To the Editor,
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of the coronavirus disease 2019 (COVID-19) and is responsible for the current global outbreak. Although COVID-19 causes viral pneumonia mainly, alarms have gone off about its potential to damage the cardiovascular system with myocardial injury as a risk factor for mortality. Several are the potential causes of COVID-19 related myocardial injury including type I and type II myocardial infarction.1
This is the case report of an apparent spontaneous coronary artery dissection (SCAD) in a patient with COVID-19 (informed consent obtained) followed by a systematic review of the medical literature available.
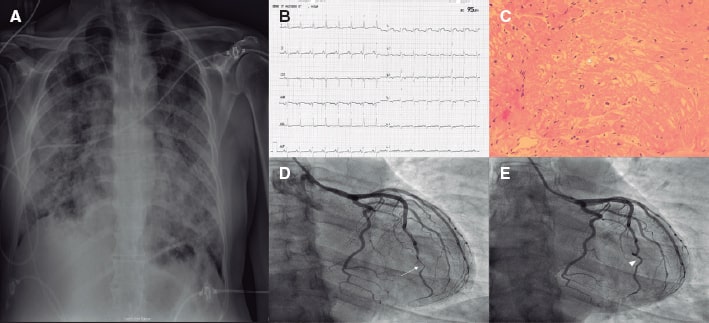
This is the case of a 40-year-old male without any known past medical history or cardiovascular risk factors who was admitted to our tertiary hospital with fever and cough. The patient tested positive in the reverse transcriptase-polymerase chain reaction test for SARS-CoV-2 infection, and the chest x-ray performed showed bilateral opacities (figure 1A). The patient was admitted to the intensive care unit due to a rapidly deteriorating clinical course within the first 72 hours despite initial supportive therapy that required early intubation. The laboratory work showed severe lymphopenia (0.5 × 103/L), troponin-T levels of 42 ng/dL, D-dimer levels > 10 000 ng/mL, CRP levels > 300 mg/dL, and ferritin levels > 3000 ng/mL. Although corticosteroids and remdesivir were administered, the patient’s hemodynamic status deteriorated with signs of acute respiratory distress syndrome and cardiogenic shock (CS). Concomitant inotropic and vasopressor support was initiated, and a transthoracic echocardiography performed revealed the presence of severe biventricular dysfunction with intraventricular thrombus without segmental wall motion abnormalities. After heart team discussion, a veno-arteriovenous extracorporeal membrane oxygenation (VAV-ECMO) was implanted. Within the first week after VAV-ECMO implantation a new electrocardiogram performed showed diffuse T-wave inversion in precordial leads (figure 1B). The patient’s successful progression allowed the withdrawal of VAV-ECMO, adrenergic drugs, and mechanical ventilation.
Figure 1. A: chest x-ray showing bilateral patchy infiltrates. B: electrocardiogram with diffuse T-wave inversion. C: endomyocardial biopsy without leukocyte infiltrate suggestive of myocarditis. Some freezing artifacts can be seen (asterisk). D: coronary angiogram, the arrow indicates the presence of significant stenosis of the distal portion of the LAD, which is compatible with a type II SCAD. E: follow-up with invasive coronary imaging showing the SCAD complete resolution (arrowhead).
Two months after the index event a cardiac magnetic resonance imaging performed discarded any signs of inflammation or fibrosis, a left ventricular ejection fraction of 35%, and resolution of the intraventricular thrombus. The endomyocardial biopsy performed tested negative for myocarditis (figure 1C). The coronary angiography performed (figure 1D,E; video 1 of the supplementary data) to discard concomitant coronary artery disease showed an isolated lesion of the distal segment in a tortuous left anterior descending coronary artery (LAD) that was compatible with a type II SCAD. Intravascular images were discarded after risk-benefit analysis, and conservative management was adopted. The patient was discharged and after an uneventful 3-month follow-up a control coronary angiography confirmed the complete resolution of the case.
Myocardial injury is a common finding in patients with COVID-19 and is associated with adverse events.1 Previous coronavirus and influenza viral infections may trigger or aggravate a wide range of major cardiovascular events though the mechanisms responsible are yet to be elucidated. SCAD is an underreported coronary event with an estimated prevalence around 4%. There are several possible triggers, but emotional and physical stressors are the ones most commonly reported.
A case of bilateral carotid artery dissection in a patient with SARS-CoV-2 infection has been reported before. Single case-control cohort studies have reported on a potential association between upper respiratory tract infections and carotid dissection;2 however, no conclusive study has been able to confirm whether infections are a predisposing condition for SCAD. Interestingly, 3 cases of SCAD and SARS-CoV-2 infection have been reported in the medical literature3-5 with different clinical course and disease severity (table 1). The potential mechanisms responsible for SCAD in patients with COVID-19 are still poorly understood, but they are probably not associated with a single factor as causality is a highly complex process.
