ABSTRACT
The occurrence of strokes after transcatheter aortic valve replacement is one of the most devastating complications. It has a multifactorial etiology and nearly half of the events occur during or immediately after the procedure. The use of periprocedural embolic protection devices to stop the emboli from reaching the cerebral vessels is a promising preventive strategy to reduce this complication. However, we still lack solid evidence supporting its systematic use. The REFLECT II clinical trial is a new randomized clinical trial that assessed the safety and efficacy profile of an embolic protection device in patients undergoing transcatheter aortic valve replacement.
Keywords: TAVI. Stroke. Embolic protection device. Prevention.
RESUMEN
El ictus después de un implante percutáneo de válvula aórtica es una de las complicaciones más devastadoras. Su etiología es multifactorial y en torno a la mitad de los casos ocurren durante el procedimiento o en el periodo inmediatamente posterior. El uso de dispositivos de protección embólica durante la intervención para prevenir que los émbolos alcancen los vasos cerebrales es una estrategia preventiva muy prometedora para reducir esta complicación. Sin embargo, la evidencia sólida que apoye su uso sistemático todavía es escasa. El estudio REFLECT II es un estudio aleatorizado que evalúa la seguridad y la eficacia de un dispositivo de protección embólica en pacientes que reciben un implante percutáneo de válvula aórtica.
Palabras clave: TAVI. Ictus. Sistemas de protección embólica. Prevención.
Abbreviations:
CEPD: cerebral embolic protection device. TAVI: transcatheter aortic valve implantation.
INTRODUCTION
The occurrence of strokes after transcatheter aortic valve implantation (TAVI) is, if not the most significant, one of the most feared and devastating complications because of its impact on the patients’ quality of life and mortality. Although it is not very common (~3%) its incidence rate has not gone down parallel to that of other complications with the development of this technique and the arrival of new devices despite the efforts and preventive measures adopted.1 Several studies using magnetic resonance imaging and transcranial Doppler ultrasound have proven that most strokes that occur after TAVI have an embolic origin from the aortic valve itself2 and at least half of them are closely related to the procedure.3 Therefore, cerebral embolic protection devices (CEPD) are used as a preventive strategy, often a mechanical barrier, to protect the cerebral vascular territory during the intervention. The results of the REFLECT II clinical trial (NCT 02536196) have recently been published. It is a randomized trial that assesses the safety and efficacy profile of the TriGUARD 3 CEPD (Keystone Heart Ltd, Caesarea, Israel) to reduce clinical events and minimize brain injuries during TAVI.4
THE REFLECT II CLINICAL TRIAL
It is a randomized clinical trial in a 2:1 allocation ratio (device vs control) with an estimated sample size of 225 patients. However, since it was completed prematurely it eventually included 179 patients (121 in the device group and 58 in the control group). The safety primary endpoint was a composite of death, stroke, life-threatening hemorrhage, acute kidney injury stage 2 or 3, major vascular complications or valvular reinterventions after 30 days. The efficacy endpoint by hierarchical order included death or stroke after 30 days, neurological deterioration according to the NIHSS (National Institute of Health Stroke Scale), lack of brain injuries, and total volume in the magnetic resonance imaging performed 2 to 5 days after the procedure. To study the safety primary endpoint, the data of 41 patients treated with the device in the early recruitment phase (162 vs 58) were included. To study the efficacy primary endpoint 63 patients of the control group from the previous DEFLECT III clinical trial (NCT02070731)5 that also studied this device were included (121 vs 121 control patients). The pre-specified per protocol analysis of efficacy was established in patients with complete coverage of the 3 sections of their brainstems, exclusively, which was finally achieved in 62 of them (59.3%). The baseline characteristics were well-balanced between both groups except for a higher percentage of patients with a past medical history of stroke in the device group. Although there were not statistically significant differences in the safety primary endpoint, the percentage of events was higher in the device group (15.9% vs 7.0%; P = .11) mainly due to a higher rate of life-threatening hemorrhages (5.7% vs 0%; P = .12) and major vascular complications (7.0% vs 0%; P = .04) associated with TAVI not with CEPD. The efficacy endpoint was also similar in both groups in all the events studied: mortality or stroke after 30 days (9.8% vs 6.7% in the control group; P = .475), worse NIHSS score (14.1% vs 7.6%; P = .176), brain injuries (85.0% vs 84.9%; P = 1.000), and brain injury volume (215.4 mm3 vs 188.1 mm3; P = .405) (table 1). The overall brain injury volume of patients with complete cerebral protection was smaller in the pre-specified protocol analysis (145.7 mm3 vs 188.1 mm3), although it was not statistically significant.
