ABSTRACT
Over the last decade, transcatheter aortic valve replacement (TAVR) has become the preferred treatment for patients with severe aortic stenosis at increased risk for surgery. Consequently, this new technology has been recently tested in low-risk subjects. The PARTNER 3 trial randomized 1000 patients (mean Society of Thoracic Surgeons score, 1.9%; mean age, 73 years) to undergo TAVR with a balloon-expandable valve or surgical aortic valve replacement showing that TAVR was superior in terms of the composite endpoint of death, stroke and re-hospitalization at 1 year. In the Evolut Low Risk trial that randomized 1468 patients with the use of a self-expandable prosthesis, TAVR was non-inferior to surgery for the primary composite endpoint of death or disabling stroke at 24 months. While the available 1-year follow up does not answer the question of transcatheter valves durability, these results will definitely change our everyday clinical practice.
Keywords: Transcatheter aortic valve replacement. Low surgical risk. Randomized trial.
RESUMEN
En la última década, el reemplazo valvular aórtico transcatéter (TAVR, por sus siglas en inglés) se ha convertido en el tratamiento preferente para los pacientes con estenosis aórtica grave y con alto o incluso moderado riesgo quirúrgico. En consecuencia, esta nueva tecnología ha sido evaluada en sujetos de bajo riesgo quirúrgico. En el estudio PARTNER 3 se aleatorizó a 1.000 pacientes (puntuación media de la Society of Thoracic Surgeons, 1,9%; edad media, 73 años) para ser sometidos a TAVR con una válvula balón expandible o a reemplazo quirúrgico de válvula aórtica, y se halló que la TAVR fue superior en términos del objetivo final compuesto de muerte, ictus y reingreso a 1 año. En el ensayo Evolut Low Risk, en el que 1.468 pacientes fueron aleatorizados a TAVR con una prótesis autoexpandible o cirugía, la TAVR no fue inferior a esta última en términos del criterio de valoración principal compuesto de muerte o accidente cerebrovascular discapacitante a los 24 meses. Si bien el seguimiento a 1-2 años disponible no responde a la pregunta sobre la durabilidad de las válvulas transcatéter, estos resultados cambiarán nuestra práctica clínica diaria.
Palabras clave: Reemplazo de válvula aórtica transcatáter. Riesgo quirúrgico bajo. Ensayo aleatorizado.
Abbreviations: AS: aortic stenosis. CAD: coronary artery disease. TAVR: transcatheter aortic valve replacement. THV: transcatheter heart valve. PVL: paravalvular leak. SAVR: surgical aortic valve replacement.
Percutaneous coronary angioplasty took approximately 30 years, from the first pioneering experience of Andreas Gruentzig back in 1977, to reach class I indication in the international clinical practice guidelines on myocardial revascularization of left main/ 3-vessel coronary artery disease (CAD) and replace coronary artery bypass grafting for the management of most patients with CAD. Similarly, 17 years have passed since the very first transcatheter aortic valve replacement (TAVR) was performed in a prohibitive-risk patient with severe aortic valve stenosis (AS) until the arrival of the contemporary randomized trial of TAVR vs surgical aortic valve replacement (SAVR) in a low-surgical risk population. This road has been paved with substantial technical improvements in transcatheter heart valves (THV), increased operators’ experience and rigorously-designed randomized trials. As a matter of fact, TAVR first proved to be superior to medical therapy in patients considered inoperable,1,2 and confirmed it was non-inferior to SAVR in high3,4 and intermediate risk5,6 patients. Actually, when performed using the transfemoral access, TAVR proved to be superior to SAVR.7 So, time was ripe to test this disruptive technology in low-risk patients who amount to 80% of the patients who, to this day, undergo SAVR procedures.8,9
The PARTNER 3 trial10 was a multicenter randomized study that compared TAVR and SAVR procedures for the management of severe symptomatic AS in low-surgical risk patients (Society of Thoracic Surgeons [STS] score < 4%). This trial randomized 1000 low-risk subjects (mean STS score 1.9%) from 71 centers (98% of the patients wer recruited in the United States) to transfemoral TAVR with the balloon-expandable SAPIEN 3 (Edwards Lifesciences, Irvine, California, United States) THV or SAVR. The primary endpoint was a composite of all-cause mortality, stroke and rehospitalization due to heart failure at 1 year. The trial was designed to test both the non-inferiority (with a prespecified margin of 6 percentage points) and superiority of the TAVR procedure, in the as-treated population. There was a 46% reduction in the rate of the primary composite endpoint at 1 year for TAVR compared to SAVR that met the criteria of both non-inferiority (8.5% vs 15.1%; absolute difference, −6.6%; 95% confidence interval [95%CI], −10.8 to −2.5; P < .001) and superiority (hazard ratio [HR], 0.54; 95%CI, 0.37-0.79; P = .001). Even excluding rehospitalization due to heart failure, arguably the weaker endpoint of the composite, TAVR had better results than surgery (death or stroke 1.8% vs 4.9%, treatment effect 0.36; 95%CI, 0.17-0,79). Several hierarchical pre-specified secondary endpoints were also tested, resulting in significantly lower 30-day rates of stroke (0.6% vs 2.4%; P = .02), death or stroke (1.0% vs 3.3%; P = .01), life-threatening or major bleeding (3.6% vs 24.5%; P < .001) and new-onset atrial fibrillation (5.0% vs 39.5%; P < .001) in the TAVR group. No statistically significant differences were seen in moderate or severe paravalvular leak (PVL) (0.8% vs 0%), need for a new permanent pacemaker (6.5% vs 4.0%) or major vascular complications (2.2% vs 1.5%) between the 2 populations.
