Abstract
Introduction and objectives: A significant amount of patients undergoing transcatheter aortic valve implantation (TAVI) have an indication for oral anticoagulation due to atrial fibrillation. In these patients the bleeding risk is often high. The purpose of this study was to compare the clinical outcomes of patients treated with low doses of apixaban or the vitamin K antagonist (VKA) acenocumarol in this setting.
Methods: Multicenter observational registry including patients treated after TAVI with low doses of apixaban (2.5 mg/12 hours) or VKA both without associated antiplatelet therapy. Propensity score matching was conducted to select 2 comparable cohorts. Data were gathered for 12 months following the procedure. Coprimary endpoints of efficacy (death, myocardial infarction, and stroke) and safety (bleeding BARC ≥ 2) were considered.
Results: A total of 236 patients were included. They were divided into 2 comparable groups of 64 patients each. Only 19 patients (30%) strictly met the dose adjustment criteria for apixaban. The rate of death, myocardial infarction, and stroke was similar at the 12-month follow-up (12.5% with VKA vs 9.3% with apixaban, P = .5), but the rate of bleeding BARC ≥ 2 was significantly higher in the VKA group (7.8% vs 0%; P = .02). Most of the events seen in the apixaban group occurred in patients with incorrect dose titration.
Conclusions: In this registry of patients treated with TAVI and atrial fibrillation the use of low-dose apixaban compared to VKA—both without antiplatelet agents—was associated to a lower rate of actionable bleeding and a similar rate of thrombotic events.
Keywords: TAVI. Anticoagulation. Apixaban. Vitamin K antagonist.
RESUMEN
Introducción y objetivos: Una proporción significativa de pacientes sometidos a implante percutáneo de válvula aórtica (TAVI) presenta indicación de anticoagulación oral por fibrilación auricular. En estos pacientes, con frecuencia el riesgo hemorrágico es alto. El objetivo del estudio fue comparar los resultados clínicos en pacientes tratados con dosis baja de apixabán o con acenocumarol, un antagonista de la vitamina K (AVK).
Métodos: Registro observacional multicéntrico que incluyó pacientes sometidos a TAVI tratados con dosis baja de apixabán (2,5 mg/12 h) o AVK, en ambos casos sin tratamiento antiplaquetario asociado. Se llevó a cabo un emparejamiento por puntuación de propensión para seleccionar dos cohortes comparables. Se recabó la información de los 12 meses posteriores al procedimiento. Se consideraron objetivos coprimarios de eficacia (muerte, infarto de miocardio e ictus) y de seguridad (hemorragias BARC ≥ 2).
Resultados: Se incluyeron 236 pacientes y se obtuvieron 2 grupos de 64 pacientes comparables en cuanto a características basales. Solo 19 (30%) cumplieron estrictamente los criterios de ajuste a la baja de la dosis de apixabán. A los 12 meses, la incidencia de muerte, infarto de miocardio e ictus fue comparable (12,5% con AVK frente a 9,3% con apixabán; p = 0,5), pero la incidencia de hemorragia BARC ≥ 2 fue significativamente mayor en el grupo de AVK (7,8 frente a 0%; p = 0,02). La mayoría de los eventos trombóticos en el grupo de apixabán se observaron en pacientes con reducción de dosis no ajustada a criterios.
Conclusiones: En este registro de pacientes con TAVI y fibrilación auricular, el uso de la dosis baja de apixabán en comparación con el uso de AVK, sin antiagregantes concomitantes, se asoció a una menor incidencia de hemorragias mayores con una incidencia similar de eventos tromboembólicos.
Palabras clave: TAVI. Anticoagulación. Apixabán. Antagonistas vitamina K.
Abbreviations: AF: atrial fibrillation. BARC: Bleeding Academic Research Consortium. DOAC: direct oral anticoagulants. MACE: major adverse cardiovascular events. TAVI: transaortic valve implantation. VKA: vitamin K antagonist.
INTRODUCTION
The growing number of transaortic valve implantation (TAVI) procedures over the last few years is the consequence of the large and solid scientific evidence available that has broadened its indications.1-3
Atrial fibrillation (AF) is a common finding in these patients.4 Its presence prior to the implant and its new appearance at the follow-up are associated with a higher mortality rate and a higher incidence of stroke,5 but also with more hemorrhages mainly due to the need for anticoagulation.6,7 The risk of hemorrhage is particularly high in patients who undergo TAVI since they are often old patients.
