ABSTRACT
Introduction and objectives: There is great interest in the development of devices for the percutaneous management of mitral regurgitation (MR). For this reason, having an experimental model that reproduces the conditions of the disease is of great importance. Our objective was to validate an experimental model of MR in a porcine model.
Methods: For the model creation phase 3, 2-month-old 25 ± 3 kg large white pigs were used. An acute myocardial infarction was caused in the circumflex artery territory that hampered the perfusion of the posteromedial papillary muscle. Then, volume overload was induced in the animal by creating an arteriovenous shunt and connecting the aorta and the pulmonary artery using a Dacron tube. Echocardiography and magnetic resonance imaging were performed before the intervention and on week 8. Afterwards, the animal was euthanized to conduct the pathological study.
Results: One out of the 3 pigs died during the intervention due to ventricular fibrillation. The remaining 2 pigs survived the procedure and were euthanized as scheduled on week 8. In both cases a transmural infarction occurred, 1 at lateral level and the other one at posteroinferior level with moderate secondary mitral regurgitation. Ventricular dimensions and volumes increased and the overall contractility was maintained despite segmental alterations.
Conclusions: The experimental model of chronic MR based on the ischemic damage of the posteromedial papillary muscle associated with volume overload is feasible, safe and reproducible. Also, it can be very useful to test the safety and efficacy of future devices for the management of this condition.
Keywords: Mitral regurgitation. Experimental model. Porcine model.
RESUMEN
Introducción y objetivos: Existe un creciente interés en el desarrollo de dispositivos para el tratamiento de la insuficiencia mitral (IM) de forma mínimamente invasiva. Para este propósito, disponer de un modelo experimental que reproduzca las condiciones de la enfermedad sería de gran utilidad. Nuestro objetivo fue validar un modelo experimental de IM en cerdos.
Métodos: Para esta fase de creación del modelo se han utilizado 3 cerdos de raza large white, de 2 meses de edad y un peso de 25 ± 3 kg. Se provocó un infarto en el territorio de la arteria circunfleja que afectó la perfusión del músculo papilar posteromedial, y posteriormente se sometió al animal a una sobrecarga de volumen mediante creación de un shunt arteriovenoso, con la conexión de la aorta y la pulmonar mediante un tubo de dacrón. Se realizó análisis mediante ecocardiografía y resonancia magnética antes de la intervención y a las 8 semanas, y posteriormente el animal fue eutanasiado para realizar el estudio anatomopatológico.
Resultados: De los 3 cerdos, 1 falleció durante la intervención por fibrilación ventricular y los otros 2 sobrevivieron al procedimiento y fueron eutanasiados como estaba previsto a las 8 semanas. En ambos se produjo un infarto transmural, uno lateral y otro posteroinferior, con IM moderada secundaria. Las dimensiones y los volúmenes ventriculares aumentaron, y la contractilidad global se mantuvo a pesar de las alteraciones segmentarias.
Conclusiones: El modelo experimental de IM crónica basado en el daño isquémico del músculo papilar posteromedial asociado a una sobrecarga de volumen es factible, seguro y reproducible, y puede ser de gran utilidad para comprobar la seguridad y la eficacia de los futuros dispositivos para el tratamiento de esta afección.
Palabras clave: Insuficiencia mitral. Modelo experimental. Modelo porcino.
Abbreviations:
MR: mitral regurgitation.
INTRODUCTION
Mitral valve repair surgery is the treatment of choice for the management of patients with severe mitral regurgitation (MR) who meet the criteria and indications proposed in the clinical practice guidelines.1 However, almost 50% of the patients referred to surgery are not operated on,2,3 mainly due to the presence of comorbidities, left ventricular dysfunction or age related issues.4 In these cases, the use of transcatheter techniques has become a valid alternative.