Another randomized study on low-risk patients with AS, the Evolut Low Risk trial,11 confirmed the non-inferiority of TAVR with a self-expandable THV (Evoult R and Pro, Evolut Medtronic Inc., Minneapolis, Minnesota, United States) compared to surgery in the primary composite endpoint of all-cause mortality or disabling stroke at 24 months.11 The 24-month estimated incidence rate of the primary endpoint was 5.3% in the TAVR group vs 6.7% in the surgery group (difference, –1.4 percentage points; 95% Bayesian credible interval for difference, –4.9 to 2.1; posterior probability of noninferiority > 0.999). In this study, TAVR was not superior to SAVR, but had numerically lower rates of hard endpoints (main results of both trials are shown on table 1). In short, patients who underwent TAVR procedures had lower incidence rates of disabling stroke, bleeding complications, acute kidney injury, and atrial fibrillation, but a higher incidence rate of moderate/severe PVL and pacemaker implantation. Although comparing the 2 trials is difficult because of differences of statistical design and endpoints (and beyond the scope of this manuscript), it is important to notice that both studies pointed in the same direction, suggesting a class effect of TAVR in this low-risk population.
Table 1. Summary of baseline characteristics and outcomes of low-risk aortic valve stenosis patients enrolled in the PARTNER 3 and Evolut Low Risk randomized trials
| PARTNER 3 | Evolut Low Risk | |||||
|---|---|---|---|---|---|---|
| TAVR (n = 496) | SAVR n = 454) | Treatment effect [95%CI] | TAVR n = 725) | SAVR (n = 678) | Difference [95%CI] | |
| Baseline characteristics | ||||||
| Age (years) | 73.3±5.8 | 73.6±6.1 | – | 74.1±5.8 | 74.1±5.8 | – |
| STS score (%) | 1.9±0.7 | 1.9±0.6 | – | 1.9±0.7 | 1.9±0.7 | – |
| Male sex (%) | 67.5 | 71.1 | – | 64.0 | 66.2 | – |
| Mean LVEF (%) | 65.7±9.0 | 66.2±8.6 | – | 61.7±7.9 | 61.9±7.7 | – |
| NYHA class III-IV (%) | 31.2 | 23.8 | – | 25.1 | 28.4 | – |
| Primary endpoint | ||||||
| All-cause mortality, stroke, or rehospitalization due to heart failure at 1 year (%) | 8.5 | 15.1 | 0.54 [0.37-0.79] | – | – | – |
| All-cause mortality or disabling stroke at 2 years (%) | – | – | – | 5.3 | 6.7 | –1.4 [–4.9- 2.1] |
| 30-day outcomes | ||||||
| All-cause mortality (%) | 0.4 | 1.1 | 0.37 [0.07-1.88] | 0.5 | 1.3 | –0.8 [–1.9- 0.2] |
| Cardiac mortality (%) | 0.4 | 0.9 | 0.46 [0.08-2.49] | 0.5 | 1.3 | –0.8 [–1.9- 0.2] |
| Disabling stroke (%) | 0 | 0.4 | N/A | 0.5 | 1.7 | –1.2 [–2.4- -0.2] |
| Life threatening/disabling bleeding (%) | 1.2 | 11.9 | 0.09 [0.04-0.22] | 2.4 | 7.5 | –5.1 [–7.5- -2.9] |
| Major vascular complications (%) | 2.2 | 1.5 | 1.44 [0.56-3.73] | 3.8 | 3.2 | 0.6 [–1.4- 2.5] |
| Stage II-III acute kidney injury (%) | 0.4 | 1.8 | N/A | 0.9 | 2.8 | –1.8 [–3.4- –0.5] |
| New-onset atrial fibrillation (%) | 5.0 | 39.5 | 0.10 [0.06-0.16] | 7.7 | 35.4 | –27.7 [–31.8- –23.6] |