Direct oral anticoagulants (DOAC) have proven a better safety and efficacy profile compared to vitamin K antagonists (VKA) in the nonvalvular AF setting. However, few studies have analyzed their role in patients with valvular AF and, today, only the guidelines on the management of valvular heart disease established by the European Society of Cardiology de 2017 recommend them with a class IIa level of evidence C 3 months after the implant of a surgical bioprosthesis.8 No obstante, to this day dabigatran is the only DOAC that has proven non-inferior to VKA in patients with surgical bioprosthesis.9
Regarding patients with AF treated with TAVI there is not much evidence available for DOAC. In one of the very few cases published, the use of apixaban was associated with a significantly lower rate of adverse events at 30 days compared to the use of VKA.10 However, there were significant differences between the groups, no statistical matching was conducted, and patients with associated antiplatelet therapy were included.
The population treated with TAVI is often old (> 80 years), shows different stages of chronic kidney disease, and at times low body weight. These conditions may justify the relatively high prevalence of low-dose apixaban (2.5 mg/12 h).
This study assessed the use of low-dose apixaban in patients with TAVI and compared its long-term clinical outcomes to patients treated with VKA. A multicenter registry was designed including patients with an indication for oral anticoagulation (without associated antiplatelet therapy) post-TAVI on VKA or apixaban at doses of 2.5 mg/12 h. The registry included propensity score matching of these patients to estimate the effect of treatment.
METHODS
Study population
A multicenter, retrospective and observational registry was designed from a review of individual TAVI registries from 4 hospitals nationwide.
The study population included all consecutive patients treated with TAVI from 2008 with a diagnosis of AF at hospital discharge and an indication for chronic oral anticoagulation only whether VKA or apixaban at a dose of 2.5 mg/12 h, and with a 1-year follow-up. Patients dead at admission were, therefore, excluded and there were no additional exclusion criteria.
The decision on the dose of apixaban was the responsibility of the patient’s treating physician. European Medicines Agency recommends low-dose apixaban in patients with non-valvular AF and glomerular filtration rate of 15-29 mL/min and in patients with, at least, 2 of the following characteristics: age ≥ 80 years, body weight ≤ 60 kg or serum creatinine levels ≥ 1.5 mg/dL (133 µmol/L).11
Study endpoints and definition of events
All clinical variables, demographic data, and cardiovascular risk factors were recorded from each case. Also, previous TAVI procedures, the presence of cardiovascular disease or previous heart surgeries, chronic lung or kidney disease, liver cirrhosis or neoplasms was also recorded in all of the patients. Variables associated with cardiovascular status such as ventricular function, aortic stenosis and coronary artery disease were included as well. Surgical risks were assessed using the following risk scores: EuroSCORE log, EuroSCORE II, and the Society of Thoracic Surgery (STS) score. Given the presence of AF at hospital discharge (whether known or de novo), the annual risks of thromboembolic events were assessed using the CHA2DS2-VASc score while bleeding risk was assessed using the HAS-BLED score in all of the patients. Other data on the procedure and complications derived from it were also recorded as defined by the updated criteria established by the Valve Academic Research Consortium-2 (VARC-2).12
The rate of major adverse cardiovascular events (MACE) at the 1-year follow-up was studied in all of the patients after TAVI implantation in each center. MACE was defined as all-cause mortality, stroke (whether ischemic or hemorrhagic), and acute myocardial infarction, all of them defined according to the criteria established by VARC-2.11 Also, the rate of hemorrhages categorized according to the classification established by the Bleeding Academic Research Consortium (BARC)12 and considered relevant if BARC ≥ 2 was also studied. The net composite endpoint of efficacy-safety including all MACE and BARC type ≥ 2 bleeding was studied as well.
Two coprimary endpoints were studied: efficacy (through MACE) and safety (BARC type ≥ 2 bleeding). Secondary endpoints were a net composite endpoint of efficacy-safety, overall mortality, cardiac death, myocardial infarction, stroke, and hemorrhagic stroke.
The adjudication of events was left at the discretion of the researchers from each center according to the definitions previously indicated. The database did not include any events that allowed the identification of patients and anonymity was guaranteed at all time. The study was approved by the coordinating center ethics committee.
Statistical analysis
Continuous variables were expressed as mean and standard deviation or median and interquartile range according to their distribution. Categorical variables were expressed as percentages. The Kolmogorov-Smirnov test was used to determine whether the distribution of continuous variables was normal. If so both groups were compared using the independent-samples t-test was used. In cases of non-normal distribution Wilcoxon test was used. Qualitative or categorical variables were compared using the chi-square test or Fisher’s exact test when appropriate. Cox proportional hazards regression model was used in the entire sample before the matching to identify predictor variables of net composite event. Variables with P values < .1 in the univariate analysis were included.