Given the complexity of the mitral valve, there are several devices in the pipeline to reduce the degree of regurgitation using transcatheter approaches.5,6 Of all the devices available, very few have been eventually used for the management of patients. Among these, only MitraClip—inspired in the Alfieri technique—has proven great clinical utility.7-9
That is why it is important to have an animal model of MR available to test the safety, efficacy, and tissue response of these new devices in a scenario that reproduces the future clinical situations we may encounter faithfully. Our objective was to assess the feasibility of creating an experimental model of MR capable of reproducing the actual conditions with an acceptable safety and efficacy profile.
METHODS
Animal model
Different experimental models have been described by the medical literature to induce MR by causing ischemic damage through the selective occlusion of the circumflex artery and rupture of a mitral chorda tendinae,10 the production of ischemia in both the circumflex and right coronary arteries11 or the production of selective ischemia in the marginal arteries that supply the papillary muscle.12 A Spanish group studied the role of atrial infarction in ischemic MR and atrial and ventricular remodeling through the occlusion of the circumflex artery before or after the origin of the atrial branch.13 The models based on the production of ischemic damage only caused moderate MR. Only the model designed by Cui et al.,10 that combined mitral chordae tendinae ruptures with the corresponding volume overload, induced severe regurgitation. Our group designed a new model to induce MR by combining ischemic damage and the creation of an aortopulmonary shunt as the mechanism of volume overload.
To create this experimental model, 3 Large White domestic pigs were used. They were 2 months old and weighted 25 ± 3 kg. All procedures were performed in full compliance with the national legislation in force (Royal Decree 53/2013 of February 1st on the basic standards for the protection of animals used for scientific purposes) and European Directive 2010/63/EU.
The echocardiographic studies were conducted using a Vivid I GE ultrasound system with 3S cardiac sector probe (1.5-4 MHz). Parasternal short-axis and long-axis slice planes and apical 4-chamber planes were acquired.
The magnetic resonance imaging study was conducted using a Signa HDx 3.0 T GE MR system through FIESTA balanced steady-state free precision multifarious sequences of specific cardiac planes (of 2, 3, and 4 chambers, and in the short axis) to assess both the anatomy and the cardiac function. All images were processed using the ReportCard 4.0 software package.
Conceiving the experimental model
Anesthetic procedure to perform the procedure destined to induce MR and magnetic resonance imaging study
On the day of the surgery, anesthetic premedication was administered based on a combination of midazolam (0.35 mg/kg, Midazolam Normon, Normon), ketamine (5 mg/kg, Imalgene 1000, Merial), and methadone (0.1 mg/kg, Semfortan, Dechra) via intramuscular access. After confirming the correct sedation of the animals, preoxygenation with oxygen mask at 100% concentration was administered. Then, venoclysis was performed in the marginal atrial vein using a 20-gauge endovenous catheter followed by maintenance fluid therapy with lactated Ringer’s solution at an infusion rate of 10 mL/kg per hour. Propofol (2-4 mg/kg, Propovet, Esteve) was used for the induction of anesthesia followed by conventional tracheal intubation. The maintenance anesthetic agent used was sevoflurane (Sevorane, Abbot) at a dose of 1-1.5 MAC. Fentanyl (Fentanest, Janssen) was the intraoperative analgesic used. It was administered through a slow IV bolus of 5 µg/kg and followed by the continuous infusion of a 6 µg/kg/hour dose during the entire procedure. Bail-out doses were administered if necessary.
Prior to the thoracotomy the neuromuscular blocking agent atracurium (Tracrium, Glaxo SmithKline) was administered intravenously at a dose of 0.25 mg/kg. This dose was repeated after 30 minutes if necessary.
As an additional analgesic measure and prior to performing the thoracotomy, intercostal nerve block was achieved using bupivacaine at 0.5% (Bupivacaine, Braun) at a dose of 2 mg/kg in 5 sites: the intercostal space of the surgical site, 2 cranial spaces, and 2 spaces immediately caudal to this one.
