To the Editor,
Chronic heart failure (CHF) is the third leading cause of cardiovascular death in developed countries, and is mostly of ischemic etiology.1
Despite the optimal medical therapy based on the clinical practice guidelines, many patients remain symptomatic for whom different procedures have become available over the last few years to stop pathological ventricular remodeling.
The Revivent system (BioVentrix Inc., United States) is a hybrid ventricular reconstruction procedure that works by implanting micro-anchors via endovascular access and left mini thoracotomy to create a longitudinal plication of scar tissue without the need for mid sternotomy or extracorporeal circulation. Basically it is indicated in patients with ischemic heart disease and anterolateral or apical aneurysmal regions with transmural scar in both the left ventricle (LV) and right septum (RS)—according to the magnetic resonance imaging—to prevent muscle tears, and who still have persistent advanced CHF and NYHA functional class (FC) > III despite the optimal medical therapy.2 Biffi et al. confirmed an in-hospital mortality rate of 1.4%, and a 1-year survival rate of 90% in 203 patients.3 The STICH4 and RESTORE5 clinical trials revealed in-hospital mortality rates of 6% and 5.1%, respectively, and 18-month survival rates of 85% and 88%, respectively, after surgical therapy in patients with a similar profile.
The objective of this letter is to report on the clinical characteristics of the procedure and the 90-day results of the first 2 patients treated at our center. Both gave their informed consent prior to publishing their cases.
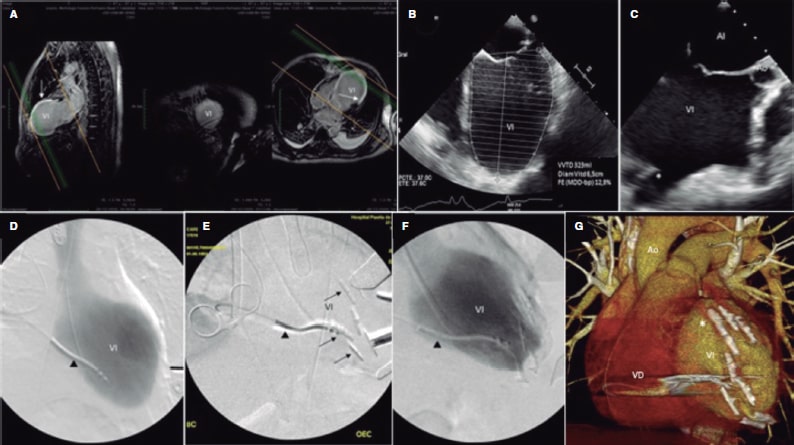
Patient no. 1 is a 67-year-old man without cardiovascular risk factors admitted with signs of anterolateral infarction with ST-segment elevation due to thrombotic occlusion in the left anterior descending coronary artery. Plain old balloon angioplasty was performed followed by dual drug-eluting stent implantation on a second stage. The patient was classified as NYHA FC II, and was on daily furosemide 180 mg, and eplerenone 50 mg. The echocardiogram revealed the presence of a dilated LV, a left ventricular ejection fraction (FEVI) of 12%, a large apical aneurysm, and severe pulmonary hypertension. The 60-day magnetic resonance imaging revealed the presence of akinesis in the anteroseptal, anterolateral, and apical segments without viability data (figure 1A). The pre-transplantation study performed anticipated unfavorable prognostic outcomes, which is why the Revivent therapy was proposed 6 months after the infarction.
Figure 1. A: preoperative magnetic resonance imaging. Two-chamber long axis view, and 3-chamber long axis view, ischemic scar (arrows). B: preoperative transesophageal echocardiography (TEE), 4-chamber view, LV end-diastolic volume. C: intraoperative TEE, 3-chamber view, remodeled LV chamber, first anchor. D: intraoperative fluoroscopy, ventriculography, implantable cardioverter-defibrillator leads (arrowhead). E: reconstruction anchors (arrows). F: ventriculography final outcomes. 3D CT scan reconstruction. G: Final outcomes (asterisks). Ao, aorta; LA, left atrium; LV, left ventricle; RV, right ventricle.