Table 1. Randomized clinical trials with cerebral embolic protection devices
| Study | Year | Device | Total number of patients/total number with CEPDs | Primary endpoint | Main results |
|---|---|---|---|---|---|
| EMBOL-X, Wendt et al.6 | 2015 | EMBOL-X (Edwards Lifesciences, United States) | 30/14 | New brain injuries Volume of injuries |
|
| DEFLECT III, Lansky et al.5 | 2015 | TriGuard (Keystone Heart Ltd, Israel) | 85/46 | Safety and efficacy Safety endpoint: death, stroke, life-threatening hemorrhage, acute kidney injury (stage 2-3), major vascular complication |
|
| MISTRAL-C, Van Mieghem et al.7 | 2016 | SENTINEL (Boston Scientific, United States) | 65/32 | New brain injuries |
|
| CLEAN-TAVI, Haussig et al.8 | 2016 | SENTINEL (Boston Scientific, United States) | 100/50 | Number and volume of brain injuries |
|
| SENTINEL, Kapadia et al.9 | 2017 | SENTINE (Boston Scientific, United States | 363/244 | Clinical safety and efficacy (MACE) of CEPD during TAVI |
|
| REFLECT II, Moses4 | 2020 | TriGUARD 3 (Keystone Heart Ltd, Israel) | 179/121 | Safety endpoint (composite) after 30 days Efficacy endpoint (death or stroke, neurological deterioration, lack of brain injuries and volume) after 30 days |
|
|
CEPD, cerebral embolic protection device; MACE, major adverse cardiovascular events; NIHSS: National Institute of Health Stroke Scale; TAVI: transcatheter aortic valve implantation. |
|||||
REFLECTIONS ON THE REFLECT II CLINICAL TRIAL
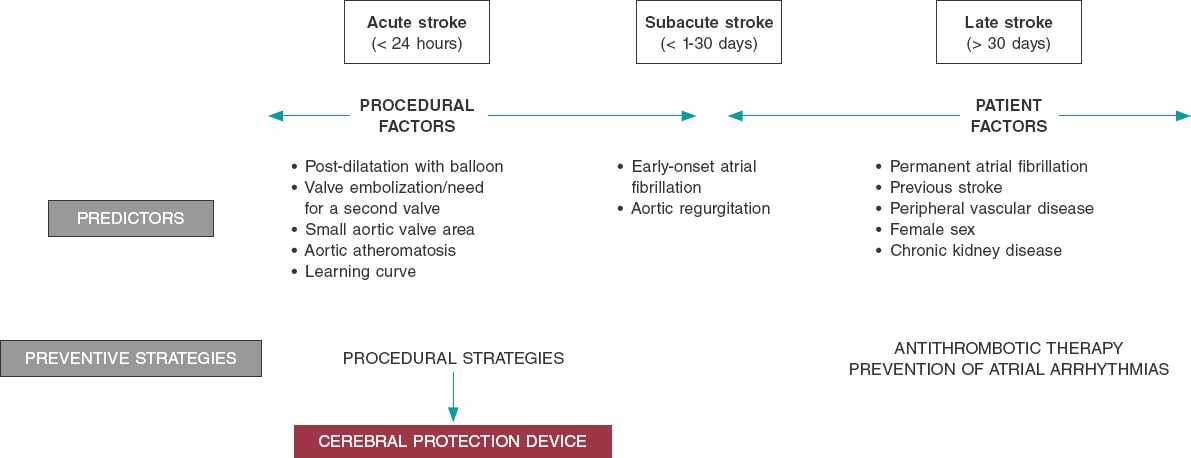
Strokes post-TAVI are a complex problem with a multifactorial etiology. Several factors impact different moments during and after TAVI such as patient factors like atrial arrhythmias or previous cerebrovascular disease, procedural risk factors like embolisms or hemodynamic instability, and antithrombotic therapy (figure 1). CEPDs can reduce procedural strokes. Six randomized clinical trials have been conducted so far (including the DEFLECT III and the REFLECT II) with CEPDs in patients treated with TAVI (table 1).4-9 This new trial confirmed the same limitations already reported by previous randomized clinical trials on CEPDs since it also included a small number of patients and rare events. Therefore, it did not have the statistical power required to study differences in clinical trials. Most primary endpoints went from surrogate endpoints to findings made by the imaging modalities (in general, rate and volume of new brain injuries in the magnetic resonance imaging).10
Figure 1. Risk factors for stroke after TAVI and possible preventive strategies.