The anticoagulant therapy used was sodium heparin at a dose of 200 IU/kg via IV access. The antiarrhythmic therapy used was an infusion of amiodarone (Trangorex, Sanofi-Aventis) at a dose of 5 mg/kg every hour.
Volume controlled ventilation was used during the entire procedure. The ventilator parameters used were: inspired oxygen fraction (0.4), tidal volume (10 mL/kg) by controlling maximal inspiratory pressure and adjusting respiratory rate based on the volume per minute and partial pressure of carbon dioxide, inspiratory/expiratory ratio (1:2-1:3) (based on arterial oxygenation and arterial pressures), inspiratory pause time (10%), and positive end-expiratory pressure of 4 that gradually went up to 8 after the thoracotomy. Alveolar recruitment maneuvers were performed every 20 minutes to avoid alveolar collapse and atelectases.
Vital signs were monitored every 10 minutes and arterial blood-gas tests were performed by measuring the ventilator parameters during the procedure.
During the immediate postoperative 1.6 mg/kg of furosemide (Seguril, Aventis) and 4 mg/kg of carprofen (Rimadyl, Pfizer) were administered via IV access. The postoperative analgesic agent used was transdermal fentanyl (Durogesic, Janssen) at a dose of 50 µg/h within the first 72 hours followed by buprenorphine (Buprex, Life) at a dose of 0.01 mg/kg via subcutaneous access every 8 hours for 3 days. Also, oral carprofen (Rimadyl) was administered at a dose of 4 mg/kg every 24 hours as anti-inflammatory therapy for 5 days followed by a 9-day course of oral amoxicillin-clavulanic acid (Synulox, Pfizer) at a dose of 20 mg/kg every 12 hours as antibiotic therapy.
The protocol to perform the magnetic resonance imaging included the administration, on the day of the procedure, of anesthetic premedication: a combination of midazolam (0.35 mg/kg, Midazolam Normon) and ketamine (5 mg/kg, Imalgene 1000) via intramuscular access. Once the correct sedation of the animals was confirmed, they were transferred to the preparation area and preoxygenation with oxygen mask at 100% concentration was started. Then, venoclysis was performed in the marginal atrial vein using a 20-gauge endovenous catheter. Propofol (2-4 mg/kg, Propovet) was used for the induction of anesthesia followed by conventional tracheal intubation. Sevoflurane (Sevorane) at 1-1.5 CAM was used as maintenance anesthesia.
Mechanical ventilation followed the same parameters as during the entire procedure with periodic monitoring of the vital signs and arterial blood-gas tests.
Inducing the infarction in the circumflex artery territory
After anesthetizing the animal, its thorax was opened, and the pericardium dissected to access the circumflex coronary artery and induce the infarction in this artery through surgical ligation. Prior to this an injection of contrast and echocardiographic study were used to see what branches of this artery were supplying the posteromedial papillary muscle. Once identified, ligation was attempted to occlude the 2 and 3 obtuse marginal arteries to avoid inducing a massive MR.
Creation of an arteriovenous shunt
After the infarction volume overload was attempted through the creation of an arteriovenous shunt by connecting a branch of the pulmonary artery to the aorta using a Dacron tube graft. This procedure was performed with clamping and without extracorporeal circulation.
After performing both procedures the thorax was closed, and the pig was transferred to its storage facility the for control and maintenance.
Follow-up
The presence of MR and the effect of cardiac remodeling were assessed through echocardiographic and magnetic resonance imaging 8 weeks after the procedure and through ventriculography during euthanasia.
The degree of MR was assessed with an ultrasound scan using semi-quantitative methods (estimation of color area, vena contracta). In these ultrasound and MRI studies the volumes of the cardiac chambers (right and left ventricular diameters and volumes, left atrial diameters and volumes) and their function were measured.
Anatomopathological study
At the 8-week follow-up, the animals were euthanized following the directives established by Royal Decree 53/2013 on animal protection.