| New pacemaker implantation (%) | 6.5 | 4.0 | 1.66 [0.93-2.96] | 17.4 | 6.1 | 11.3 [8.0-14.7] |
| Moderate-severe paravalvular leakage (%) | 0.8 | 0 | N/A | 3.4 | 0.4 | – |
| Mean aortic valve area (cm2) | 1.7±0.02 | 1.8±0.02 | –0.1 [–0.1- 0] | 2.2±0.06 | 2.0±0.06 | – |
| Mean aortic valve gradient (mmHg) | 12.8 | 11.2 | 1.5 [0.9-2.0] | 3.4 | 0.4 | – |
| 1-year outcomes | ||||||
| All-cause mortality (%) | 1.0 | 2.5 | 0.41 [0.14-1.17] | 2.4 | 3.0 | –0.6 (–2.6-1.3) |
| Cardiac mortality (%) | 0.8 | 2.0 | 0.40 [0.12-1.30] | 1.7 | 2.6 | –0.9 (−2.7- 0.7) |
| Disabling stroke (%) | 0.2 | 0.9 | 0.22 [0.03-2.00] | 0.8 | 2.4 | –1.6 (–3.1- –0.3) |
| Rehospitalizations due to heart failure (%) | 7.3 | 11.0 | 0.65 [0.42-1.00] | 3.2 | 6.5 | –3.4 (–5.9- –1.0) |
|
95%CI, 95% confidence interval; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SAVR, surgical aortic valve replacement; STS, Society of Thoracic Surgeons; TAVR, transcatheter aortic valve replacement. |
||||||
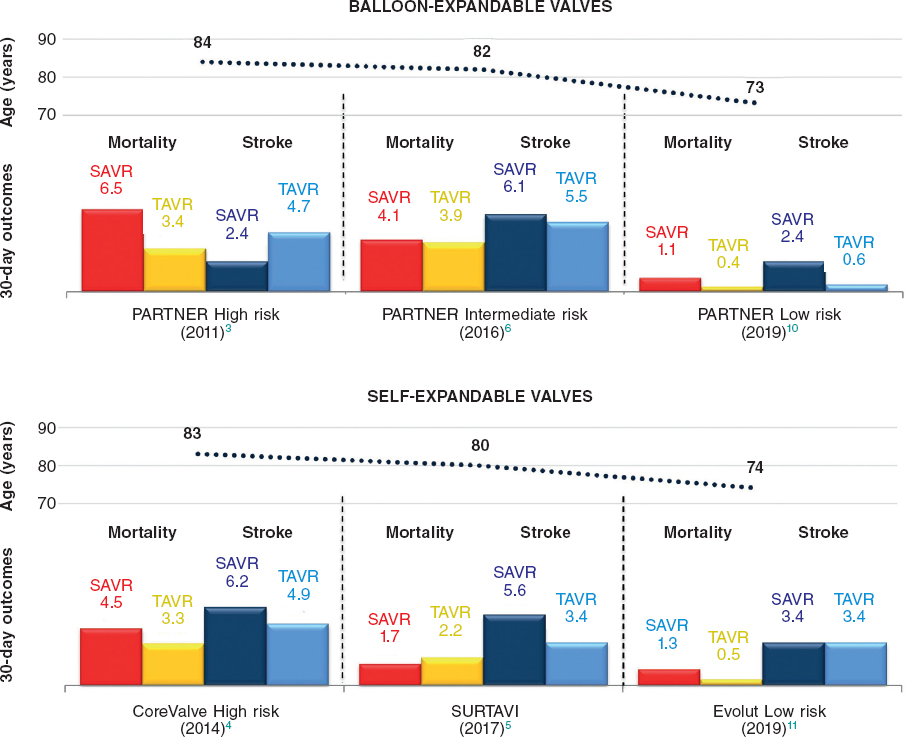
These trials represent a landmark in interventional cardiology and, broadly speaking, in modern medicine for two reasons. Firstly because it is the last step down the surgical risk ladder for TAVR, finally proving that the transcatheter management of severe AS has similar (if not superior) results compared to SAVR regardless of the surgical risk. Secondly, and more importantly, these are the first randomized trials to put the transcatheter management of AS to the test in a younger population with a longer life expectancy. As a matter of fact, the mean age of the study population was 73-74 years, with roughly 10% of the subjects < 65 years of age in the PARTNER 3 trial. Prior to these studies, despite a reduction in the surgical risk score, the mean age of patients treated with TAVR had been largely over 80 years of age (Figure 1).8 Focusing on the PARTNER 3 trial, in this younger and “healthier” population the surgical control arm had very good outcomes, with 30-day mortality rates as low as 1.1% and 30-day disabling stroke rates of 0.4% (non-disabling stroke 2.0%). Nevertheless, the TAVR group had even lower 30-day mortality rates (0.4%) and no disabling stroke rates (non-disabling stroke 0.6%). To this regard, the extremely low rate of strokes seen in the TAVR group questions the need for the routine use of cerebral embolic protection devices. Also, 1-year all-cause mortality was extremely low in both groups compared to previous PARTNER trials. If we examine the data carefully, it is clear that almost all of the very few deaths reported had to do with cardiac causes (with a 0.8% cardiac mortality seen in the TAVR group vs 2.0% in the SAVR group). This finding is new compared to previous TAVR trials in which cardiac mortality accounted for less than 60% of deaths at 1-year, which is likely due to the younger age and low prevalence of comorbidities in the PARTNER 3 population.
