To the Editor,
The anomalous origin of coronary arteries is a rare entity (overall rate < 1 for every 300 000 live births), and often appears concomitantly with other congenital heart defects.1 The anomalous origin of the left circumflex artery (LCx) from the right coronary artery (RCA), the right sinus of Valsalva or the separate origin of the left anterior descending coronary artery (LAD) and LCx all have been reported.2,3 However, an anomalous origin of the LCx from the pulmonary artery (PA) or its branches is extremely rare.4
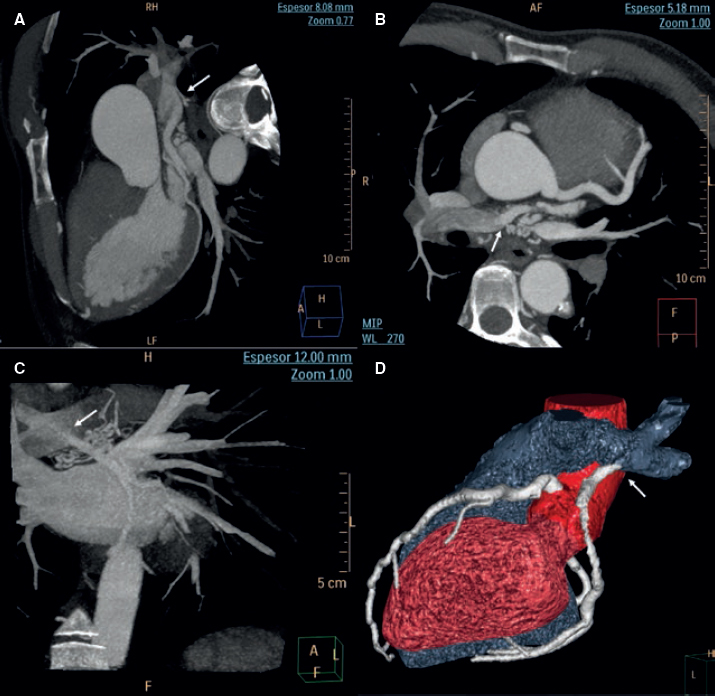
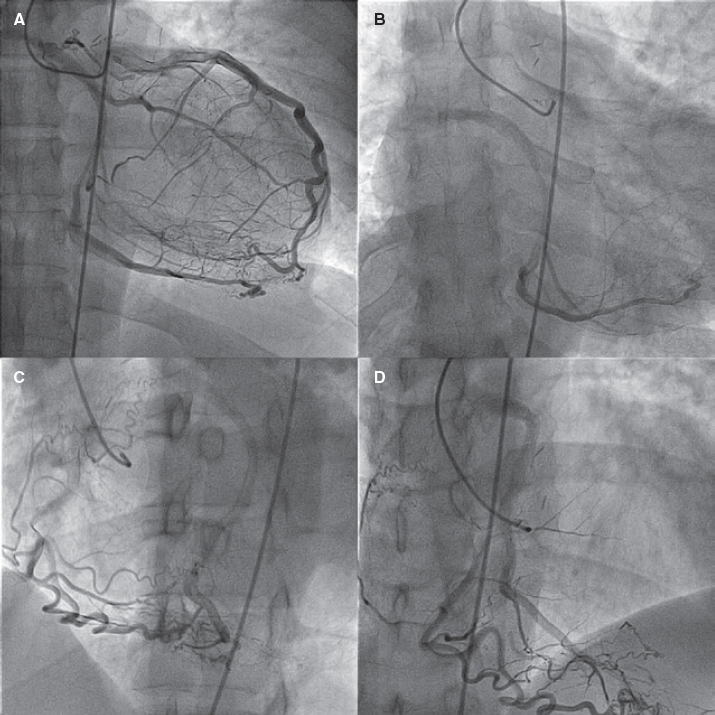
A 39-year-old male presented with palpitations and atypical chest pain of 12-hour duration in a context of vomits, diarrhea, and abdominal pain. Three weeks prior to admission he had reported the presence of catarrhal symptoms. Prior to the current admission the patient recounted several episodes of chest tightness while performing high-intensity physical exercise. The patient’s past medical history included a non-dysfunctional bicuspid aortic valve and aortic coarctation. Coarctation was initially corrected (1995) with a subclavian artery-to-descending aorta bypass graft. Ten years later he underwent further surgical repair using an interposition tube graft. A diagnosis of acute myocarditis was considered likely. Upon admission the electrocardiogram showed sinus rhythm with normal progression of R waves and no repolarization abnormalities. The creatine kinase myocardial band (CK-MB) and troponin I levels were positive. Other laboratory tests looked normal too. The echocardiogram showed normal left ventricular contractility, bicuspid aortic valve without stenosis or regurgitation, normal right ventricle, normal pulmonary pressure, and normal gradients in the left anterior descending coronary artery. The cardiovascular magnetic resonance (CMR) imaging performed confirmed the absence of myocardial edema or late gadolinium enhancement of the left ventricle. The coronary computed tomography angiography (CCTA) performed revealed the anomalous origin of the LCx from the right PA without coronary atherosclerosis (figure 1). The CCTA confirmed the presence of isolated coronary artery ectasia of the LAD originating from the left sinus, normal non-dominant RCA, and a dominant LCx originating from the right PA with retrograde filling receiving collateral circulation from both the LAD and the RCA (figure 2). The therapeutic options were discussed with the heart team and the patient who refused surgery. Therefore, medical therapy was the strategy of choice. Patient's informed consent to diagnostic tests and consent to publish were granted.
