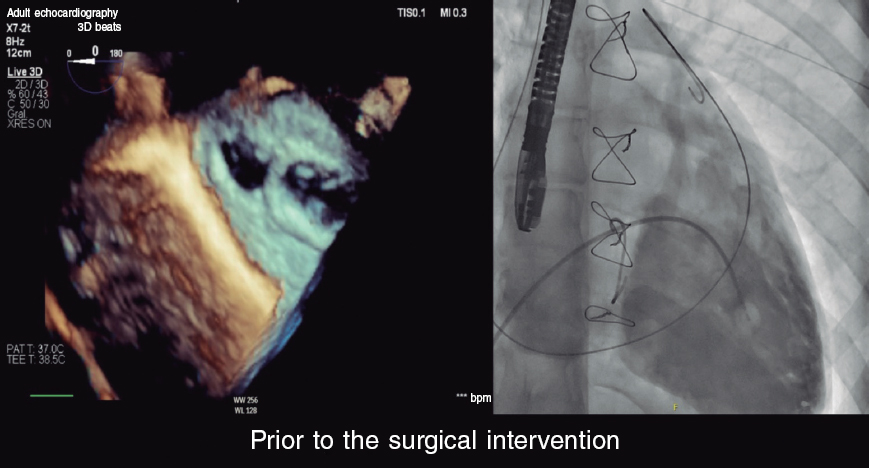
HOW WOULD I APPROACH IT?
There is no doubt that, procedurally, the case of fistulization reported between the pulmonary artery trunk and the right pulmonary branch is an interesting case similar to common situations reported in the routine clinical practice where pulmonary trunk surgical ligation is not complete thus leaving residual passage between the heart and Fontan circulation. This is unsought because it sends already oxygenated blood back to the lung, which overloads a circulation so sensitive as the Fontan one that lacks heart pump and works through venous pressure gradient.
In this type of procedures, closing the junction between the branch and the pulmonary trunk is often enough. Different strategies exist for this purpose. First, we need to think about the approach that should be used to close the defect. In this case, although the existence of fenestration (communication between Fontan circulation and the systemic atrial region) facilitates occlusion using the antegrade (through the ventricle) and retrograde (coming from the pulmonary artery) approaches, the latter is often easier and faster to use. Procedure can be performed via femoral access. The bigger the patient the easier the procedure. However, in very small patients, it is often easier to perform via jugular access that allows direct and straight access to the pulmonary branch in Fontan circulation. Also, it provides great support to perform the procedure.
While closing the defect, if there is a stenosis in that region, a polytetrafluoroethylene (PTFE) covered stent can be implanted to both treat the stenosis and close the antegrade passage. In the absence of stenosis, we can proceed by closing the defect directly. Since it is a region previously ligated through surgery, it is often too rigid and gives devices enough support, which means that significant device oversizing or large retention discs to achieve stability are not usually needed. If there are doubts on the consistency of the defect, a compliant balloon can be used for assessment purposes.
We often use the Amplatzer Vascular Plug II occluder device (Abbot Cardiovascular, United States) that, in this particular case, could be 12 mm or 14 mm in size—meaning having to use 5-Fr or 6-Fr sheaths capable of navigating with standard Teflon coated or a little more rigid guidewires like the ones used to implant IAC devices. Coming from the pulmonary branch, implantation is based on leaving the distal disc and the body of the device inside the aneurysm, retrieving device and sheath en bloc until the device reaches the end, and eventually releasing the proximal disc that will stay inside the right branch. Type II Amplatzer occluder devices—that have a better profile—can be used in defects ≤ 5 mm leaving in this case the body in the defect and a large retention disc in each side. If compliance of the defect and stability worry us due to its specific characteristics, interatrial communication occluder devices can be used. For the diameters needed in these cases, IAC devices have very large discs compared to the body of the device and defect that should be occluded. Depending on the diameter selected—7-Fr or 9-Fr sheaths—are needed, and probably more rigid guidewires, especially from femoral access.
In most cases, the exclusive closure of the connection with the branch is performed by preserving Fontan circulation, and opening a way for a seesaw high-pressure passage in the trunk. This type of flow barely generates any complications. In this case, and given the aneurysmal dilation of the trunk, and to prevent its progression, its closure at valvular and subavalvular stenosis level seems reasonable prior to the closure of the connection between trunk and branch (in procedures performed from the pulmonary artery). Such closure could be performed with an AVP-II device, and probably with a 6-Fr sheath and a standard or a little more rigid guidewire. We could even think of using an Amplatzer VSD occluder device. Its advantages are that it is more rigid and contains polyester fabric in the nitinol mesh thus promoting an earlier closure of flow through it compared to devices that don’t have it except for a mesh like the AVP II. The use of an Amplatzer VSD occluder device would require 7-Fr or 8-Fr sheaths, and a more rigid guidewire.
In conclusion, very many different approaches can be used: antegrade (by fenestration), retrograde, femoral or jugular being the process of selection in elderly patients with non-complex anatomies less important. However, it is more relevant in younger patients or with complex anatomies in whom retrograde and jugular accesses often guarantee immediate support, which facilitates the procedure significantly.
FUNDING
None whatsoever.
CONFLICTS OF INTEREST
None.