Table 1. Systematic review of the medical literature on spontaneous coronary artery dissections in patients with COVID-19
| Authors | Journal/year | Age/sex | LVEF at admission | Previous predisposing factors* | Symptoms/signs at admission | COVID-19 severity | In-hospital treatment | Coronary angiogram | Management of SCAD |
|---|---|---|---|---|---|---|---|---|---|
| Courand P-Y et al.3 | JACC Cardiovasc Interv 2020 | 55/male | Preserved | Peripheral artery disease | Fever, dyspnea, and cough | Mild | Unreported | Mid-RCA dissection (confirmed on the OCT) | Conservative Aspirin, statins, and beta-blockers |
| Gasso LF et al.4 | Eur Heart J 2020 | 39/male | 50% to 55% | None | Fever, dyspnea, cough, chest pain, and myalgia | Severe | Hydroxycloroquine, azitromycin, lopinavir/ritonavir, tocilizumab | Multivessel dissection (no intracoronary imaging) | Conservative Dual antiplatelet therapy |
| Kumar K et al.5 | Catheter Cardio Int 2020 | 48/female | 45% to 55% | Migraine Dyslipidemia | Chest pain | Mild | Unreported | Mid-to-distal LAD dissection (confirmed on the computed tomography scan) | Conservative Dual antiplatelet therapy LifeVest, beta-blockers, and amiodarone after sustained PVT |
| Reported patient | 2020 | 40/male | Severe (< 30%) | None | Fever, and dyspnea | Severe (mixed shock) | Hydroxycloroquine, azitromycin, lopinavir/ritonavir, corticosteroids, remdisivir, inotropic and vasopressor agents, VAV-ECMO | Distal LAD dissection (no intracoronary imaging) | Conservative Aspirin, and guideline-directed medical therapy for HF The follow-up CA confirmed the complete resolution |
|
CA, coronary angiogram; COVID-19, coronavirus disease 2019; HF, heart failure; LAD, left anterior descending coronary artery; LEVF, left ventricular ejection fraction; OCT: optical computed tomography; PVT, polymorphic ventricular tachycardia; RCA, right coronary artery. * Prior to admission. |
|||||||||
SCAD has been associated with autoimmune and inflammatory diseases, which may be the result of eosinophilic infiltration with lytic enzyme secretion.6 The key of COVID-19 is an intense inflammatory burden and endothelial dysfunction.1 However, SCAD may be due to other contributing factors typically seen in critically ill patients. For instance, an overactive sympathetic system can cause intimal dissection. Also, SCAD could be the result of high-dose corticosteroid therapy, broadly used in COVID-19, due to the spontaneous rupture of a weakened arterial wall.6 Finally, a direct SARS-CoV-2 related endothelial damage cannot be discarded either.
Although SCAD is more common in females, 3 out of 4 cases reported are males, which could be explained by the higher incidence of COVID-19 reported in males. Chest pain is the most common symptom of SCAD and is present in 2 of the cases reported; however, the case reported by Courand PY et al. and our own case did not show specific symptoms of acute coronary syndrome.3 Whether SCAD is the cause of CS and severe ventricular dysfunction, in our case, is still under discussion. A previous unknown cardiomyopathy or transient ventricular dysfunction are possible causes since the SCAD was found in a distal small portion of the LAD. In any case, regardless of the severity of COVID-19, conservative management is a safe strategy. Morbidity is high as Kumar K et al. reported polymorphic ventricular tachycardia,5 and our patient presented with CS. We chose single antiplatelet therapy with aspirin, and guideline-directed medical therapy for heart failure like Courand PY et al. did,3 but different from the other 2 cases reported that chose dual antiplatelet therapy.4,5 It may raise concerns whether anticoagulation is a safe strategy as COVID-19 is associated with prothrombotic state.
In conclusion, SCAD is a potential cause of type II myocardial infarction in patients with COVID-19, but more studies are needed to establish causality. Infection-related SCAD may occur at any time during index events and could be difficult to diagnose. Conservative management seems like a safe strategy, although CS and ventricular arrhythmias can occur.
FUNDING
None.
AUTHORS' CONTRIBUTION
Á. Aparisi, C. Ybarra-Falcón, P.E. García-Granja, drafting the article or revising it critically for important intellectual content. All authors contributed substantially to the conception and design, and interpretation of data, and to the final approval of the version to be published.
CONFLICTS OF INTEREST
Nothing to declare.
SUPPLEMENTARY DATA
Vídeo 1. Aparisi A. DOI: 10.24875/RECICE.M20000185
REFERENCES
1. Sandoval Y, Januzzi JL, Jaffe AS. Cardiac Troponin for the Diagnosis and Risk-Stratification of Myocardial Injury in COVID-19:JACC Review Topic of the Week. J Am Coll Cardiol. 2020;76:1244-1258.
2. Morassi M, Bigni B, Cobelli M, Giudice L, BnàC, Vogrig A. Bilateral carotid artery dissection in a SARS-CoV-2 infected patient:causality or coincidence?J Neurol. 2020. http://dx.doi.org/10.1007/s00415-020-09984-0.
3. Courand P-Y, Harbaoui B, Bonnet M, Lantelme P. Spontaneous Coronary Artery Dissection in a Patient with COVID-19. Jacc Cardiovasc Interventions. 2020;13:e107-e108.
4. Gasso LF, Melon NMM, Cebada FS, Solis J, Tejada JG. Multivessel spontaneous coronary artery dissection presenting in a patient with severe acute SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41:3100-3101.
5. Kumar K, Vogt JC, Divanji PH, Cigarroa JE. Spontaneous coronary artery dissection of the left anterior descending artery in a patient with COVID?19 infection. Catheter Cardio Inte. 2021;87:E249-E252.
6. Krittanawong C, Kumar A, Johnson KW, et al. Conditions and Factors Associated with Spontaneous Coronary Artery Dissection (From a National Population-Based Cohort Study). Am J Cardiol. 2018;123:249-253.