A complete, organized, and systematic necropsy of each animal corpse was conducted to identify and diagnose any possible conditions associated with the procedure. The samples obtained were fixed in formaldehyde at 10% for histopathological study. In the macroscopic study of the heart, its weight was recorded and its cavities, walls, papillary muscles, mitral chordae tendinae, annulus, and valve leaflets analyzed. All the possible anomalies seen in these structures were documented photographically. Afterwards, the leaflets were extracted from their insertion location and up to their free borders including their chordae tendinae and they were fixed in formaldehyde at 10% and included in paraffin for histopathological study. Three µm thick serial sections were stained with the usual hematoxylin and eosin technique; the Van Gieson elastin stain protocol was used to study elastic and collagen fibers; the Masson trichrome stain protocol was used to differentiate muscular from collagen fibers; finally, the alcian-blue PAS staining protocol was used for the detection of mucopolysaccharides. The histopathological changes identified were semi-quantitatively assessed by establishing the different degrees of damage.
After collecting the leaflets to characterize the infarction, another 4 cross-sectional cuts were performed from the vertex of the heart towards its base. They were weighted and stained with triphenyltetrazolium chloride histochemical staining to enhance the viable area (red color) of the necrotic region (white color). For that purposes, the levels established were submerged in a solution of triphenyltetrazolium chloride (Sigma-Aldrich) at 1% in a phosphate buffered saline solution (pH 7.4) for 5 to 10 minutes at 37 °C, and then they were submerged in formaldehyde at 10%. The sections were photographed, and the areas measured using the Image J system. Samples of the infarction region, limit, and non-infarcted region were collected from every level, submerged in paraffin, and stained using the hematoxylin and eosin technique and the Masson trichrome stain protocol to characterize ischemic damage.
RESULTS
The first animal died during the procedure due to an unresponsive ventricular fibrillation; the remaining 2 completed the 8-week follow-up without complications.
Echocardiographic study
Animal #1
Echocardiographic data at baseline and at the 8-week follow-up from the parasternal short-axis and apical 4-chamber planes are shown on table 1. In the baseline study, ventricular thickness at anteroseptal level was 10.6 mm and at posteroinferior level, 9 mm. The mitral valve was morphologically normal with thin normally moving leaflets and no regurgitation on the color Doppler ultrasound. At the 8-week follow-up there were segmental alterations of contractility that were seriously hypokinetic in the 3 segments of the lateral side with hypercontractility of the remaining segments. Also, a mitral valve with a thickened posterior leaflet and low motility, and moderate mitral regurgitation in the form of posteriorly directed eccentric regurgitation jet was seen too.
Table 1. Echocardiographic data at baseline and at the 8-week follow-up (animal #1)
| LVEDD (mm) | LVESD (mm) | FS (%) | EF (%) | Color area (cm2) | Vena contracta (mm) | |
|---|---|---|---|---|---|---|
| Baseline | 46 | 30 | 34 | 62 | 0 | 0 |
| 8-week follow-up | 49 | 32 | 34 | 60 | 4 | 4 |
|
EF, ejection fraction; FS, fractional shortening; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter. |
||||||
Animal #2
Echocardiographic data at baseline and at the 8-week follow-up are shown on table 2. In the baseline study, ventricular thickness at anteroseptal level was 9 mm and at posteroinferior level, 6 mm. The mitral valve was morphologically normal with thin normally moving leaflets and no sign of regurgitation on the color Doppler ultrasound. At the 8-week follow-up, segmental alterations of contractility were seen in the medium and basal segments of the posterior side, a mitral valve with thickening of both leaflets, and moderate mitral regurgitation in the form of a mitral regurgitation central jet.