Figure 1. Maximum intensity projection images of coronary computed tomography angiography (A, B, C) and volume-rendering imaging (D) showing the anomalous origin of the left circumflex artery coming from the right pulmonary artery (arrow) with posterior trajectory.
Figure 2. Coronary angiography in the (A) anteroposterior, (B) caudal, (C) left anterior oblique and (D) cranial projections showing the independent origin of left anterior descending coronary artery that supplies collateral circulation and retrograde filling to the left circumflex artery that originates in the right pulmonary artery, and the right coronary artery of normal origin that supplies collateral circulation and retrograde filling to the left circumflex artery.
The anomalous origin of the LCx from PA or any of its branches is often accompanied by heart conditions like aortic coarctation, patent ductus arteriosus, Tetralogy of Fallot, aortopulmonary window, truncus arteriosus, subaortic fibrous membrane stenosis, ventricular septal defect, and pulmonary valve stenosis.1,2
Clinical presentation depends on age. In some cases it is symptomatic within the first week of life. In others, it is asymptomatic until adulthood or presents with sudden cardiac death (SCD).2 Depending on coronary collateralization, the clinical course may be silent.5 In our case, the diagnosis was an incidental finding despite having 2 aortic surgeries. The patient had no prior coronary angiograms as he had undergone surgery at 13 and 23 years old.
In patients who undergo a coronary angiography due to ischemic heart disease, this anomaly can be incidentally noted. Few adult cases have been reported with different presentations like new-onset angina, dyspnea, abnormal ischemic changes on the electrocardiogram, abnormal stress electrocardiography, nuclear scintigraphy or single-photon emission computed tomography.2,6 Our patient reported chest tightness while performing high-intensity physical exercise. Cardiac arrest has been reported as a very rare presentation in adults.1,4
SCD can occur after myocardial ischemia during exercise or ventricular arrhythmias triggered by ventricular scar tissue.4 Endothelial injury of the anomalous coronary artery with subsequent sudden coronary spasm or modification in the physiology of blood circulation have been proposed as probable pathophysiological mechanisms to explain ischemia and SCD in patients with coronary arteries of anomalous origins.3
The gold standard for diagnosis here is coronary angiography that allows good visualization of collateral vessels and the degree of shunting,1,5 and allows us to exclude concomitant atherosclerotic disease.6 CMR or CCTA imaging give us the correct anatomical location of the coronary origin. The CCTA has better spatial resolution, but the CMR can give us information on the direction of flow in an anomalous vessel, on myocardial viability and perfusion.1,3,5,6
Some criteria for surgical treatment are the presence of anginal symptoms, the ventricular area supplied by the artery, and homocoronary and/or heterocoronary collateral vessels.5 In this patient a conservative approach was decided due to the patient’s refusal to undergo surgical treatment and in view of the absence of clear signs of ischemia and late enhancement on the CMR. Surgical options can include the simple ligation of the anomalous vessel, reimplantation of the anomalous vessel into the aorta, a coronary artery bypass graft or transpulmonary artery aortocoronary reconnection.2 At the 12-month follow-up our patient has no cardiac symptom at all.
FUNDING
No funding was received for this work.
CONFLICTS OF INTEREST
The authors declare no conflicts related to this work.
REFERENCES
1. Aktas¸D, Erdem A, Çelik N, KamalıH, Sarıtas¸T. A rare coronary anomaly with masked diagnosis:Anomalous left circumflex artery from right pulmonary artery. Turk Kardiyol Dern Ars. 2015;43:551-553.
2. Danov V, Kornovski V, Hazarbasanov D, Panayotov P. Anomalous Origin of Left Circumflex Coronary Artery from the Right Pulmonary Artery in Adult. Thorac Cardiov Surg. 2009;57:110-118.
3. Schicchi N, Fogante M, Giuseppetti GM, Giovagnoni A. Diagnostic detection with cardiac tomography and resonance of extremely rare coronary anomaly:A case report and review of literature. World J Clin Cases. 2019;7:628-635.
4. Harky A, Bashir M, Garner M, Hsia TY. Anomalous origin of the circumflex coronary artery presenting with ventricular fibrillation cardiac arrest. BMJ Case Rep. 2017;2017:bcr2016219184.
5. Daylan A, Ertugay S, Apaydın AZ, Og˘uz E. Circumflex coronary artery originating from the right pulmonary artery in adult. Asian Cardiovasc Thorac Ann. 2017;25:528-530.
6. Korosoglou G, Ringwald G, Giannitsis E, Katus HA. Anomalous origin of the left circumflex coronary artery from the pulmonary artery. A very rare congenital anomaly in an adult patient diagnosed by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2008;10:4.