ABSTRACT
Vascular access is an essential part of all interventional procedures whether coronary or structural. Over the last 15 to 20 years, in coronary interventions, traditional femoral access has been mostly replaced by the radial approach. Nonetheless, the femoral approach through both artery and vein is still the main approach for structural heart procedures. Over the last few years, femoral access has evolved from a puncture guided by anatomical references to more accurate ultrasound-guided approaches. The relatively recent introduction of interventions such as transcatheter aortic valve replacement has conditioned the use of large introducers and ultimately the need for specific hemostatic systems, above all, percutaneous closure devices. This manuscript reviews different anatomical concepts, puncture techniques, diagnostic assessments, and closure strategies of the main arterial and venous approaches for the diagnosis and treatment of different structural heart procedures.
Keywords: TAVI. Vascular. Accesses. Structural.
RESUMEN
El acceso vascular es una parte esencial de cualquier procedimiento intervencionista coronario o estructural. En procedimientos coronarios, el acceso femoral tradicional prácticamente ha sido sustituido por el radial desde hace 15-20 años. No obstante, el acceso femoral, tanto arterial como venoso, sigue siendo la principal vía de abordaje para el intervencionismo estructural. El acceso femoral ha ido evolucionando con el paso del tiempo de una punción mediante referencias anatómicas a una punción mucho más precisa guiada por ecografía. La llegada de técnicas como el recambio valvular aórtico percutáneo ha condicionado el uso de introductores arteriales de gran tamaño y, por tanto, la necesidad de sistemas de control de la hemostasia, principalmente los sistemas percutáneos de cierre vascular. Este artículo revisa diversos conceptos anatómicos, técnicas de punción, evaluación diagnóstica y estrategias de cierre de las principales vías de acceso arterial y venoso utilizadas en el diagnóstico y el tratamiento de diferentes patologías estructurales.
Palabras clave: TAVI. Vascular. Accesos. Estructural.
Abbreviations CFA: common femoral artery. PDA: patent ductus arteriosus. TAVI: transcatheter aortic valve implantation.
ARTERIAL ACCESSES
Arterial puncture technique
Vascular access is an essential part of all interventional procedures whether coronary or structural. Over the last 15 to 20 years, traditional femoral access has been replaced by the radial approach in coronary interventions. However, the femoral approach is still the most widely used regarding structural hear procedures. Brachial, cubital, axillary or carotid accesses are also used, but to a lesser extent. Knowledge of anatomy and the puncture technique is essential. This is particularly relevant in accesses different from the radial/cubital one where the rate of complications is higher especially if large catheters and devices are used.
Femoral artery access
Common femoral artery (CFA) is the best puncture site because of its larger size and location on the femoral head favoring its palpation and compression view. The CFA is in the lateral femoral sheath, the common femoral vein is in the medial sheath while the femoral nerve rests outside the sheath, lateral to the artery. Distally, it can be divided into superficial and deep femoral arteries. High punctures above the inguinal ligament complicate arterial compression and trigger possible retroperitoneal bleeding. Low punctures in the superficial or deep femoral artery, however, increase the chances of pseudoaneurysm, hematoma or ischemia and arteriovenous fistula because, at that level, the vein and the artery often overlap, and can be crossed inadvertently.
There are 3 basic ways to catheterize the CFA:
1) Skin-based punctures
The most widely used in the past. Typically, here we’d be palpating the arterial beat 2-to-3 cm underneath the inguinal skin fold. Local anesthesia is administered followed by a needle using the modified Seldinger technique. Then, the anterior wall is punctured to prevent bleeding into the artery posterior region. Once pulsatile flow is obtained, the guidewire is inserted towards the abdominal aorta under fluoroscopy guidance. Alternatively, a micropuncture system can be used to open a smaller orifice (almost 60% smaller) with the potential to minimize complications. Afterwards, a 0.018 in guidewire is used followed by a 4-Fr introducer sheath through which a 0.035 in guidewire can be inserted. Skin-based punctures are not optimal if accuracy is what we’re after.