Table 2. Echocardiographic data at baseline and at the 8-week follow- (animal #2)
| LVEDD (mm) | LVESD (mm) | FS (%) | EF (%) | Color area (cm2) | Vena contracta (mm) | |
|---|---|---|---|---|---|---|
| Baseline | 44 | 28 | 36 | 65 | 0 | 0 |
| 8-week follow-up | 47 | 30 | 36 | 63 | 6 | 5 |
|
EF, ejection fraction; FS, fractional shortening; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter. |
||||||
Magnetic resonance imaging
The baseline study showed ventricles of normal dimensions, thickness, and also normal overall and segmental contractility for our cath lab in similar populations.
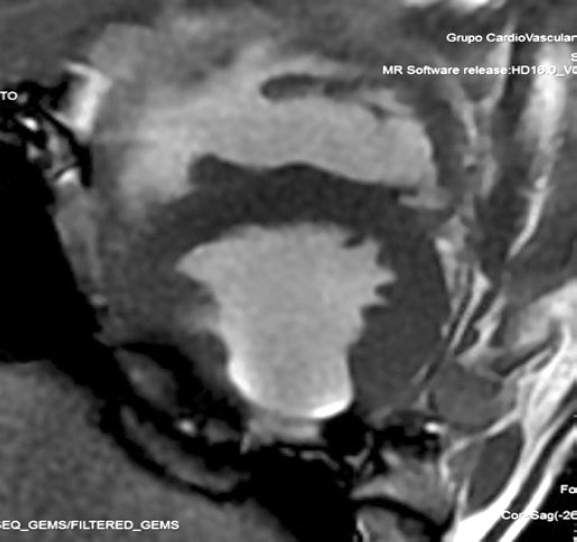
The 8-week follow-up revealed segmental alterations of contractility, lateral wall thinning, and fat transformation at posterior level in pig #1 and at posteroinferior level in pig #2 (figure 1). Ventricular volumes grew 10% and 7%, respectively.
Figure 1. Magnetic resonance imaging of animal #2. Short axis of the left ventricle. Presence of fat transformation at posterior level.
The values found in this study are shown on table 3 and table 4.
Table 3. MRI study at baseline and at the 8-week follow-up (animal #1)
| LVEDD (mm) | LVESD (mm) | LVEDV (ml) | LVESV (ml) | EF (%) | Left atrium (cm2) | |
|---|---|---|---|---|---|---|
| Baseline | 46 | 31 | 68 | 29 | 57 | 12 |
| 8-week follow-up | 49 | 32 | 75 | 30 | 60 | 14 |
|
EF, ejection fraction; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVESD, left ventricular end-systolic diameter; LVESV, left ventricular end-systolic volume. |
||||||
Table 4. MRI study at baseline and at the 8-week follow-up (animal #2)
| LVEDD (mm) | LVESD (mm) | LVEDV (ml) | LVESV (ml) | EF (%) | Left atrium (cm2) | |
|---|---|---|---|---|---|---|
| Baseline | 42 | 29 | 58 | 29 | 50 | 10 |
| 8-week follow-up | 44 | 31 | 62 | 30 | 52 | 11 |
|
EF, ejection fraction; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVESD, left ventricular end-systolic diameter; LVESV, left ventricular end-systolic volume. |
||||||
Anatomopathological study
Animal #1
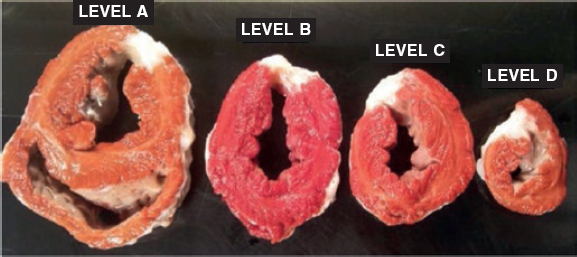
The macroscopic examination revealed an infarction region in the lateral side from apical to basal level. The use of triphenyltetrazolium chloride stain confirmed the occurrence of a transparietal infarction (figure 2) whose size is shown on table 1 of the supplementary data together with the weight of each level.