The procedure was transesophageal echocardiography (TEE) and fluoroscopy guided. The LV apex and anterolateral side were accessed via left mini thoracotomy. A 14-Fr introducer sheath was implanted via right jugular vein to advance a Swan-Ganz catheter. Afterwards, the EnSnare device with 3 interlaced loops (EnSnare Merit Medical Systems Inc., United States) was inserted until the RV. A transseptal guidewire was inserted from the LV through fluoroscopy and TEE guidance that was captured using the snare in the RV and then removed via jugular vein. This circuit is used to implant the first endocavitary anchor that, once cinched, allows the partial obliteration of the aneurysm. Ventricular reduction was completed by implanting 4 pairs of additional extracardiac anchors. The proper reduction and plication was confirmed via fluoroscopy and TEE (figure 1B,F).
After an immediate postoperative without complications the patient was discharged from the hospital on day 13 after the procedure and classified as NYHA FC I-II. After 3 months he was classified as NYHA FC I, which reduced the need for furosemide down to 20 mg/day. The control computed tomography (CT) scan is shown on figure 1G.
Patient no. 2 is a 50-year-old man with a past medical history of peripheral arterial vasculopathy and dilated cardiomyopathy of ischemic origin. He presented with residual LVEF after the infarction of 15% with a LV anterolateral aneurysm. The patient was classified as NYHA FC III and was on daily furosemide 180 mg, eplerenone 50 mg, and chlorthalidone 25 mg.
The procedure was performed in a similar way compared to the former case. No significant complications were reported, and the patient was discharged on day 20 of the postoperative period and classified as NYHA FC II that improved at 3 months (I-II), which is why the dose of furosemide was reduced to 120 mg/day. In both cases ventricular parameters improved (table 1).
Table 1. Volumes, ventricular diameters, and left ventricular ejection fraction before and after implantation
| Patient no. 1 | Patient no. 2 | |||||
|---|---|---|---|---|---|---|
| Before implantation | After implantation | Third month | Before implantation | After implantation | Third month | |
| LVEDV (mL) | 285 | 224 | 200 | 178 | 131 | 73 |
| LVESV (mL) | 227 | 161 | 139 | 150 | 108 | 54 |
| LVEDD (mm) | 70 | 63 | 65 | 68 | 53 | 50 |
| LVEF (%) | 12 | 28 | 28 | 15 | 20 | 26 |
|
LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume. |
||||||
These 2 patients are the first cases ever reported in the medical literature treated with the Revivent system in Spain. Cardiac surgeons and interventional cardiologists alike participated in the procedure. Although an initial learning curve is required, no complications were reported, and both the functional class, and the volumes improved. The 2 patients had long hospital stays, which were attributed to the management of hydroelectrolytic balance in patients with severe CHF. Since control echocardiograms were performed 3 months after surgery, it is anticipated that left ventricular end-diastolic volume (LVEDV) will be reduced even further.6
Randomized clinical trials are needed with a large number of patients to determine whether the Revivent system is an effective, safe, and long-lasting therapeutic option in patients with post-ischemic severe ventricular dilatation and dysfunction.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
J. E. De Villarreal: drafted the manuscript, processed, and edited the images, 1st, 2nd, and 3rd reviews; M. del Trigo: edited the manuscript 2nd review; C. Esteban Martín: edited the manuscript 1st review; J. Goicolea Ruigómez: edited the manuscript 2nd review; S. Mingo: collaborated to acquire the images and the echocardiography volumes; A. Forteza Gil: edited the manuscript 1st and 3rd reviews.
CONFLICTS OF INTEREST
None reported.
REFERENCES
1. Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020; 141:e139-e596.
2. Brinza C, Cinteza M, Popa IV, Burlacu A. Safety and efficacy of less-invasive ventricular enhancement procedure with the transcatheter Revivent TCTM system in patients with left ventricular aneurysm: a systematic review. Rev Cardiovasc Med. 2021;22:445-452.
3. Biffi M, Loforte A, Folesani G, et al. Hybrid transcatheter left ventricular reconstruction for the treatment of ischemic cardiomyopathy. Cardiovasc Diagn Ther. 2021;11:183-192.
4. Hassanabad A, MacQueen K, Ali I, et al. Surgical Treatment for Ischemic Heart Failure (STICH) trial: A review of outcomes. J Card Surg. 2019;34:1075-1082.
5. Athanasuleas C, Buckberg G, Stanley A, et al. Surgical ventricular restoration: the RESTORE Group experience. Heart Fail Rev. 2004;9:287-297.
6. Naar J, Skalský I, Kru“ger A, et al. Long-Term Results of Hybrid Left Ventricular Reconstruction in the Treatment of Ischemic Cardiomyopathy. J Cardiovasc Transl Res. 2021;14:1043-1050.