2) Based on radiographic references
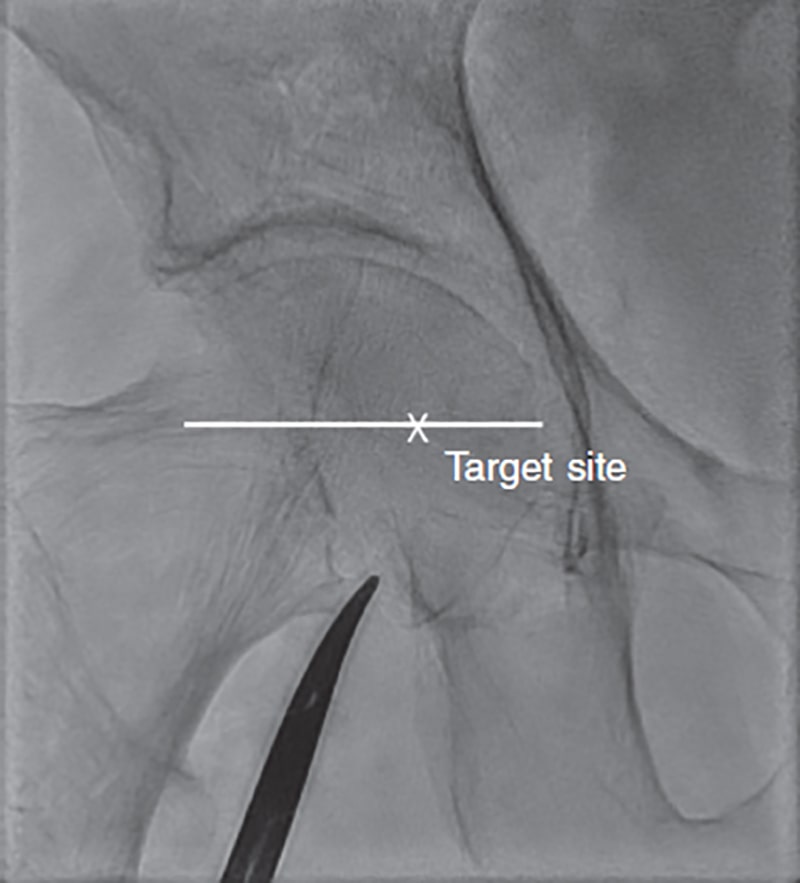
The femoral head is seen on the fluoroscopy and a radiopaque marker is placed in its inferior border as a height reference. If punctured with an inclined needle between 30º to 45°, this becomes be the perfect spot to insert the needle and try to pinch the artery halfway through the femoral head (figure 1). If punctured a little more vertically, the skin should be accessed a little more cranially. Inguinal ligament is often found 15 mm above the medial femoral head. In most patients, femoral artery bifurcations can often be found distal to the femoral head inferior side. That’s why the medial femoral head is the target here. If large caliber introducer sheaths should be needed like for transcatheter aortic valve implantation (TAVI), a variation of this technique should be used and contrast injected through an advanced catheter via a different arterial access towards the CFA to spot it correctly.
Figure 1. Radiographic references of punctures on the femoral artery.
3) Ultrasound-guided
Most interventional cardiologists trained over the last 15 years have limited experience with the femoral approach following the popularity gained by the radial access. Therefore, it seems logical somehow to think that femoral access training should be based on ultrasound guidance. An 8-to-12 MHz vascular linear probe is introduced into a sterile bag. 2D echo shows the CFA directly, its bifurcation, and the femoral head. The CFA should be assessed in the long axis from its bifurcation until it later enters the pelvis by measuring its caliber and assessing the presence of atheromatous plaques. The artery should be assessed in its short or cross-sectional axis that shows the typical «Mickey mouse head» look in the arterial bifurcation with the medial vein and the superficial femoral artery on the deep femoral artery, and the entire CFA cut section to assess which section is healthier by moving the transducer cranially (video 1 of the supplementary data). The vein looks different from the artery because it is much more compressible and for the direction and velocity of flow on the color Doppler echocardiography. Ultrasound-guided assessment allows us to select the arterial region with less calcium in the anterior wall. Infiltration with a local anesthetic at this spot and ultrasound-guided puncture—that reveals the entry of the needle in the center of the artery—facilitate the proper functioning of the closure systems. After introducing the 0.035 in guidewire, the ultrasound shows that the puncture site is the proper one since the guidewire is particularly echogenic and easily visible. This approach does not require the use of contrast or x-rays during puncture. In the FAUST trial1—a prospective multicenter trial that randomized 1004 patients to fluoroscopy or ultrasound-guided femoral access for TAVI—ultrasound-guided puncture was associated with a higher rate of success in the first attempt (83% vs 46%; P < .01), fewer attempts (1.3 vs 3.0; P < .01), less risk of venous puncture (2.4 vs 15.8; P < .01), shorter mean access times (136 vs 148 seconds; P < .01), and fewer access site related complications (1.4% vs 3.6%; P = .04).
The basic complications of arterial puncture in structural heart procedures (TAVI) are shown on table 1. Old age, feminine sex, low weight or obesity, peripheral vascular disease, kidney disease, hemorrhagic diathesis, baseline anticoagulation, and introducer sheaths of a larger size are associated with more complications.2 Although the rates of vascular complications were high in the past, they have dropped significantly over the last few years.2
Table 1. Main complications of femoral artery access
| Incidence rate % | |
|---|---|
| Hematoma | 2.2-12.5 |
| Retroperitoneal hemorrhage | 1-2.2 |
| Iliofemoral rupture | 0.7-7.1 |
| Pseudoaneurysm | 2-6 |
| Arterial dissection | 2-7.4 |
| Local infection | 1.6-6.3 |
ARTERIAL CLOSURE DEVICES
Arterial closure devices were introduced for the first time at the beginning of the 1990s. For arterial accesses with > 8-Fr introducer sheaths the closure devices available are suture-mediated or bioresorbable implantation-based. Table 2 describes the 3 large caliber vascular closure devices most widely used to this date.