Given the limitations and interpretation biases of the possible associations in the comparison of unadjusted variables of an observational study like this propensity score adjustment was made using the logistics regression model. The type of high anticoagulation was established (apixaban 2.5 mg vs VKA) as a dependent variable and the baseline characteristics shown on table 1 were included in the analysis as independent variables. Since the number of patients was clearly lower in the apixaban group, the goal of the propensity score matching to estimate the effect of treatment was to match each patient from the apixaban group with a patient from the VKA group. This procedure included 2 stages: 1) Propensity scores were estimated using the logistics regression model and treatment with apixaban was used as the outcome variable. All the covariables analyzed were used as predictors; and 2) patients were matched on a 1:1 ratio using the nearest neighbor matching based on a correlation algorithm that categorizes observations in the apixaban group based on the estimated propensity score. Then each unit was sequentially combined with a unit from the VKA group with the nearest propensity score. All the differences seen in the standardized means after matching were < 10%. Calibration was estimated using the Hosmer-Lemeshow test while precision was assessed using the area under the ROC (Receiver Operating Characteristic) curve. The PS Matching software and SPSS statistical package version 22.0 (IBM, United States) were used. The PS Matching software performs all R analyses using the SPSS R-Plugin. Event-free survival was studied using the Kaplan-Meier method. The log-rank test was used for group comparison. All analyses were 2-tailed and P values < .05 were considered statistically significant. Statistical analyses were conducted using the SPSS statistical package version 22.0.
Table 1. Baseline characteristics before and after propensity score adjustment
| Overall cohort | Matched cohort | |||||
|---|---|---|---|---|---|---|
| Apixaban (n = 64) | VKA (n = 172) | P | Apixaban (n = 64) | VKA (n = 64) | P | |
| Male sex | 41 (64) | 60 (35) | < .001 | 41 (64) | 38 (59) | .6 |
| Age (years) | 82 ± 6 | 83 ± 6 | .3 | 82 ± 6 | 81 ± 7.5 | .7 |
| Weight (kg) | 72 ± 12 | 71 ± 17 | .6 | 72 ± 12 | 74 ± 14 | .5 |
| Height (cm) | 161 ± 10 | 158 ± 14 | .1 | 161 ± 10 | 160 ± 9 | .32 |
| HBP | 55 (86) | 140 (81) | .4 | 55 (86) | 48 (75) | .11 |
| Diabetes mellitus | 27 (42) | 51 (30) | .07 | 27 (42) | 19 (30) | .14 |
| Baseline glomerular filtration rate (mL/min) | 58 ± 20 | 61 ± 23 | .3 | 58 ± 20 | 60 ± 25 | .6 |
| Previous ACS | 4 (6) | 10 (6) | .9 | 4 (6) | 4 (6) | 1 |
| Previous PTA | 9 (14) | 10 (6) | .04 | 9 (14) | 8 (13) | .8 |
| Previous CABG | 3 (5) | 12 (7) | .52 | 3 (5) | 4 (6) | .7 |
| Previous AVR | 7 (11) | 6 (4) | .03 | 7 (11) | 4 (6) | .3 |
| Previous stroke | 3 (5) | 26 (15) | .03 | 3 (5) | 3 (5) | 1 |
| COPD | 19 (30) | 39 (23) | .27 | 19 (30) | 15 (23) | .4 |
| Cirrhosis | 0 | 4 (2) | .6 | 0 | 1 (1.6) | 1 |
| Neoplasm | 11 (17) | 16 (9) | .09 | 11 (17) | 6 (9) | .2 |
| Peripheral vascular disease | 7 (11) | 4 (5) | .15 | 7 (11) | 2 (6) | .4 |
| NYHA III-IV | 30 (51) | 111 (65) | .06 | 30 (51) | 37 (58) | .4 |
| Baseline LVEF (%) | 53 ± 12 | 56 ± 13 | .1 | 53 ± 12 | 56 ± 12 | .3 |
| EuroSCORE log | 13.3 ± 9 | 18.5 ± 13 | .002 | 13.3 ± 9 | 14 ± 8 | .7 |
| EuroSCORE II | 4.2 ± 5 | 6.4 ± 6 | .013 | 4.2 ± 5 | 4.7 ± 4 | .55 |
| STS mortality | 6 [2-10] | 7 [5-15] | .4 | 6 [2-10] | 11 [3-11] | .5 |
| CHA2DS2-VASc | 4.5 ± 1.1 | 4.6 ± 1.4 | .55 | 4.5 ± 1.1 | 4 ± 1.3 | .07 |
| HAS-BLED | 2.8 ± 1 | 2.74 ± 1 | .8 | 2.8 ± 1 | 2.5 ± 1.1 | .3 |
|
ACS, acute coronary syndrome; AVR, aortic valve replacement; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; HBP, high blood pressure; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PTA, percutaneous transluminal angioplasty; STS, Surgeon Thoracic Score; VKA, vitamin K antagonists. Data are expressed as no. (%) or mean ± (standard deviation) or median [interquartile range]. |
||||||
RESULTS
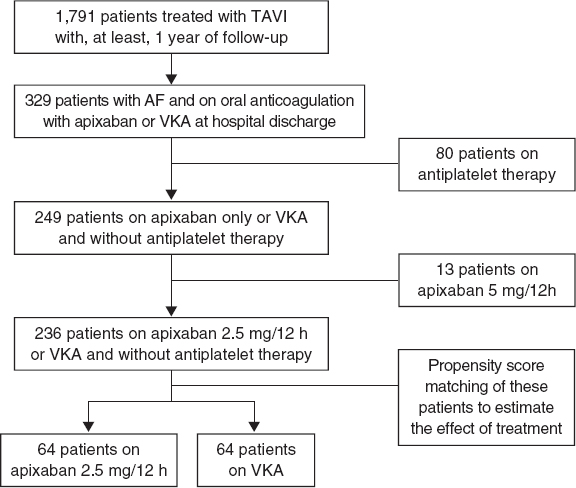
Of a total of 1791 patients a final cohort of 236 patients who met the inclusion criteria was obtained. Of these 64 (27%) were treated with low-dose apixaban at discharge after TAVI, and 172 (73%) were treated with VKA. Using propensity score matching to estimate the effect of treatment 2 groups of 64 patients each were made. The flow chart of patients is shown on figure 1. The center-based distribution of cases with apixaban was 60%, 20%, 14%, and 3%.