Figure 1. Thirty-day mortality and stroke rates in major transcatheter aortic valve replacement (TAVR) vs surgical aortic valve replacement (SAVR) trials across the surgical risk spectrum. Notably, mean age of the study population decreased significantly in the PARTNER 3 (PARTNER Low Risk) and and Evolut Low Risk trials.
Another striking finding of this trial was that previous TAVR setbacks such as vascular complications, need for new pacemaker implantations, and moderate/severe PVL rates were as low as those of SAVR. This is likely due to the major technical advances made in new-generation THVs12 (with the arrival of external sealing skirts and lower sheath profile compatibility), careful pre-procedural CT assessments (thus reducing prosthesis oversizing), and greater operators’ experience (resulting in more precise THV implantations). The reduction in the number of periprocedural complications together with the adoption of a minimally invasive approach (only one third of the TAVRs were performed under general anesthesia, for the most part not even requiring intensive care unit admission) resulted in significantly shorter hospital stays of TAVR patients (3.0 vs 7.0 days) and higher discharge or self-care rates (95.8% vs 73.1%) compared to SAVR. Thus, 30-day functional status and quality of life were better among TAVR patients.
With regard to the echocardiographic findings, although moderate/severe PVL was similar in the 2 groups, TAVR had significantly higher rates of mild PVL compared to surgery (28.7% vs 2.9%). It should be noted that the impact of mild PVL on long-term outcome of younger patients is still unknown. Moreover, TAVR patients had lower mean aortic valve areas and higher transvalvular gradients at 30-day compared to SAVR (1.7 cm2 vs 1.8 cm2 and 12.8 mmHg vs 11.2 mmHg, respectively). This finding, which was not described in any of the previous PARTNER trials, is probably explained by the greater use of larger bioprostheses in the surgical arm (80% of the prosthesis were ≥ 23 mm). If the larger valve area of the surgical group will translate into hemodynamic or clinical benefits at a longer follow up remains to be seen. We should mention here that in the Evolut Low Risk trial, patients undergoing TAVR with a supra-annular self-expandable THV had lower aortic-valve gradients (8.6 mmHg vs 11.2 mmHg) and larger effective orifice areas (2.3 cm2 vs 2.0 cm2) compared to the patients in the surgical group at 12 months.
The major limitation of these trials is that the short term follow-up does not answer the question of THV durability, which becomes of course of paramount importance when treating younger, low-risk subjects. It is remarkable that the long-term data available (up to 8-year follow up)13,14 do not seem to show any signs of early deterioration of theTHVs. Although this still represents a major concern for many clinicians, we should mention that many surgical bioprostheses that are currently used worldwide have even fewer long-term data compared those available for THVs. To address this issue, the trial protocol includes an annual evaluation of up to 10 years, at least, after the index procedure, which will finally shed light on the long-term hemodynamic performance (in terms of bioprosthetic valve dysfunction and failure rates) of both transcatheter and surgical heart valves. Moreover, a prespecified computed tomography angiography sub-analysis of the PARTNER 3 trial will look at valve-leaflet disfunction and asymptomatic valve thrombosis. On this isse, at 1-year, five patients in the TAVR arm vs one patient in the surgical arm had evidence suggestive of valve thrombosis.