Table 2. Main devices for percutaneous vascular closure
| Company | Name | Type | FDA indication | Characteristics |
|---|---|---|---|---|
| Abbot | Perclose Proglide | Suture-based | CFA accesses (5-Fr-to-21-Fr), vein (5-Fr-to-24-Fr) | Monofilament polypropylene suture with premounted knot Minimum residual intravascular material Keeps access guidewire No re-access restrictions Preclosure with 2 devices if > 8-Fr |
| Abbot | Prostar XL | Suture-based | CFA accesses (8.5-Fr-to-24-Fr) | 2 braided polyester sutures 4 nitinol needles Minimum residual intravascular material Keeps access guidewire Preclosure if > 10-Fr |
| Teleflex | Manta | Bioresorbable implantation | CFA accesses 10-Fr to-20-Fr devices | No need for preclosure Residual intravascular anchor |
|
CFA, common femoral artery; FDA, Food and Drug Administration. |
||||
Proglide
The Perclose/Proglide (Abbott Vascular, United States) is the most widely used suture-mediated device today as it is easier to use compared to the Prostar XL. It is inserted into the artery through a 0.035 in guidewire until pulsatile blood flow is seen through the lateral port. A lever releases feet inside the lumen that are pulling the artery anterior wall while releasing needles and creating a knot. The closure of the artery ties the knot. Introducer sheaths > 8-Fr require preclosure before inserting the introducer sheath following the steps already mentioned but sparing the knot tying, a maneuver that is performed at the end of the procedure. Regarding the suture-mediated device proper tunneling of subcutaneous cellular tissue is performed to make sure that the suture knot comes down. Overall, regarding TAVI procedures, preclosure is often performed using 2 devices that are released in different orientations (usually perpendicular) that are tied when the device is eventually removed resulting in an X-shape suture on the arterial surface.
Manta
The Manta device (Teleflex, United States) is available in 2 different sizes (14-Fr and 18-Fr) for arteriotomies of 10-Fr-to-14-Fr, and 15-Fr-to-20-Fr, respectively. After pinching the artery, its depth should be measured using a specific sheath. Although its performance is better in non-calcified arteries, some operators rather use it in calcified arteries because that’s where suture-mediated closure systems work worse. Closure device is mounted on a specific introducer sheath until a click sound is heard. Afterwards, the whole kit is removed until the previously measured depth and the intraarterial anchor is released using a lever. Device is pulled until a green-yellowish color can be seen in a tension indicator and, while keeping the tension, a blue cylinder is advanced that lowers a radiopaque closure and fixes the collagen material over the arterial surface. After checking hemostasis, the guidewire is removed (usually a 0.035 in high-support guidewire) and suture is cut. The anchor is then resorbed, and metal closure is useful to pinch > 2.5 cm above or below if re-accessing the artery is required.
PARTICULARITIES OF ARTERIAL ACCESS IN TAVI
TAVI has revolutionized the management of severe aortic stenosis turning into the treatment of choice for a great deal of patients. In TAVI selecting this or that access route conditions the results, which is why proper planning and selection is of paramount importance.
Results from the different studies published have proven unfavorable for transthoracic compared to transfemoral accesses, which is why the latter should always be prioritized.3 Also, the ongoing technological advances made, and the operators’ increased experience have reduced the rate of major vascular complications from > 10% in the early series down to < 3% over the last few years.4
Transfemoral access
Planning
In most cases, transfemoral access can be performed completely percutaneously under superficial sedation. To guarantee success, meticulous planning through coronary computed tomography angiography (CCTA) is essential and, ideally, volumetric reconstruction and analysis using specific software. Such analysis should assess, above all, the vessel minimum diameter from femoral bifurcation until the origin of the common iliac artery. A minimum of 5.5 mm for 14-Fr devices and 6 mm to 6.5 mm for the 18-Fr ones are required. However, expert operators can use accesses of smaller diameters for the lack of calcification in the 360° of the arterial wall. Tortuosities, the presence of calcified plaques, and the quality of distal beds should also be assessed. Similarly, the entire descending aorta should be studied considering transfemoral access as an entire entity from the femoral artery until the aortic annulus. When in doubt on the actual puncture site, performing an in-situ ultrasound often helps since the size of the vessel and quality of the arterial wall can be assessed very precisely. Therefore, we can have a severely diseased vessel where the ultrasound shows the presence of an area spared for puncture and posterior percutaneous closure.
Technical aspects
The puncture site extends 1 cm above the femoral bifurcation until the origin of the epigastric artery. Distally, the ideal thing to do is to get away from the bifurcation to prevent damaging the ostium of the deep femoral artery during puncture or closure. Also, to have enough space if a bailout covered stent should eventually be implanted. Proximally, puncture limit is set by the epigastric artery that—on its way down towards the anterior rectum muscle—marks the outside of the abdomen.