Figure 1. Study flow chart. AF, atrial fibrillation; TAVI, transaortic valve implantation; VKA, vitamin K antagonist.
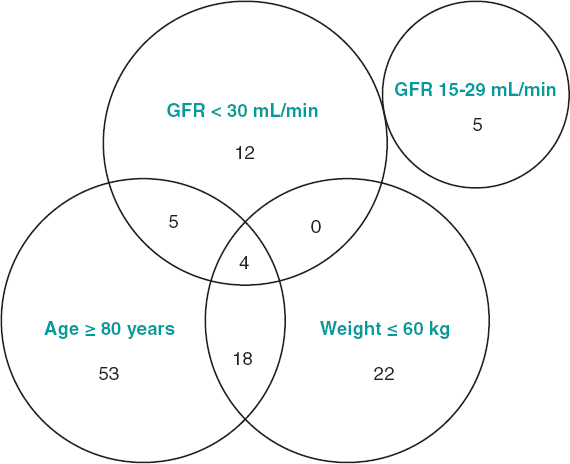
Regarding the adequacy of low-dose apixaban with respect to the degree of compliance of the characteristics recommended by the European Medicines Agency,11 it was confirmed that only 19 patients (30%) strictly met the criteria (figure 2). Sixty-eight per cent of the patients ≥ 80 years had chronic kidney disease stage IIIA (glomerular filtration rate 30-59 mL/min) and that factor was considered enough to indicate a dose of 2.5 mg.
Figure 2. Group of patients treated with low-dose apixaban according to the variables contemplated for the reduction of doses. The number of patients who met each and every criterion and the possible combination of these is shown too. The European Medicines Agency recommends low-dose apixaban in patients with non-valvular atrial fibrillation and glomerular filtration rates (GFR) of 15-29 mL/min, as well as in patients with, at least, 2 of the following characteristics: age ≥ 80 years, body weight ≤ 60 kg or serum creatinine levels ≥ 1.5 mg/dL (133 µmol/L).11
Table 1 shows the baseline characteristics of the overall cohort and those adjusted by propensity score. In the overall cohort the group on apixaban included more males, more patients with previous coronary and valve interventions and less patients with a past medical history of strokes. The values of EuroSCORE were lower compared to the VKA group, but bleeding risk scores were similar. No significant differences were seen between the matched groups.
The procedural characteristics and complications seen in the groups already matched are shown on table 2. No significant differences were found for any of the aspects studied. It should be mentioned here that patients from the apixaban group showed higher hemoglobin levels at hospital discharge. Also, in this group, 10 patients had made it to TAVI on anticoagulant and antiplatelet therapy (suspended after TAVI) compared to only 1 patient from the VKA group. Regarding the timeline of AF, all the patients from the VKA group already showed it before TAVI, while only 5 patients from the apixaban group developed it after TAVI.
