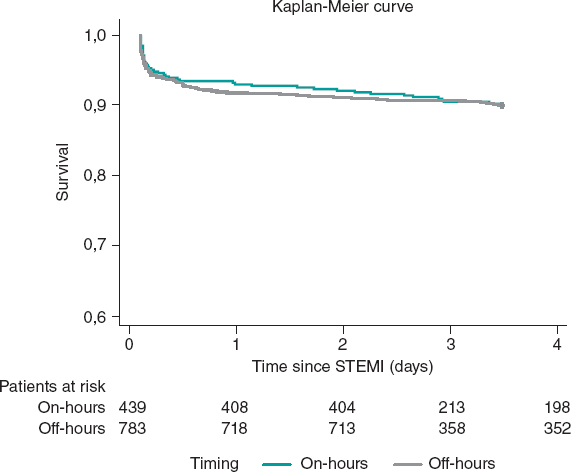
A.: The PROTECTED TAVR5 was a multicenter randomized trial that included a total of 3000 patients treated with TAVI and randomized on a 1:1 ratio to undergo the procedure with or without a CPD (control group). The study primary endpoint was to assess the rate of strokes 72 hours after TAVI or before discharge, whatever came first, and the difference was not significant between the 2 groups (absolute difference, −0.6%; relative difference, −20.7%). However, in 1 of the 15 secondary endpoints, the rate of disabling strokes dropped significantly in the CPD group (0.5% vs 1.3% in the control group). The number needed to treat to prevent an disabling stroke was 125 patients. This study has its pros and cons. Its main strength is that neurological examinations were conducted before and after TAVI, and events were adjudicated by an independent event adjudication committee.14 However, these examinations were not always conducted by expert neurologists. Also, no imaging modalities were systematically performed on all the patients, thus leading to misdiagnosed asymptomatic strokes. We should mention that hemorrhagic strokes were also included. However, they were only found in 2 patients from each group. The study main weakness is that the size of the sample was estimated to have a rate of strokes of 4%. However, the actual rate of strokes of the control group was much lower than expected (2.9%). A reason that may explain the low rate of strokes reported in the control group is the risk profile of the patients included. In this study, the Society of Thoracic Surgeons (STS) mean score in the control group was 3.4 ± 2.8. Also, over 50% of the patients included in both groups had STS scores < 3 meaning that they were low-risk patients. The results of the PROTECTED TAVR trial do not provide scientific evidence for the systematic use of cerebral protection devices. However, we should mention that, across the years, the improvements made in both the TAVI implantation technique and the design of the devices used haven’t reduced the rate of strokes significantly.15 It’s plain to see that the rate of strokes of the different studies conducted drops because the risk profile of the patients included is better. However, if the SENTINEL device eventually manages to reduce the rate of disabling strokes in low-risk patients, we’d be reducing the occurrence of one of the most dreaded complications for patients treated with TAVI both due to the increased mortality associated and the greater morbidity it adds on the patient who, on many occasions, becomes disabled. However, not everything has been said and done in this field. The results of the BHF Protect TAVI randomized clinical trial are still pending.16 It is still recruiting patients and will include twice the number of patients since the size of the sample was estimated for a rate of strokes in the control group of 3%, something more in tune with the actual rate of patients treated with TAVI. There are still unsolved issues like what impact CPD have on patients with high risk of stroke and whether the protective effect of CPD treating asymptomatic cerebral lesions is associated with the patients’ cognitive function in both the mid- and long-term.17
Q.: Are you for a widespread use of cerebral protection devices or do you think that some patients are more eligible than others?
A.: To this date, with the results currently available, there is no robust scientific evidence backing the systematic use of CPD in patients treated with TAVI. I think that those who would benefit the most from this kind of devices are individuals with a higher risk of stroke like patients with previous strokes, renal injuries, bicuspid aortic valves, severe aortic valve calcification and valve-in-valve procedures, porcelain aorta, and young patients.15,18 Also, patients with thrombi in the left atrial appendage or fibroelastoma or loose material dependent on the aortic leaflets or ascending aorta that could embolize during predilatation or valve implantation.
Q.: What are the main differences of the devices currently available?
A.: The 2 devices currently available in Spain are the SENTINEL and the TriGUARD (Keystone Heart Ltd, Israel). The main differences between the 2 are:
- – Access route: the SENTINEL devices always uses a 6-Fr right radial access, and the TriGUARD a 8-Fr access route.
- – The degree of protection of supraaortic trunks: the SENTINEL device protects the brachiocephalic trunk and the left carotid artery only sparing the left subclavian artery. However, the TriGUARD device protects all 3 supraaortic trunks.
- – Anatomical limitations regarding implantation: the SENTINEL device requires brachiocephalic trunk and left carotid artery diameters of 9 mm to 15 mm and 6.5 mm to 10 mm, respectively, and no tortuosity or severe stenosis in the 3 cm from the ostia of the vessels. Also, there are certain anatomical variants of supraaortic trunks that, though rare, would contraindicate its use. Therefore, a computed tomography scan including supraaortic trunks is advised to measure and assees the anatomy and see if device implantation is feasible. Regarding the TriGUARD, the anatomical limitations are iliac artery and abdominal artery diameters > 3.7 mm and > 10 mm, respectively, a distance < 76 mm from the femoral head up to 3 cm to 4 cm beyond the brachiocephalic trunk (this measurement complies with almost 100% of the population in our country), and the so-called «security gap» consisting of a distance > 65 mm between the aortic annulus and the ostium of the brachiocephalic trunk to make sure that the device does not interfere with TAVI.