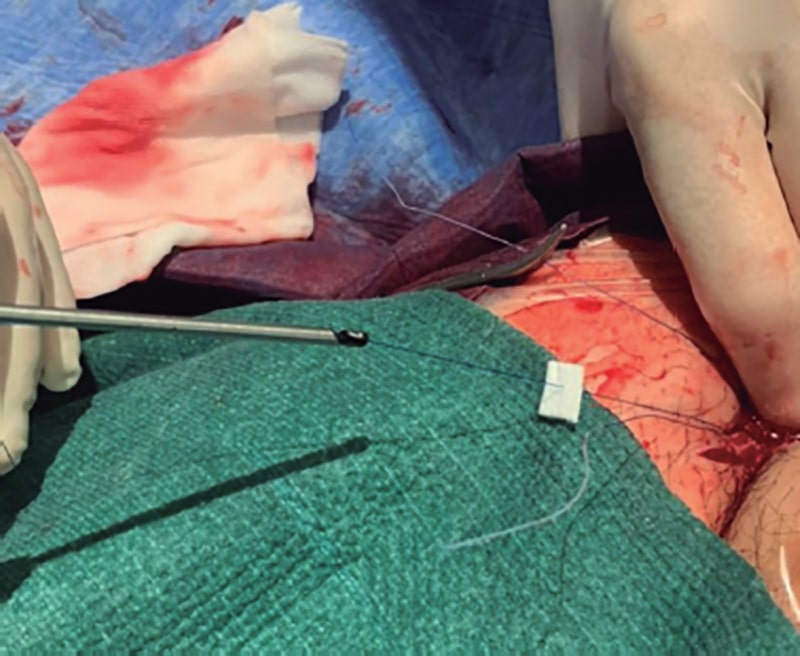
Ultrasound-guided puncture reduces the number of complicatios.1 The common femoral artery should be screened to select the segment with the lowest degree of calcification and smallest plaque especially in the anterior wall. The presence of anterior extensive calcification and eccentric plaques immediately proximal to the puncture site can be significant limitations to suture-mediated closure devices; in these cases, surgical approach can be considered (figure 2). Other alternatives like micropuncture or placing a pigtail catheter at the puncture site via contralateral femoral access are less common. Some centers place a safety guidewire anterogradely from the radial artery or via contralateral femoral access to perform an emergency occlusion with a balloon or implant a covered stent if closure fails; in these cases, balance between the potential benefit of the safety guidewire and the risk of vascular complications associated with a secondary femoral access should be observed.
Figure 2. Pull down of Teflon pledgets through a suture of the Proglide device.
If femoral access is achieved successfully, but there is stenosis at a more proximal level (external or common iliac artery) successive dilatations using sheaths of growing sizes or balloon dilatations can be considered. In some cases, intravascular lithotripsy can be useful. In the presence of severe tortuosities, a very high-support guidewire can be used (Lunderquist, Cook Medical, United States).
The 3 most common closure modalities are a) 2 suture-based closure devices (Proglide) in a twirl motion (one at the 11 o’clock position and the other one at the 1 o’clock position); b) 1 suture-mediate device and 1 collagen device (AngioSeal, Terumo, Japan); and c) 1 collagen closure device (Manta). The use of suture-mediated closure devices has a greater learning curve. However, it is associated with fewer serious complications or open bailout surgeries. Occasionally, more than 2 devices for complete closure are required.5 If closure system fails—with suture—keeping the guidewire inside the artery facilitates placing new devices (1 or several Proglide devices with different rotation or a new collagen-based device). If the early failing device is a collagen-based device and a bailout parallel guidewire hasn’t been left in place we won’t have a guidewire meaning that the fastest solutions available will be stent-graft implantation (stent covered with a membrane to prevent bleeding) or a call to the surgery unit for bailout surgical closure. Otherwise, in case of unfailed closure with residual bleeding, half the dose of protamine can be added followed by prolonged compression. Also, it is very effective to pull down small Teflon pledgets through the Proglide suture (figure 2). Protamine at full doses can trigger the thrombosis of the arterial system, which is why half the dose of sodium heparin administered is advised. At the end of the procedure, some operators perform control CCTAs via secondary arterial access. However, for the lack of external bleeding, the lack of complications and presence of distal flow can be confirmed on an ultrasound. Regarding secondary access for angiographies while the valve is being placed, some centers select the contralateral femoral access. However, if possible, radial access should always be prioritized since it is associated with a lower risk of bleeding and vascular complications.6
Transaxillary access
Axillary/subclavian access should not be used as the priority access in patients with good transfemoral access. Although some studies have shown good results, the Spanish registry showed a higher rate of complications compared to transfemoral access.7 Therefore, it is considered as the alternative access of choice when the transfemoral one is not good enough.
Overall, left access is the preferred one because it does not share a common origin with the carotid artery, and access looks more like the femoral one because of the greater curvature of the aorta and perpendicularity to the annular view. However, right access is barely used as it’s spared for patients with patent left internal mammary artery graft or severe stenosis of the left subclavian artery. Horizontalization of the annular view is ill-advised (> 30º to 45°) since access often occurs through the aorta lesser curvature side and is misaligned with the valve plane.