Table 2. Procedural characteristics, complications, and characteristics at hospital discharge of the matched cohort
| Apixaban (n = 64) | VKA (n = 64) | P | |
|---|---|---|---|
| Balloon-expandable aortic valve | 51 (80) | 50 (78) | .8 |
| Femoral access | 64 (100) | 62 (97) | .5 |
| Successful implant | 64 (100) | 64 (100) | 1 |
| Transfusions | 7 (11) | 5 (8) | .5 |
| Coronary occlusion | 0 | 0 | |
| Valve embolization | 0 | 0 | |
| Annular rupture | 0 | 0 | |
| Periprocedural stroke | 1 (1.6) | 1 (1.6) | 1 |
| Vascular complication | 7 (11) | 10 (16) | .4 |
| BARC type ≥ 2 bleeding | 4 (6.3) | 4 (6.3) | 1 |
| Periprocedural ACS | 0 | 0 | |
| Need for pacemaker | 8 (13) | 10 (16) | .6 |
| De novo atrial fibrillation | 5 (8) | 0 | .06 |
| Hemoglobin levels (g/dL) at hospital discharge | 12 ± 1.6 | 11 ± 1.4 | .05 |
| Platelet levels at hospital discharge (109/L) | 133 ± 92 | 160 ± 60 | .2 |
| Glomerular filtration rate (mL/min) at hospital discharge | 68 [53-82] | 66 [40-82] | .5 |
|
ACS, acute coronary syndrome; BARC, Bleeding Academic Research Consortium; VKA, vitamin K antagonists. Data are expressed as no. (%) or mean ± (standard deviation) or median [interquartile range]. |
|||
No patients were lost to the follow-up. Table 3 shows the cardiovascular events seen from hospital discharge until 1 year later without significant differences between both groups regarding MACE. Four deaths occurred in the VKA group (6%) of which 2 were cardiac deaths (heart failure and sudden death) and the remaining 2 were due to major hemorrhages (hemorrhagic stroke and hypovolemic shock after a fall with hip fracture). The 3 deaths from the apixaban group were caused by pulmonary disease. It is interesting to see the events that occurred in the apixaban group based on the use of adjusted low doses and not on criteria since MACE occurred in the group with unadjusted doses.
Table 3. Major cardiovascular adverse events and hemorrhages at the 12-month follow-up
| Apixaban | VKA (n = 64) | P * | ||||
|---|---|---|---|---|---|---|
| LD (n = 64) | ALD (n = 19) | NALD (n = 45) | ||||
| MACE | 6 (9.3) | 1 (5.3) | 5 (11) | 8 (12.5) | .5 | |
| Death | 3 (4.7) | 0 | 3 (4.7) | 4 (6.2) | .7 | |
| Cardiac death | 0 | 0 | 0 | 2 (3.1) | .3 | |
| Myocardial infarction | 1 (1.6) | 0 | 1 (2.2) | 1 (1.6) | 1 | |
| Stroke | 2 (3.1) | 1 (5.3) | 1 (2.2) | 3 (4.7) | .9 | |
| Hemorrhagic stroke | 0 | 0 | 0 | 2 (3.1) | .5 | |
| BARC type > 2 bleeding | 0 | 0 | 0 | 5 (7.8) | .02 | |
| Safety-efficacy target** | 4 (6.2) | 0 | 4 (8.8) | 8 (12.5) | .23 | |
|
ALD, adjusted low dose; LD, low dose; MACE, major cardiovascular adverse events; NALD, non-adjusted low dose; VKA, vitamin K antagonists. Data are expressed as no. (%). * P values for the comparison between apixaban LD vs VKA. ** Composite of death, myocardial infarction, stroke, and BARC ≥ type 2 bleeding. |
||||||
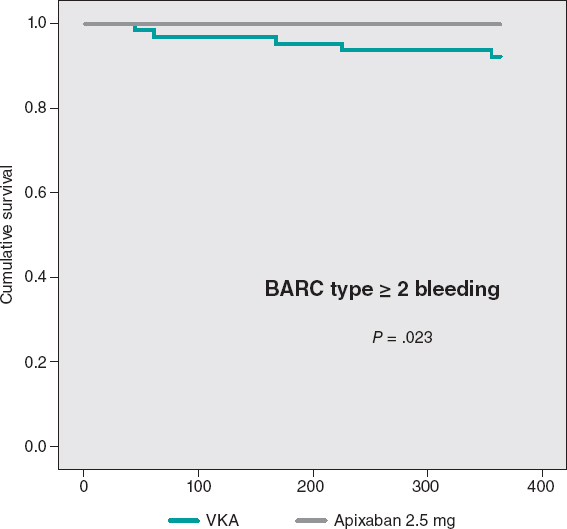
Only relevant hemorrhages were reported (BARC ≥ 2) in the VKA group with a significant difference compared to the apixaban group. Events occurred during months 1, 3, 5, 6, 11 after TAVI. Three of these major hemorrhages were due to digestive problems, 1 due to subarachnoid hemorrhage, and the other due to a spontaneous hematoma in the anterior rectus abdominis muscle. All of these patients required admission, surgery, and received 4 blood transfusions. In the apixaban group the rate of BARC type ≥ 2 bleeding seen (0%) was lower than anticipated by the HAS-BLED score (3.4%), but in the VKA group it was exactly the other way around: the rate seen (7.8%) was higher than expected (2.8%).