Figure 2. Animal #1. Presence of transparietal infarction areas from basal (A) to apical level (D). Triphenyltetrazolium chloride stain.
The mitral valve showed a thickened posterior leaflet without damage to the anterior one. Microscopically, the posterior leaflet showed focal thickening with increased deposition of mucopolysaccharides and vascularization of the proximal part with distal reduction in the number of vessels. The infarction regions were histologically characterized by the presence of mature connective tissue including islets of cardiac muscle fibers and inflammatory cells.
Animal #2
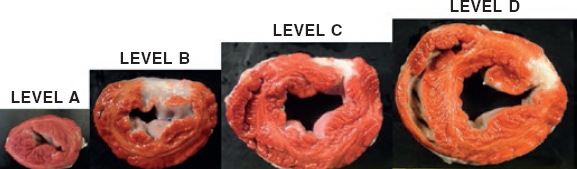
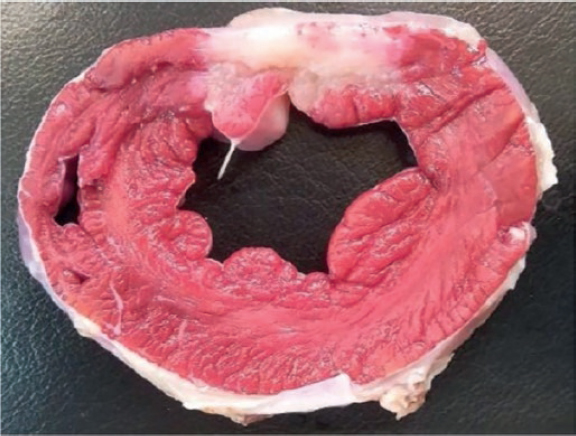
The macroscopic evaluation revealed the presence of a transparietal infarction region in the posterior side damaging the medium and basal segments (figure 3) and papillary muscle (figure 4). The spread of this lesion into the different levels is shown on table 2 of the supplementary data.
Figure 3. Animal #2. Presence of transparietal infarction areas from medium (B) to basal level (D). Triphenyltetrazolium chloride stain.
Figure 4. Papillary muscle lesion. Lower part of level C. Triphenyltetrazolium chloride stain.
In the macroscopic examination, the mitral valve showed thickened leaflets with hemorrhages in their atrial surface (figure 1A of the supplementary data). Histologically and added to the already mentioned hemorrhages, both leaflets appeared thickened due to the deposition of mucopolysaccharides, especially in the middle layer (figure 1B,C of the supplementary data). Small caliber vessels were seen together with a mild inflammatory response figure 2 of the supplementary data. The infarction regions were characterized by the presence of mature connective tissue including islets of cardiac muscle fibers with similar characteristics compared to animal #1.
DISCUSSION
Our group developed a safe and feasible experimental porcine model to induce ischemic MR after causing an infarction associated with volume overload by creating an aortopulmonary shunt.
Currently, several studies on experimental models (sheep and pigs, basically) have been conducted to induce and maintain MR. All of them have pros and cons and imitate different etiologies of MR such as dilated cardiomyopathy, ischemic MR, and even rupture of a mitral chorda tendinae.
In the model of ischemic MR, Llaneras et al.14 were able to induce MR in sheep through obtuse marginal artery ligation. The authors said that for this event to appear 2 prerequisites are required: a) the papillary muscle needs to be infarcted; b) the ventricle needs to be dilated. With just 1 of the 2 requirements no MR would be induced. In their results, the ligation of marginal arteries 2 and 3 induced a gradually developing MR. On the other hand, the ligation of marginal arteries 2, 3 plus the posterolateral artery led to the development of a massive MR with high lethality.