Same as it happens with transfemoral access planning using CCTA is essential to assess the presence of calcifications, stenosis, and minimum caliber, especially at the origin of the subclavian artery for being the region more prone to atherosclerosis. We should also mention the subclavian artery different histological make-up including a tunica media with more elastic fibers and a thinner adventitia layer compared to the femoral one that includes a tunica media with smooth muscle cells and a thicker and more fibrous adventitia layer.8 These characteristics turn the subclavian into a fragile artery that is more prone to ruptures or dissections. Access is often attempted using surgical techniques although percutaneous access has been reported in different series.9 The introducer sheath should not be advanced too much leaving, at least, 5 cm until the valve plane for the correct deployment of the prosthesis. In some cases, a Dacron tube graft can be sutured proximally to the artery and distally to the introducer sheath. Using the percutaneous technique, a radial-femoral loop should be created before accessing to place an occlusion balloon in case of bleeding or during the device exchange.
Transcarotid access
It’s considered as an alternative access of choice in patients without suitable transfemoral accesses in some experienced centers. Although the risk of stroke is similar to that of transfemoral approach, the main risk here is damage to peripheral nerves like the facial or recurrent laryngeal nerves—branch of the vagus nerve—complications reported in up to 2.2% of the cases.10,11
Technique that should be used here is basically surgery, and the right side is often used by performing a small 5 cm incision along the anterior border of the sternocleidomastoid muscle. Afterwards, the muscle needs to be retracted for direct exposure and puncture of the artery. Proximal clamp can be used to confirm the presence of collateral circulation and there is often continuous cerebral monitorization during the procedure. Same as it happens with subclavian access, the introducer sheath should not be advanced too much leaving enough space for the correct deployment of the valve.
Transcaval access
Transcaval access has been recently developed for alternative vascular access in percutaneous coronary interventions. Goal here is to prevent the morbidity associated with transthoracic access and add the advantages associated with venous transfemoral access (almost no complications at femoral level and the possibility of conscious sedation). However, this access requires the capacity to perform punctures from the vena cava towards the abdominal aorta through the retroperitoneum and then advance the introducer sheath and the TAVI release system. This step requires meticulous preoperative planning with CCTA. Transcaval access is feasible because the interstitial hydrostatic pressure of retroperitoneal space exceeds venous pressure, which is why the blood that comes out of the abdominal aorta during the procedure returns to venous circulation without accumulating in the retroperitoneum. On the other hand, the abdominal aorta entry zone should not have calcifications to advance the material properly and effectively close this cavoaortic shunt at the end of the procedure using an Amplatzer occluder device (often a VSD Occluder, Abbott Vascular).
The main data on transcaval access come from a multicenter, prospective registry of 100 patients.12 This registry confirmed a rate of procedural success of 99%, yet rates of potentially fatal hemorrhages and vascular complications of 7% and 13%, respectively. Therefore, to this date, its use is basically marginal.
Direct aortic surgical access
Direct aortic surgical access requires general anesthesia and was developed as an alternative to transapical access to overcome the complications and myocardial damage associated with apical access. It requires partial upper sternotomy towards the second or third right intercostal space. It is barely used today.
Particularities of arterial access in other structural heart procedures
Paravalvular leaks
Paravalvular leak closure is probably one of the most complex techniques out there and with greater heterogenicity among operators. Paravalvular leaks can be divided into aortic and mitral.
Aortic paravalvular leaks
Access is basically retrograde (aorta-ventricle). Therefore, arterial puncture is essential. In most cases, an Amplatzer Vascular Plug 3 device (AVP3) (Abbott Vascular) is implanted. These devices require introducer sheaths between 6-Fr and 7-Fr. The Amplatzer Vascular Plug 4 device is suitable for smaller leaks because it can be advanced through a 4-Fr diagnostic catheter. These procedures can be performed via radial access13 although most operators rather use the femoral artery to prevent the risk of spasm in cases where significant catheter manipulation is required. Overall, the use of a high-support guidewire in the left ventricle is enough to provide support to advance the delivery catheters. In this sense, high-support guidewires like the ones used in TAVI procedures are advised to prevent left ventricular perforations. Otherwise, the creation of an arteriovenous loop may be required and even an arterioarterial prosthetic loop (especially useful in leaks over self-expanding TAVIs).14 In both cases, additional specific venous or arterial accesses will be needed. Finally, we should mention that despite the limited size of introducer sheaths, the use of vascular closure systems is advised since we’re mostly dealing with patients with mechanical valves who, therefore, need to restart anticoagulation early.
Mitral paravalvular leaks
Most operators use antegrade access (left atrium-ventricle) through a femoral vein and via transseptal access. To perform this technique, it is essential to use good 3D transesophageal echocardiography imaging support plus a catheter with flexion capabilities to guide the latter over the origin of the leak. In most cases, placing a high-support guidewire inside the left ventricle makes the creation of an arteriovenous loop unnecessary (that would require arterial access). Therefore, in most cases, 1 single venous access is often enough. The devices used are often the same ones used in aortic leaks and the necessary catheters have the same size. We should mention that occasionaly mitral leaks require multiple device implantation. If simultaneous implantation of 2 or more devices is required, it may be necessary to perform as many venous accesses as devices will be eventually implanted. Alternatively, mitral paravalvular leaks can be crossed retrogradely (ventricle-left atrium). This technique requires manipulating catheters inside the left ventricle with the corresponding high risk of arrhythmia. It can be an alternative when antegrade crossing becomes complicated, and is easier in posterior compared to anterior leaks (since the guidewire always moves through the aorta). Obviously, this technique arterial puncture and the creation of an arteriovenous loop at left atrium level.