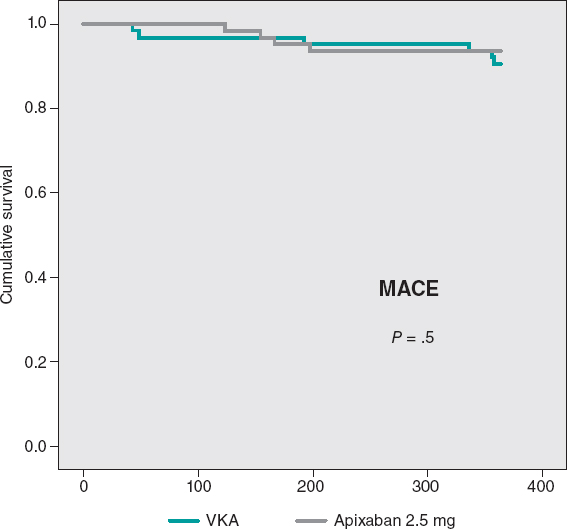
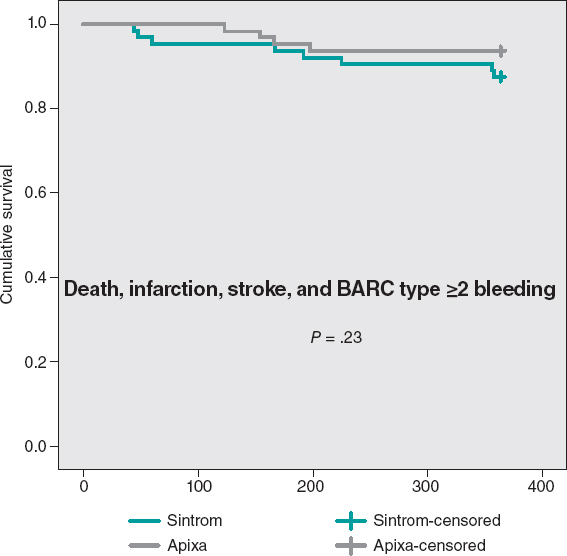
The cumulative MACE-free survival at 1 year showed no significant differences between the groups as shown on figure 3. Figure 4 shows the hemorrhage-free survival curves (BARC ≥ 2) with significant differences that favor the apixaban group. Mortality-free survival, infarction, stroke, and BARC type ≥ 2 bleeding curves did not show significant differences, but they did favor apixaban (figure 5). The Cox multivariate analysis conducted on the overall sample prior to case matching identified the use of apixaban as an independent predictor for the net composite endpoint of efficacy and safety (hazard ratio, 0.56; 95% confidence interval, 0.23-0.98; P = .04).
Figure 3. Major cardiovascular events (MACE)-free Kaplan-Meier survival curves at the 1-year follow-up. VKA, vitamin K antagonist.
Figure 4. BARC type ≥ 2 bleeding-free Kaplan-Meier survival curves. VKA, vitamin K antagonist.
Figure 5. Mortality-free Kaplan-Meier survival curves, infarction, stroke, and BARC type ≥ 2 bleeding.
DISCUSSION
The main study findings are: a) the use of apixaban is still not widespread compared to VKA in the population with TAVI and AF; b) the use of low-dose apixaban is very common in this context, but on many occasions it does not strictly adjust to the instructions for use and underdosing is a common thing; c) compared to VKA treatment with low-dose apixaban was associated with very similar rates of thrombotic-ischemic events and a significantly lower risk of major bleeding.
These findings together with the reservations derived from the study limitations would be consistent with the results of the ARISTOTLE trial subanalysis13 in patients with bioprosthesis. These results would confirm the safety and efficacy profile of apixaban in valvular patients, although only 31% of these were > 75 years old. A substudy of the ENGAGE AF-TIMI 48 trial14 of 191 patients with previous implant of a surgical or transcatheter bioprosthesis and AF showed a significant reduction of major bleeding with low-dose edoxaban (30 mg) compared to warfarin. The low and high doses (60 mg) of edoxaban were associated with a reduced composite of stroke, systemic embolism, major bleeding or death.
The population treated with TAVI includes elderly patients with multiple comorbidities who are not very well represented in the clinical studies of other contexts such as non-valvular AF.
The presence of previous or de novo AF after TAVI is not an uncommon finding in this population, and embolic and hemorrhagic risk is higher compared to other populations, which poses a significant challenge when having to decide what the best antithrombotic treatment should be.
There are still few studies that compare DOAC to VKA in the TAVI setting, and they are often registries. In the aforementioned Seeger et al.,10 141 patients treated with apixaban (most of them with doses of 5 mg) and 131 with VKA, the safety profile was better with apixaban and efficacy was similar. However, in this registry the low-dose apixaban and statistical matching were not studied and cases with concomitant antiplatelet therapy were included. In our study we thought it was very important to exclude patients with associated antiplatelet therapy and perform propensity score matching to estimate the effect of treatment. That is so because both aspects reduce significantly the load of biases associated with registry-based comparative studies. On the other hand, the low-dose study was very pertinent given its frequency of use in this population.
