HOW WOULD I APPROACH IT?
Pseudoaneurysm is defined as a partial rupture of the arterial wall that originates a fragile aneurysm sac of incomplete wall that communicates with the arterial lumen through the neck. The pseudoaneurysm of this case refers to the so-called anastomotic aneurysms (AA) that occur as a complication after surgery with synthetic valve implantation. The AA is due to the appearance of a dehiscence between the valve and the artery, which allows a pulsatile blood flow that is often contained by the fibrous reaction of surrounding tissues. The AA is not a rare entity and it is more common during the postoperative period to the point that 15 years after implanting the valve it is still present in one third of the patients, as it happened with this case.
Early AAs should lead us to think of an infectious origin. Late AAs are due to valve deterioration (specially Dacron grafts). Sometimes both causes overlap as in the case of AA fistulization towards a contaminated cavity. This is important because in the presence of an infection the endovascular solution is not possible being surgery the right option.
A high percentage of AAs are found when they are symptomatic (in this case due to aortobronchial fistulization), but even with incidental findings and given the unpredictability of the moment of rupture, treatment should be prescribed fast. In most cases this means using the endovascular option because in most cases—especially in emergent situations—surgery is often associated with a high morbimortality rate.
The imaging modality of choice to assess AA is the coronary computed tomography angiography (CCTA). In order to plan endovascular treatment, the following data should be obtained from the angiographic study:
– Location of dehiscence and aneurysm sac: proximal anastomosis, distal anastomosis or both. Relation to surrounding anatomical structures and eventual presence of fistulization. Arterial vascular anatomy in the valve juxtaposed area and, in particular, the presence of nearby bifurcation areas.
– Larger and smaller diameter of the sac; length of the neck.
– Diameter of the valve and vascular segment adjacent to the dehiscence.
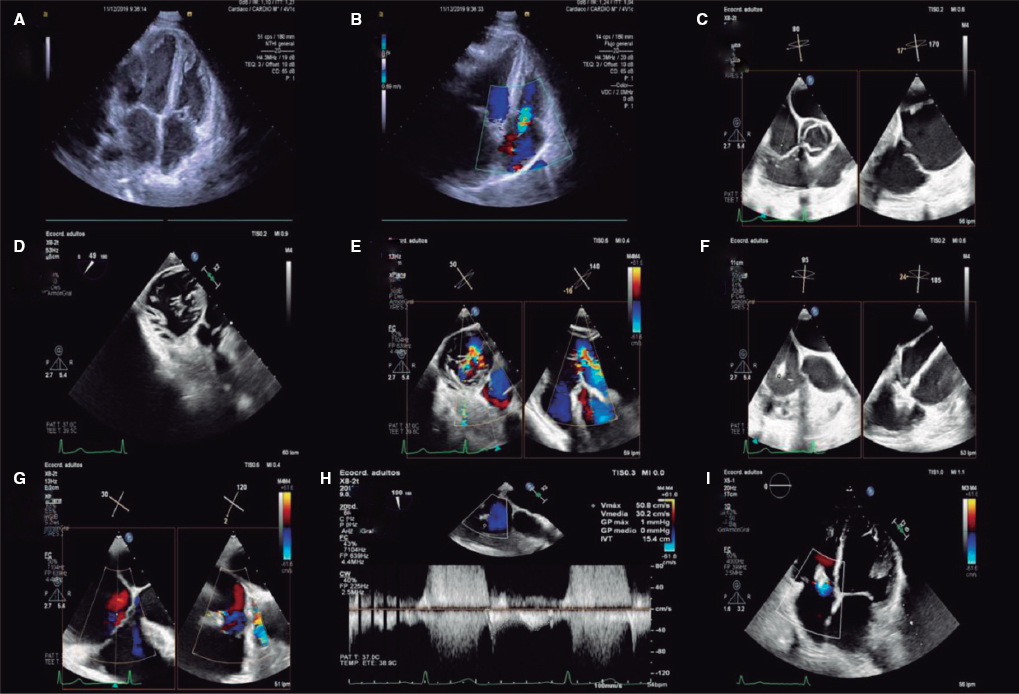
The CCTA of the case shows the presence of a proximal AA of an interposed bridge graft between the aortic arch and the descending thoracic aorta. The sac has saccular morphology and discrete size with a possibly well-defined neck. The valve dehiscence is in contact with the origin of the left subclavian artery.
There are 2 endovascular approaches often used to seal a pseudoaneurysm: close the dehiscence by implanting a covered stent or fill the sac with a metal (coil) or chemical (glue) structure. In the second option the intrasaccular administration of thrombin is included too.