Coarctation of aorta
The percutaneous treatment of choice of coarctation of aorta is stenting. Therefore, large size arterial access is required (10-Fr-to-14-Fr) often via femoral access. The caliber of the femoral introducer sheath depends on the selection of balloons and stents that will eventually be used. Stents can be covered or not. Covered stents are often used for complex coarctations like those with complete obstructions or critical stenoses with risk of rupture, those associated with aneurysms, pseudoaneurysms, ductus arteriosus or diseased wall (bicuspid Valve, Turner). In elderly patients the use of covered stents can cover dissections or ruptures. Apart from the risk of proximal branch occlusion, the setback of using covered stents is that these need sheaths that should be > 3-Fr compared than the ones needed for the balloon. Overall, the sheaths should be 2-Fr-to-3-Fr larger than the minimum size required by the balloon to give the stent enough space to move freely inside.
Regarding femoral arterial access, we should not forget that the arterial vasculature of patients with coarctation of aorta has a smaller than normal diameter in the lower limbs. Also, an additional radial arterial access can be useful for visualization purposes, as well as the angiography during the procedure. Also, to cross critical coarctations or complete occlusions. In rare cases, carotid access can be necessary to reach the descending aorta (neonates, critical stenoses).15
Regarding closure, since the sheath is often 12-Fr-to-14Fr, vascular closure with the aforementioned specific devices or else delayed manual compression after heparinization has been reversed is often advised.16
Closure of the ductus arteriosus
To perform the closure of ductus arteriosus, the femoral vein and artery are often catheterized. Left and right heart catheterization is advised to register pulmonary and systemic pressures, which is why it is reasonable to use venous access using a 7-Fr introducer sheath. Large sized occlusion devices for ductus arteriosus are also compatible with 7-Fr and often implanted through the venous side, which is why an early 5-Fr arterial access can be planned—often via femoral access—thus, the need for an arteriovenous loop can be anticipated. However, it can also be performed via radial access with a potential reduction of vascular complications. When femoral venous access is not possible (femoral bilateral occlusion or the inferior vena cava) and access underneath the right atrium is preferred (as in the case of the percutaneous closure of ductus arteriosus or interatrial septum defects), the use of other access routes like the transhepatic one have been described.17
Regarding the caliber of vascular accesses, we should take into consideration both the technique selected and the type and size of device used. There are 2 different percutaneous treatment options available regarding persistent ductus arteriosus: coils or occlusion devices. If we’re dealing with a small ductus (< 4 mm) 1 or several controlled-release coils compatible with small sized catheters (4-Fr) and even microcatheters can be used. For larger ductus, occlusion devices are preferred. They are all self-expandable nitinol coils compatible with 5-Fr-to-7-Fr introducer sheaths depending on their size.
Catheterization of ductus arteriosus is performed via antegrade access (from the pulmonary artery) or retrogradely (from the aorta). If so, an arteriovenous loop is required. In both cases, the device introducer sheath is inserted through the antegrade venous side from where it is implanted. Regarding closure, since these are not large caliber accesses—the largest one being via venous access—manual compression is often performed.
VENOUS ACCESS
Ultrasound-guided venous puncture technique
Transfemoral venous access is the most widely used to perform non-TAVI percutaneous structural heart procedures. Right heart chambers can be accessed via femoral vein. Left heart chambers, however, are accessed through transseptal punctures.
Traditionally, venous puncture has been performed using anatomy-guided references. Experienced operators achieve reasonable rates of success through this method. However, there is a non-negligible chance of complications like inadvertent arterial puncture, venoarterial fistula, pneumothorax (in the internal jugular venous access), nervous lesion or multiple failed catheterization attempts. The risk and the consequences of these complications depend on the type of patients treated. Risk factors like obesity, cachexia, previous radiotherapy or previous surgical scars, among others, can impact the success of catheterization and the appearance of complications.18
The safest technique for venous catheterization is ultrasound guided. To identify the vein that should be punctured and establish its association with the accompanying artery pressure with the ultrasound probe should be exerted in such a way that the vein—not the artery—will often collapse (see sections above).
There are 2 techniques available to perform ultrasound-guided venous punctures: the cross-sectional approach (out-of-plane) and the longitudinal one (in-plane).19 Both have advantages and disadvantages. The former allows us to see, in the same view, the adjacent structures we should avoid during puncture. However, with this approach it is more difficult to see the tip of the puncture needle. Therefore, the angle of the probe should be adjusted to make the views of needle and probe meet. The latter allows us to follow the trajectory of the needle since it first enters the skin until it contacts the target vein. However, the adjacent structures—above all the accompanying artery—cannot be seen in the same view. The target vein can be better seen using the Valsalva maneuver.