A different study compared the clinical progression of 154 patients treated with several DOAC and 172 treated with VKA always without antiplatelet therapy15 without statistical matching but with significant differences between the groups. The authors found very similar efficacy and safety profiles, although the DOAC group had a more adverse hemorrhagic and thrombotic baseline risk profile.
Finally, in the RESOLVE (The assessment of transcatheter and surgical aortic bioprosthetic valve thrombosis and its treatment with anticoagulation) and SAVORY (Subclinical aortic valve bioprosthesis thrombosis assessed with four-dimensional computed tomography) registries no significant differences were found regarding the move of the valve leaflets between DOAC and warfarin (3% vs 4%, respectively; P = .72), although both seemed better than non-anticoagulation.16
The high prevalence of underdosing of apixaban in this population is worth mentioning. We believe that it can be conditioned by the perception of a very high bleeding risk in these patients (most of them 80-year-old patients with a high prevalence of chronic kidney disease and other conditions) and a variable degree of frailty. Although it is not an established criterion per se to change the type and dose of anticoagulation it can certainly influence the decision-making process.
The fact that we found differences that favored apixaban at doses of 2.5 mg compared to VKA may lead us to think that underdosing does not penalize as much as in the general context of patients with non-valvular AF;13 as a matter of fact, it is associated with a better safety profile without affecting efficacy. However, the use of low doses cannot be recommended openly and we believe in the rigor of dose adjustment. Although the size of subgroups based on correct dose adjustment was not small and we cannot draw conclusive results, more thrombotic-ischemic events were seen within the apixaban group in the incorrect adjustment group. However, we should mention that dosing needs to be dynamic since dose adjustment conditions vary across follow-up and that is how the treatment of these patients is optimized at all time.
Having said this, the indications for treatment with these drugs are changing due to recent real-world data available. Until a year ago, apixaban was contraindicated in patients with acute kidney injury in whom VKA were still the treatment of choice. The studies most recently published show a clear benefit regarding hemorrhages with doses of apixaban of 2.5 mg, and thromboembolic events and mortality with doses of 5 mg in patients with acute kidney injury and dialysis.17
The difficulty of this was seen in the results reported by the GALILEO trial18 (Global study comparing a rivaroxaban-based antithrombotic strategy to an antiplatelet-based strategy after transcatheter aortic valve replacement to optimize clinical outcomes). This study showed that in patients without indication for oral anticoagulation after TAVI, a treatment strategy including rivaroxaban at doses of 10 mg/day plus antiplatelet therapy within the first 90 days was associated with a higher risk of death or thromboembolic complications and hemorrhages compared to a 90-day course of dual antiplatelet therapy and then single antiplatelet therapy.
The ongoing studies that are being conducted now will provide more solid evidence on what the best antithrombotic strategies are in patients after TAVI with and without AF.19
Limitations
The main limitation of this study is that it was not randomized. As any other observational registry, it is subject to more and less evident confounding factors. Although the use of propensity score matching to estimate the effect of treatment produced 2 groups with very similar baseline characteristics, the chances of bias were still present.
The size of the sample is the second most important limitation. The volume of patients with TAVI plus an indication for oral anticoagulation only is not high in any centers in our setting, especially if we wish to include those specifically treated with low-dose apixaban. Therefore, a multicenter study with high-volume centers was designed (compared to the average of the country), but still the size of the sample could not be higher. This creates an underpowered study that should be considered exploratory and hypothesis-generating only. This limitation is even more evident for subgroup comparisons based on dose adjustment. However, until statistically powered studies become available, the results from registries like this contribute to expand our knowledge base. Event adjudication was not centralized although the previously established standardized definitions were adjusted.11
CONCLUSIONS
The use of low-dose apixaban (2.5 mg/12 h) in patients treated with TAVI often did not strictly match the official recommendations. In this registry, the use of low-dose apixaban was associated with very similar figures of thrombotic-ischemic events compared to the use of acenocoumarol, but a significantly lower risk of major hemorrhages. This study suggests that, in patients treated with TAVI who have AF, the use of low-dose apixaban (if adequately prescribed) is safer and equally efficient compared to acenocoumarol.
CONFLICTS OF INTEREST
J.M. de la Torre Hernández has received unconditional institutional research grants and fees for as a counselor for Bristol-Myers Squibb. Also, he is the editor-in-chief of REC: Interventional Cardiology; the journal’s editorial procedure to ensure impartial handling of the manuscript has been followed.
WHAT IS KNOWN ABOUT THE TOPIC?