Importantly, the findings of these trials should not be generalized to all AS patients at low surgical risk. For example, patients with bicuspid aortic valve –who are representative of a relevant portion of younger subjects with AS– were excluded from the analysis, mainly because of concerns related to the presence of an elliptic annulus and asymmetric leaflet calcifications possibly leading to eccentric prosthesis expansion and higher rates of PVL and risk of annular rupture.15,16 In a recent propensity-matched analysis of the STS/TVT registry, TAVR in bicuspid vs tricuspid valve was associated with a higher risk of aortic injury and conversion to open heart surgery (although the overall rate was < 1.0%) but similar survival at 30 days and 1 year. A dedicated randomized trial in patients with bicuspid AS is needed at this point. Also, these studies excluded patients with an unsuitable transfemoral access, low-flow low-gradient AS, severe coronary artery disease (SYNTAX score > 32), absence of symptoms.17 Finally, the patients recruited were treated by experienced operators at high volume centers. In this sense, such low rates of events might not be reproducible in smaller centers with less experienced physicians.
The PARTNER 3 and the Evolut Low Risk trials will have profound implications in clinical practice, and will likely lead to class I indication of TAVR also in low-risk subjects in the upcoming international clinical practice guidelines. Treatment choices in patients with severe symptomatic AS should not rely on surgical risk anymore, but rather be influenced by clinical and anatomical considerations and patient preference. Unless there is a clear anatomic characteristic driving the choice towards SAVR (e.g. bicuspid aortic valve, high SYNTAX score, no feasible transfemoral approach), from now on every patient considered for SAVR with a bioprosthetic valve should be informed about the possibility to undergo TAVR. Transcatheter aortic valve replacement may soon become the preferred therapy for most of AS patients, thus leading to a long-awaited change of paradigm: Rather than asking ourselves if a patient is candidate for TAVR, we will have to justify if a patient is eligible for surgery. We will have to wait for the long-term durability data, but it looks like SAVR is on the brink of becoming an endangered species.
CONFLICTS OF INTEREST
G. Tarantini has received lecture fees from Edwards Lifesciences, Medtronic, Boston Scientifics, Abbott. L. Nai Fovino has declared no conflicts of interest whatsoever.
REFERENCES
1. Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597-1607.
2. Popma JJ, Adams DH, Reardon MJ, et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63:1972-1981.
3. Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187-2198.
4. Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790-1798.
5. Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2017;376:1321-1331.
6. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016;374:1609-1620.
7. Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients:a propensity score analysis. Lancet. 2016;387:2218-2225.
8. Tarantini G, Nai Fovino L, Gersh BJ. Transcatheter aortic valve implantation in lower-risk patients:what is the perspective?Eur Heart J. 2018;39:658-666.
9. Tarantini G, Lefèvre T, Terkelsen CJ, et al. One-Year Outcomes of a European Transcatheter Aortic Valve Implantation Cohort According to Surgical Risk. Circ Cardiovasc Interv. 2019;12:e006724.
10. Mack MJ, Leon MB, Thourani VH, et al.;PARTNER 3 Investigators. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019;380:1695-1705.
11. Popma JJ, Deeb GM, Yakubov SJ, et al.;Evolut Low Risk Trial Investigators. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med. 2019;380:1706-1715.
12. Nai Fovino L, Badawy MRA, Fraccaro C, et al. Transfemoral aortic valve implantation with new-generation devices:the repositionable Lotus versus the balloon-expandable Edwards Sapien 3 valve. J Cardiovasc Med (Hagerstown). 2018;19:655-663.
13. Barbanti M, Costa G, Zappulla P, et al. Incidence of Long-Term Structural Valve Dysfunction and Bioprosthetic Valve Failure After Transcatheter Aortic Valve Replacement. J Am Heart Assoc. 2018;7:e008440.
14. Tarantini G, Purita PAM, D'Onofrio A, et al. Long-term outcomes and prosthesis performance after transcatheter aortic valve replacement:results of self-expandable and balloon-expandable transcatheter heart valves. Ann Cardiothorac Surg. 2017;6:473-483.
15. Tarantini G, Basso C, Fovino LN, Fraccaro C, Thiene G, Rizzo S. Left ventricular outflow tract rupture during transcatheter aortic valve implantation:anatomic evidence of the vulnerable area. Cardiovasc Pathol. 2017;29:7-10.
16. Tarantini G, Fabris T, Cardaioli F, Nai Fovino L. Coronary access after transcatheter aortic valve replacement in bicuspid aortic valve:lights and shades. JACC Cardiovasc Interv. 2019. http://doi.org/10.1016/j.jcin.2019.03.031.
17. Tarantini G, Nai Fovino L, Tellaroli P, Fabris T, Iliceto S. Asymptomatic Severe Aortic Stenosis and Noncardiac Surgery. Am J Cardiol. 2016;117:486-488.