Percutaneous closure devices via venous access
Traditionally, venous puncture wound hemostasis has been performed through prolonged manual compression followed by the application of compressive bandage. With the use of larger introducer sheaths to perform structural heart procedures—above all in femoral venous access—safer and more effective methods to achieve hemostasis are under way.
Figure-of-eight subcutaneous suture technique
This technique consists of passing a subcutaneous suture proximally and cross-sectionally to the entry of the venous introducer sheath. Afterwards, the opposite side is crossed, and a subcutaneous suture is performed distally to the sheath entry. Suturing creates a skin and subcutaneous cellular tissue-cinching effect by exerting pressure on the femoral vein. This technique is complemented with mild compressive bandage. A modified technique has been described by performing the subcutaneous suture longitudinally—not cross-sectionally—to the trajectory of the vein looking to minimize the possibility of inadvertent puncture of the vein.20
Vascular closure devices
Angioseal has been used via femoral venous access with up to 8-Fr sheaths with good results.21 The use of percutaneous suture devices like the Proglide has proven safe and effective in the femoral venous access with sheaths of up to 24-Fr.22 Implantation technique is the same as the one used in the artery (see sections above). Depending on the result of the closure, it can be combined with the subcutaneous «figure-of-eight» suture in cases when early hemostasis is not complete. It is often completed with mild compressive bandage.
Particularities of percutaneous mitral valve repair
The most widely used percutaneous coronary intervention on the mitral valve is the so-called «edge-to-edge» repair using the MitraClip (Abbott Vascular, United States) or Pascal devices (Edwards Lifesciences; United States). However, there are direct annuloplasty devices available that replicate a similar repair compared to the surgical one. Also, other transcatheter mitral valve repair options are being developed—some of them completely percutaneous—with good results.
The most widely used vascular access regarding percutaneous coronary interventions on the mitral valve is the femoral vein given its caliber, accessibility, and how easy it is to close after the procedure has been completed. Selecting the left or the right femoral vein depends on the patient’s clinical circumstances (having 2 accesses available, previously operated vascular disease in either one of the 2 accesses, etc.), and the operator’s preference regarding implantation. Therefore, the most widely used access is the right femoral vein that is more comfortable for the operator and uses less radiation.
The possibility of performing implantation via right jugular access has been reported. However, only anecdotal cases have been published due to the difficulties associated with femoral access like the presence of an occluded filter in the inferior vena cava or very sharp angulations of venous iliofemoral axis.23 Technically, implantation is more difficult and has multiple considerations although puncture is basically the same as the routine one.
Ultrasound-guided venous puncture limits its possible complications and should be generalized. In most cases, preclosure devices are implanted before starting the procedure. After inserting a high-support guidewire until the superior vena cava (or inferior if access is jugular) access dissection with forceps is attempted followed by access predilatations with different caliber dilators. Then, the guide catheter is advanced until it reaches the right atrium. Its advance is less complicated compared to the arterial access and with less resistance too. The caliber of the MitraClip guide catheter can be up to 24-Fr—22-Fr with the Pascal device for valve implantation (mitral or aortic in the mitral position)—varies depending on the type of device that should be implanted.
Particularities of tricuspid valve interventional procedures
Percutaneous coronary interventions of the tricuspid valve have evolved over the last decade acting efficiently on leaflet coaptation with suture or ring annuloplasties and eventually with orthotopic or heterotopic percutaneous valve repair.24
The access most widely used is venous access via femoral or jugular vein or both. Depending on the type of procedure used, an additional venous or arterial access or should be attempted—preferably radial—given its lower rate of vascular complications.
Currently, the most widely used device to treat tricuspid regurgitation is the TriClip device (Abbott Vascular) since it was awarded the CE marking back in 2020. With the development of the specific TriClip device—that has a specific wheel to distance itself from the interatrial septum—the right or left venous access does not affect implantation. Therefore, most operators use the right femoral vein as the access route.25
Regarding the implantation of orthotopic or heterotopic valves, access of choice is right femoral access with different calibers depending on the device that should be used (between 14-Fr and 30-Fr).
Particularities of interventional pulmonology procedures
Percutaneous coronary interventions on the pulmonary valve or artery always require a highly variable venous access in its diameter depending on the technique that should be used. Access via femoral vein is the most common one. Therefore, increasing the size of introducer sheaths is not such a big deal as it is the case with arterial accesses.
Percutaneous pulmonary valve implantation requires the use of 16-Fr-to-22-Fr introducer sheaths depending on the model. In many cases, high-support guidewires are required to rectify curvatures in the trajectory of right heart chambers.
Acute treatment of pulmonary thromboembolic disease requires the use of thrombus extraction systems. Since the main determinant of the system that should be used is the size of target thrombus and since the pulmonary artery can accommodate large thrombi, some systems required large caliber accesses. For example, the Penumbra system (Penumbra Inc, United States) can navigate through 8-Fr, Nautilus system (iVascular, Spain) through 10-Fr, and the Flowtriever system (Inari Medical Inc, United States) through 16-Fr-to-24 Fr.