- – The SENTINEL device captures particles in its filter baskets while the TriGUARD does not. Instead, it steers them towards the descending aorta.
- – Finally, another significant difference is that the TriGUARD device does not protect the coronary arteries from the radial access. If there is risk of coronary occlusion during TAVI, 2 different ipsilateral femoral accesses plus the therapeutic one should be used. This is not the case with the SENTINEL device because the left radial access can be used, if necessary,
FUNDING
None whatsoever.
CONFLICTS OF INTEREST
None reported.
REFERENCES
1. Kawakami R, Gada H, Rinaldi MJ, et al. Characterization of cerebral embolic capture using the SENTINEL device during transcatheter aortic valve implantation in low to intermediate-risk patients: the SENTINEL-LIR study. Circ Cardiovasc Interv. 2022;15:e011358.
2. Van Mieghem NM, van Gils L, Ahmad H, et al. Filter-based cerebral embolic protection with transcatheter aortic valve implantation: the randomised MISTRAL-C trial. EuroIntervention. 2016;12:499-450.
3. Haussig S, Mangner N, Dwyer MG, et al. Effect of a cerebral protection device on brain lesions following transcatheter aortic valve implantation in patients with severe aortic stenosis: the CLEAN-TAVI randomized clinical trial. JAMA. 2016;316:592-601.
4. Kapadia SR, Kodali S, Makkar R, et al. Protection against cerebral embolism during transcatheter aortic valve replacement. J Am Coll Cardiol. 2017;
69:367-377.
5. Kapadia SR, Makkar R, Leon M, et al. Cerebral Embolic Protection during Transcatheter Aortic-Valve Replacement. N Engl J Med. 2022;387:1253-1263.
6. Giustino G, Mehran R, Veltkamp R, et al. Neurological outcomes with embolic protection devices in patients undergoing transcatheter aortic valve replacement: a systematic review and meta-analysis of randomized controlled trials. JACC Cardiovasc Interv. 2016;9:2124-2133.
7. Pagnesi M, Martino EA, Chiarito M, et al. Silent cerebral injury after transcatheter aortic valve implantation and the preventive role of embolic protection devices: A systematic review and meta-analysis. Int J Cardiol. 2016;221:97-106.
8. Bagur R, Solo K, Alghofaili S, et al. Cerebral embolic protection devices during transcatheter aortic valve implantation: systematic review and meta-analysis. Stroke. 2017;48:1306-1315.
9. Giustino G, Sorrentino S, Mehran R, et al. Cerebral embolic protection during TAVR: a clinical event meta-analysis. J Am Coll Cardiol. 2017;69:
465-466.
10. Mohananey D, Sankaramangalam K, Kumar A, et al. Safety and efficacy of cerebral protection devices in transcatheter aortic valve replacement: a clinical end-points meta-analysis. Cardiovasc Revasc Med. 2018;19(7 Pt A):
785-791.
11. Wang N, Phan K. Cerebral protection devices in transcatheter aortic valve replacement: a clinical meta-analysis of randomized controlled trials.
J Thorac Dis. 2018;10:1927-1935.
12. Testa L, Latib A, Casenghi M, et al. Cerebral Protection During Transcatheter Aortic Valve Implantation: An Updated Systematic Review and Meta-Analysis. J Am Heart Assoc. 2018;7:e008463.
13. Ndunda PM, Vindhyal MR, Muutu TM, Fanari Z. Clinical Outcomes of Sentinel Cerebral Protection System Use During Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Cardiovasc Revasc Med. 2020;2:717-722.
14. Carroll JD, Saver JL. Does Capturing Debris during TAVR Prevent Strokes? N Engl J Med. 2022;387:1318-1319.
15. Vlastra W, Jimenez-Quevedo P, Tchétché D, et al. Predictors, Incidence, and Outcomes of Patients Undergoing Transfemoral Transcatheter Aortic Valve Implantation Complicated by Stroke. Circ Cardiovasc Interv. 2019;
12:e007546.
16. Kharbanda RK, Perkins AD, Kennedy J, et al. Routine cerebral embolic protection in transcatheter aortic valve implantation: rationale and design of the randomised British Heart Foundation PROTECT-TAVI trial. EuroIntervention. 2023;EIJ-D-22-00713.
17. Woldendorp K, Indja B, Bannon PG, Fanning JP, Plunkett BT, Grieve SM. Silent brain infarcts and early cognitive outcomes after transcatheter aortic valve implantation: a systematic review and meta-analysis. Eur Heart J. 2021;42:1004-1015.
18. Armijo G, Nombela-Franco L, Tirado-Conte G. Cerebrovascular Events After Transcatheter Aortic Valve Implantation. Front Cardiovasc Med. 2018;5:104.