The REFLECT II is a complex clinical trial regarding design and analysis with different population groups to study safety and efficacy endpoints and several interconnected factors that can mask the possible benefits derived from the device. As it happened in previous trials, the implantation success rate was very high (> 90%) and without serious associated complications. We should mention that since this is a preventive strategy, safety should be of paramount importance and the number of complications associated with its use should be close to none. Regarding efficacy, we should consider that the percentage of patients with complete cerebral coverage was low (~ 60%) even though the device was designed to cover the 3 supra-aortic trunks. Whether the previous analysis of the CT scan performed at aortic arch and supra-aortic trunk level can contribute to a better selection of patients eligible for this device is still to be elucidated. In other studies, the percentage of patients with material captured inside the filters of the SENTINEL device (Boston Scientific, Corp., United States) has been systematically high (> 90%). Another limitation of this study is that the amount and nature of the embolized material remain unknown since the design of the device acts as a deflector stopping embolic material from entering the supra-aortic trunks. Finally, the neutral results obtained from analyzing the 4 efficacy endpoints recommend a selective use of CEPDs in patients of high-risk of sustaining embolic events (eg, heavily calcified aortic valve or bicuspid aortic valve, valve-in-valve procedures or previous strokes). We should mention that several observational studies with historic cohort comparison11 and risk-propensity score-based registries12 have confirmed a lower overall rate of in-hospital ischemic strokes (or within the first 72 hours). However, these studies have possible biases and limitations. Therefore, randomized clinical trials with enough statistical power are needed to be able to detect clinical differences and establish an ultimate indication for this preventive measure. Two ongoing randomized clinical trials (PROTECTED TAVR [NCT04149535] and BHF PROTECT-TAVI [ISRCTN16665769]) with large sample sizes and systematic evaluations by a neurologist will shed light on all these issues. The participation of neurologists to assess patients is an essential aspect in the design of these studies that should help non-specialists find «silent» clinical events with possible neurological deterioration with clinical implications in the mid- and long-term follow-up, particularly of young patients with longer life expectancies.
The importance and impact of strokes post-TAVI is undisputed, and the ultimate goal should be to reduce their incidence rate. In most patients, the procedure itself causes the migration of embolic material towards the cerebral territory. The current evidence behind CEPDs comes from randomized clinical trials and is based on reducing the volume of silent brain injuries as a surrogate marker of cerebral disease. The clinical benefit of these devices relies on observational studies only, which is why their universal vs selective use to reduce clinical events is still under discussion. Future larger clinical trials with proper methodologies and enough statistical power are needed to find differences in clinical events. As a matter of fact, they will eventually set the pace for CEPDs in the prevention of strokes after TAVI.
FUNDING
No funding was received for this work.
CONFLICTS OF INTEREST
L. Nombela-Franco is a proctor for Abbott Vascular and has received funding for his consulting work for Abbott Vascular, and Boston Scientific.
G. Tirado-Conte has reported no conflicts of interest.
REFERENCES
1. Huded CP, Tuzcu EM, Krishnaswamy A, et al. Association Between Transcatheter Aortic Valve Replacement and Early Postprocedural Stroke. JAMA. 2019;321:2306-2315.
2. Armijo G, Nombela-Franco L, Tirado-Conte G. Cerebrovascular events after transcatheter aortic valve implantation. Front Cardiovasc Med. 2018;5:104.
3. Nombela-Franco L, Webb JG, de Jaegere PP, et al. Timing, predictive factors, and prognostic value of cerebrovascular events in a large cohort of patients undergoing transcatheter aortic valve implantation. Circulation. 2012;126:3041-3053.
4. Moses JW. A randomized evaluation of the TriGUARD3 cerebral embolic protection device to reduce the impact of cerebral embolic lesions after transcatheter aortic valve implantation:the REFLECT II trial. In:Transcatheter Cardiovascular Therapeutics 2020 (TCT 2020). October 15, 2020. Available online: https://www.tctmd.com/slide/randomized-evaluation-triguard3tm-cerebral-embolic-protection-device-reduce-impact-cerebral. Accessed 4 Nov 2020.
5. Lansky AJ, Schofer J, Tchetche D, et al. A prospective randomized evaluation of the TriGuard™HDH embolic DEFLECTion device during transcatheter aortic valve implantation:results from the DEFLECT III trial. Eur Heart J. 2015;36:2070-2078.
6. Wendt D, Kleinbongard P, Knipp S, et al. Intraaortic protection from embolization in patients undergoing transaortic transcatheter aortic valve implantation. Ann Thorac Surg. 2015;100:686-691.
7. Van Mieghem NM, van Gils L, Ahmad H, et al. Filter-based cerebral embolic protection with transcatheter aortic valve implantation:the randomised MISTRAL-C trial. EuroIntervention. 2016;12:499-507.
8. Haussig S, Mangner N, Dwyer MG, et al. Effect of a cerebral protection device on brain lesions following transcatheter aortic valve implantation in patients with severe aortic stenosis:the CLEAN-TAVI randomized clinical trial. JAMA. 2016;316:592-601.
9. Kapadia SR, Kodali S, Makkar R, et al. Protection against cerebral embolism during transcatheter aortic valve replacement. J Am Coll Cardiol. 2017;69:367-377.
10. Bagur R, Solo K, Alghofaili S, et al. Cerebral embolic protection devices during transcatheter aortic valve implantation:systematic review and meta-analysis. Stroke. 2017;48:1306-1315.
11. Seeger J, Gonska B, Otto M, et al. Cerebral embolic protection during transfemoral aortic valve replacement significantly reduces death and stroke compared with unprotected procedures. J Am Coll Cardiol Intv. 2017;10:2297-2303.
12. Megaly M, Sorajja P, Cavalcante JL, et al. Ischemic Stroke With Cerebral Protection System During Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2020;13:2149-2155.