- The performance of TAVI has experienced significant growth. The prevalence of AF among patients with TAVI is high. DOAC have proven better safety and efficacy profile compared to VKA in the non-valvular AF setting. There are few studies analyzing the role of patients with TAVI and AF, and in particular none assessing low-dose apixaban.
WHAT DOES THIS STUDY ADD?
- This multicenter registry shows that the use of apixaban is not widespread compared to the use of VKA in the population with TAVI and AF. In patients treated with apixaban the use of low doses is very common, but many times, it does not strictly follow the instructions for used. In this sense compared to VKA, treatment with low-dose apixaban was associated with very similar rates of thrombotic-ischemic events and a significant lower risk of major hemorrhages.
REFERENCES
1. Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187-2198.
2. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609-1620.
3. Waksman R, Rogers T, Torguson R, et al. Transcatheter Aortic Valve Replacement in Low-Risk Patients With Symptomatic Severe Aortic Stenosis. J Am Coll Cardiol. 2018;72:2095-2105.
4. Koniari I, Tsigkas G, Kounis N, et al. Incidence, pathophysiology, predictive factors and prognostic implications of new onset atrial fibrillation following transcatheter aortic valve implantation. J Geriatr Cardiol. 2018;15:50-54.
5. Biviano AB, Nazif T, Dizon J, et al. Atrial Fibrillation Is Associated With Increased Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement:Insights From the Placement of Aortic Transcatheter Valve (PARTNER) Trial. Circ Cardiovasc Interv. 2016 ;9:e002766.
6. Kapadia S, Agarwal S, Miller DC, et al. Insights Into Timing, Risk Factors, and Outcomes of Stroke and Transient Ischemic Attack After Transcatheter Aortic Valve Replacement in the PARTNER Trial (Placement of Aortic Transcatheter Valves). Circ Cardiovasc Interv. 2016;9. pii:e002981.
7. Tarantini G, Mojoli M, Urena M, Vahanian A. Atrial fibrillation in patients undergoing transcatheter aortic valve implantation:epidemiology, timing, predictors, and outcome. Eur Heart J. 2017;38:1285-1293.
8. Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739-2791.
9. Durães AR, de Souza Roriz P, de Almeida Nunes B, et al. Dabigatran Versus Warfarin After Bioprosthesis Valve Replacement for the Management of Atrial Fibrillation Postoperatively:DAWA Pilot Study. Drugs R D. 2016;16:149-154.
10. Seeger J, Gonska B, Rodewald C, et al. Apixaban in Patients With Atrial Fibrillation After Transfemoral Aortic Valve Replacement. JACC Cardiovasc Interv. 2017;10:66-74.
11. Agencia Europea de Medicamentos. Anexo I. Ficha técnica o resumen de las características del producto. Disponible en:https://www.ema.europa.eu/en/documents/product-information/eliquis-epar-product-information_es.pdf. Consultado 12 Mar 2020.
12. Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation:the Valve Academic Research Consortium-2 consensus document. J Thorac Cardiovasc Surg. 2013;145:6-23.
13. Guimarães PO, Pokorney SD, Lopes RD, et al. Efficacy and safety of apixaban vs warfarin in patients with atrial fibrillation and prior bioprosthetic valve replacement or valve repair:Insights from the ARISTOTLE trial. Clin Cardiol. 2019;42:568-571.
14. Carnicelli AP, De Caterina R, Halperin JL, et al. ENGAGE AF-TIMI 48. Edoxaban for the Prevention of Thromboembolism in Patients With Atrial Fibrillation and Bioprosthetic Valves. Circulation. 2017;135:1273-1275.
15. Geis NA, Kiriakou C, Chorianopoulos E, Uhlmann L, Katus HA, Bekeredjian R. NOAC monotherapy in patients with concomitant indications for oral anticoagulation undergoing transcatheter aortic valve implantation. Clin Res Cardiol. 2018;107:799-806.
16. Chakravarty T, Søndergaard L, Friedman J, et al. RESOLVE and SAVORY In-vestigators. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves:an observational study. Lancet. 2017;389:2383-2392.
17. Siontis KC, Zhang X, Eckard A, et al. Outcomes Associated With Apixaban Use in Patients With End-Stage Kidney Disease and Atrial Fibrillation in the United States. Circulation. 2018;138:1519-1529.
18. Dangas GD, Tijssen JGP, Wöhrle J, et al. A Controlled Trial of Rivaroxaban after Transcatheter Aortic-Valve Replacement. N Engl J Med. 2020;382:120-129.
19. Guedeney P, Mehran R, Collet JP, Claessen BE, Ten Berg J, Dangas GD. Antithrombotic Therapy After Transcatheter Aortic Valve Replacement. Circ Cardiovasc Interv. 2019;12:e007411.