This model has been modified later on by inducing MR through the rupture of a mitral chordae tendinae and the association of an ischemic event in the territory of the circumflex artery by implanting an aneroid.10 This would induce an ischemic lesion with a dysfunctional papillary muscle and volume overload. However, the uncontrolled rupture of a mitral chorda tendinae may lead to a high mortality rate in animals when inducing massive MR, which is often poorly tolerated. The adverse events of the animals or if some of them died during the procedure was not reported in this study.
Considering the pros and cons of the models described, our objective was to create a sustainable model of ischemic MR that, according to the medical literature, seems to be reproducible. To that end, taking into consideration what has already been described in former studies, the model of ischemic damage to the posteromedial papillary muscle associated with volume overload seems to be the safest and most effective one. Since volume overload following the rupture of a chorda or the production of a major myocardial infarction can induce massive MR and severe deterioration of the animal, our objective was to create an arteriovenous shunt as a safe way to induce volume overload since former studies have proven that the creation of a systemic-to-pulmonary shunt induces biventricular remodeling.15
In our study we induced a small size acute myocardial infarction probably due to the isolated occlusion of the obtuse marginal arteries. Other authors occluded the marginal and posterolateral arteries too, which induced bigger acute myocardial infarctions, but at a price of a significantly higher mortality rate, which is why in our study we decided to occlude obtuse marginal arteries only.
Maybe the small size of the acute myocardial infarction was the cause for the moderate MR and discrete ventricular remodeling induced (10% and 7% increase in pigs #1 and #2) yet despite the segmental alterations of contractility seen. However, the possibility that an arteriovenous shunt of inadequate magnitude contributed to this cannot be discarded.
Finally, the possibility that in this model there is a mixed etiology for mitral regurgitation cannot be discarded either: the anatomopathological analyses revealed morphological anomalies in mitral leaflets, meaning that regurgitation would not be strictly functional only. This brings about new hypotheses on the repercussions of hemodynamic overload on mitral leaflets that may go beyond annular dilatation or the ischemic restriction of its movement.
Limitations
The limitations of our study are associated with its small sample size, which is a problem when trying to draw definitive conclusions. However, we think it is very useful to disclose this new experimental model to induce ischemic MR through coronary ligation and volume overload by the creation of an aortopulmonary shunt. However, the results should be confirmed in future studies.
Whether ventricular remodeling impacts the creation of the aortopulmonary shunt is still unknow. In light of this study results, future phases of this model should analyze whether the magnitude of the shunt truly impacts ventricular remodeling.
Infarcts created through surgical ligation of the circumflex artery were small. Maybe the implantation of a coil or other occlusion devices into the proximal circumflex artery would have induced bigger infarctions. In any case, the study design anticipated the surgical approach since a thoracotomy would be needed to create the arteriovenous shunt.
Another possible limitation may be the short period of time animals were followed (8 weeks). This may explain why the remodeling process after the acute myocardial infarction was not completed, which is the reason why ventricular volumes did not reach greater dimensions.
CONCLUSIONS
In our own early experience, the experimental model of chronic MR based on ischemic damage to the posteromedial papillary muscle and associated with volume overload is feasible, safe, and reproducible. It may be useful to assess the safety and efficacy profile of future devices for the management of this heart disease.
FUNDING
This study was partially funded through a grant from the Regional Healthcare Management of Castille and León, Spain (GRS1396_A_16).
CONFLICTS OF INTEREST
R. Estévez-Loureiro is a proctor for MitraClip and received a research grant from Abbott Vascular. A. Pérez de Prado participated and received funding for his consultancy job done for Boston Scientific and iVascular SL, and lectures given for Abbott, Braun Surgical, Terumo Medical Corporation, and Philips Volcano. The remaining authors declared no conflicts of interest whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- MR is the second most common valve disease. Mitral valve repair surgery is the standard treatment, but over 50% of the patients are not operated on due to their comorbidities.