Thanks to its efficacy and greater simplicity, the first-line therapy to resolve an AA is often the implantation of a covered stent. The decision between a self-expandable covered stent and a balloon-expandable stent is made on 2 questions basically. The first one is whether the target area to be treated is subject to movements of flexion or a potential extrinsic compression. Nearly 90% of all AAs are found in the groin region. Here self-expandable stent should be used. The second question is the degree of precision required in the implant; balloon-expandable stents are clearly more precise.
Covered stents, self-expandable stents, and balloon-expandable stents are available today. They are easy to use technically and they can be used in vessels/valves of up to 12 mm to 13 mm. For larger diameters, the devices require the use of a more complex technique. Aortic endoprostheses are considered as a self-expandable alternative and the covered Cheatham Platinum stent as a balloon-expandable stent.
As a general rule, the anchorage of the covered stent should be delivered among healthy vascular segments (whether arterial or intraprosthetic); in the case of a self-expandable stent the largest diameter of the anchorage is oversized by 1 mm and postdilatation of the stent nominal diameter is advised.
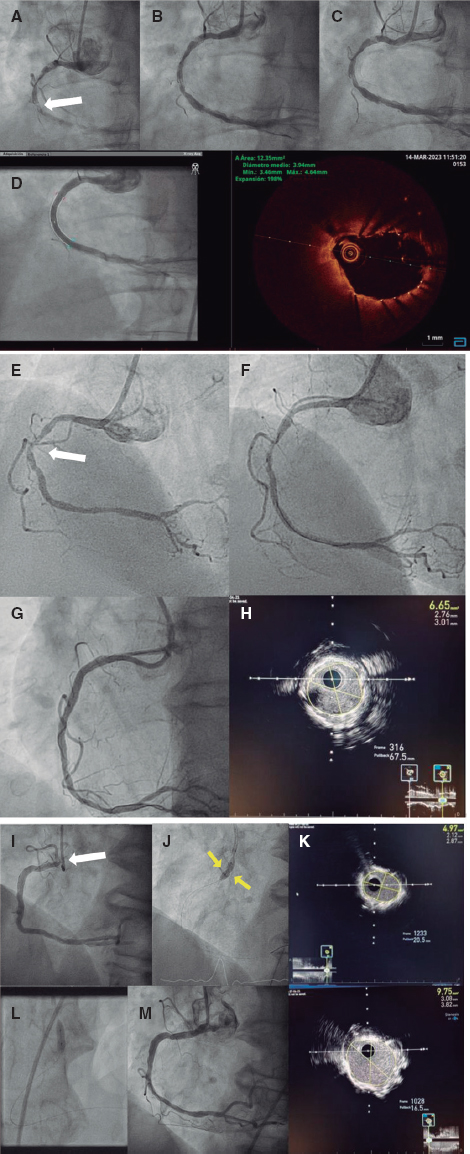
Is it possible to treat this case using a covered stent? It is not, at least it is not easily. To protect the origin of the left subclavian artery the chimney technique would be required (implantation of self-expandable covered stent from the aortic arch to the thoracic aorta with protection of a balloon-expandable stent from the left subclavian artery to the aortic arch) or else build a customized covered self-expandable prosthesis with fenestration towards the subclavian artery. The first option—more available—is limited by the large diameter of the subclavian artery, which would make it difficult to avoid the risk of leak through the self-expandable stent.
In order to consider the possibility of AA filling, the use of glue (cyanoacrylate) an even thrombin (often through percutaneous administration although it can also be administered through a microcatheter, personal experience) has been reported. With both the sac can be sealed immediately. The lack of control during their delivery and, therefore, their potential distal embolization is an important limitation here. In our own experience, these are the perfect agents to occlude pseudoaneurysms in distal vessels or in situation of multiple afferent and efferent vessels to the aneurysm sac, but this was not the case.
«Sac packing» the pseudoaneurysm with a coil can be the best option in this case, especially because the size of the sac is discrete, and the neck seems small. The stability during delivery recommends the use of a telescopic technique (5-6-Fr/90 cm guide catheter, 5-Fr Bernstein diagnostic catheter, and microcatheter). However, the secret for a proper coil embolization is to properly select the first coil: its diameter should be nominal to the sac largest diameter (it is very important to avoid excessive oversizing that may rupture its frail wall) and always larger than the diameter of the neck (to minimize the risk of embolization) with the largest possible length to occupy the maximum volume of the pseudoaneurysm. It is advised that the first coil should be a controlled delivery coil to keep all the repositioning options open. The first coil is followed by others to fill in the holes and complete the packing.
Corresponding author: Servicio Endovascular, Hospital Universitario Virgen Macarena, Avda. Doctor Fedriani 3, 41009 Seville, Spain.
E-mail address: rjruizsalmeron@yahoo.es (R.J. Ruiz Salmerón).