- There are several devices available today to reduce the degree of regurgitation through transcatheter approaches. Also, there are several studies on experimental models to induce and maintain MR in order to test these devices. All of them have pros and cons and imitate different etiologies of MR such as dilated cardiomyopathy, ischemic MR, and even rupture of a mitral chorda tendinae.
- After studying the models already published, 2 are the prerequisites to induce a sustainable model of MR: ischemic lesion with damage to the papillary muscle, and ventricular dilatation.
WHAT DOES THIS STUDY ADD?
- A new experimental model to induce ischemic MR by combining the production of ischemic damage through the coronary occlusion of the branches supplying the papillary muscle and left ventricular volume overload with aortopulmonary sunt following the implantation of a Dacron tube graft between the aorta and a pulmonary branch.
- We should mention that none of the animals survived surgery and died at the follow-up, which is indicative of a feasible and safe model of ischemic MR.
REFERENCES
1. Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739-2791.
2. Mirabel M, Iung B, Baron G, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery?Eur Heart J. 2007;28:1358-1365.
3. Borger MA, Alam A, Murphy PM, Doenst T, David TE. Chronic ischemic mitral regurgitation:repair, replace or rethink?Ann Thorac Surg. 2006;81:1153-1161.
4. Jamieson WR, Edwards FH, Schwartz M, Bero JW, Clark RE, Grover FL. Risk stratification for cardiac valve replacement. National Cardiac Surgery. Ann Thorac Surg. 1999;67:943-951.
5. Herrmann HC, Maisano F. Transcatheter therapy of mitral regurgitation. Circulation. 2014;130:1712-1722.
6. Maisano F, Alfieri O, Banai S, et al. The future of transcatheter mitral valve interventions:competitive or complementary role of repair vs. replacement?Eur Heart J. 2015;36:1651-1659.
7. Mauri L, Foster E, Glower DD, et al. 4-year results of a randomized controlled trial of percutaneous repair versus surgery for mitral regurgitation. J Am Coll Cardiol. 2013;62:317-328.
8. Nickenig G, Estevez-Loureiro R, Franzen O, et al. Percutaneous Mitral Valve Edge-to-Edge Repair:In-Hospital Results and 1-Year Follow-Up of 628 Patients of the 2011-2012 Pilot European Sentinel Registry. J Am Coll Cardiol. 2014;64:875-84.
9. Maisano F, Franzen O, Baldus S, et al. Percutaneous mitral valve interventions in the real world:early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the MitraClip therapy in Europe. J Am Coll Cardiol. 2013;62:1052-1061.
10. Cui YC, Li K, Tian Y, et al. A pig model of ischemic mitral regurgitation induced by mitral chordae tendinae rupture and implantation of an ameroid constrictor. PloS One. 2014;9:e111689.
11. Minakawa M, Robb JD, Morital M, et al. A model of ischemic mitral regurgitation in pigs with three-dimensional echocardiographic assessment. J Heart Valve Dis. 2014;23:713-720.
12. Hamza O, Kiss A, Kramer AM, Tillmann KE, Podesser BK. Characterization of a novel percutaneous closed chest swine model of ischemic mitral regurgitation guided by contrast echocardiography. Eurointervention. 2019. pii:EIJ-D-19-00095.
13. Aguero J, Galan-Arriola C, Fernandez-Jimenez R, et al. Atrial Infarction and Ischemic Mitral Regurgitation Contribute to post-MI Remodeling of the Left Atrium. J Am Coll Cardiol. 2017;70:2878-2889.
14. Llaneras MR, Nance ML, Streicher JT, et al. Large animal model of ischemic mitral regurgitation. Ann Thorac Surg. 1994;57:432-439.
15. Pereda D, García-Lunar I, Sierra F, et al. Magnetic Resonance Characterization of Cardiac Adaptation and Myocardial Fibrosis in Pulmonary Hypertension Secondary to Systemic-To-Pulmonary Shunt. Circ Cardiovasc Imaging. 2016;9:e004566.