In other cases of more distal thrombectomy or pulmonary angioplasty in chronic thromboembolic disease much smaller introducer sheaths are required (6-Fr-to-7-Fr). Guide catheter extension systems (catheters inside catheters) can be very useful in some cases of difficult access and may require larger introducers sheaths.
Particularities of the left atrial appendage closure
Percutaneous closure of left atrial appendage is often performed via femoral venous access. Some operators perform an additional arterial access to monitor arterial pressure invasively. This arterial access should be performed via radial access to reduce hemorrhagic complications. In some cases, the guidewire is performed with intracardiac ultrasound guidance. In this case, an additional venous femoral access is required.
A key aspect to select the caliber of the venous introducer sheath through which the device should be implanted is the type and size of the introducer sheath. The most widely used devices in our setting are the Amplatzer/Amulet (Abbott Vascular), the Watchman (Boston Scientific, United States), and the Lambre (Lifetech, China).26 Size of introducer sheaths goes from 8-Fr-to-10-Fr (with Lambre) up to 12-Fr-to-14-Fr (with Amulet and Watchman Flx).
Regarding vascular closure, most operators still use manual compression or «figure-of-eight» suture for venous access. However, the aforementioned vascular closure devices can also be used.
Particularities of venous access in other structural heart procedures
There are other structural heart procedures that require venous access, mainly via femoral vein. Some of the most prevalent ones are the closure of patent foramen ovale and interatrial communications. In some cases, these are intracardiac ultrasound-guided procedures, meaning that they require an additional venous femoral access. There are several devices manufactured by different companies to close these entities. However, the most common ones are the Amplatzer PFO occluder, the Amplatzer ASD occluder, and Gore devices, above all, the Gore Cardioform device (WL Gore & Associates, United States). In case of the Amplatzer devices, 8-Fr-to-12-Fr introducer sheaths are required. The Gore system requires short 11-Fr introducer sheaths that are already pre-mounted on a delivery sheath. Overall, the rate of vascular complications is low since these are often young patients who require 1 single femoral venous access only. In case of inferior vena cava occlusion, jugular vein implantation has been reported.27
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
All the authors contributed to the manuscript draft and critical review.
CONFLICTS OF INTEREST
X. Freixa is a proctor for Abbott Medical. R. Romaguera is an associate editor of REC: Interventional Cardiology; the journal’s editorial procedure to ensure impartial handling of the manuscript has been followed. Also, he is a proctor for Boston Scientific and has received conference fees from Medtronic. R. Trillo is a proctor for Medtronic and Boston Scientific. A. Jurado-Román has received conference fees from Boston Scientific.
SUPPLEMENTARY DATA
Vídeo 1. Freixa X. DOI: 10.24875/RECICE.M22000331
REFERENCES
1. Seto AH, Abu-Fadel MS, Sparling JM, et al. Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (Femoral Arterial Access With Ultrasound Trial). JACC Cardiovasc Interv. 2010;3:751-758.
2. Toggweiler S, Leipsic J, Binder RK, et al. Management of vascular access in transcatheter aortic valve replacement: part 2: Vascular complications. JACC Cardiovasc Interv. 2013;6:767-776
3. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016;374:1609-1620.
4. Forrest JK, Deeb GM, Yakubov SJ, et al. 2-Year Outcomes After Transcatheter Versus Surgical Aortic Valve Replacement in Low-Risk Patients. J Am Coll Cardiol. 2022;79:882-896.
5. van Wiechen MP, Tchetche D, Ooms JF, et al. Suture- or Plug-Based Large-Bore Arteriotomy Closure: A Pilot Randomized Controlled Trial. JACC Cardiovasc Interv. 2021;14:149-157.
6. Junquera L, Urena M, Latib A, et al. Comparison of Transfemoral Versus Transradial Secondary Access in Transcatheter Aortic Valve Replacement. Circ Cardiovasc Interv. 2020;13:e008609.
7. Jimenez-Quevedo P, Nombela-Franco L, Munoz-Garcia E, et al. Early clinical outcomes after transaxillary versus transfemoral TAVI. Data from the Spanish TAVI registry. Rev Esp Cardiol. 2021;75:479-487.
8. Schafer U, Ho Y, Frerker C, et al. Direct percutaneous access technique for transaxillary transcatheter aortic valve implantation: “the Hamburg Sankt Georg approach”. JACC Cardiovasc Interv. 2012;5:477-486.
9. Amat-Santos IJ, Santos-Martinez S, Conradi L, et al. Transaxillary transcatheter ACURATE neo aortic valve implantation - The TRANSAX multicenter study. Catheter Cardiovasc Interv. 2021;98:E291-E298.
10. Hameed I, Oakley CT, Hameed NUF, et al. Alternate accesses for transcatheter aortic valve replacement: A network meta-analysis. J Card Surg. 2021;36:4308-4319.
11. Panagides V, Kalavrouziotis D, Dumont E, et al. Cranial nerve injury during transcarotid transcatheter aortic valve replacement. Int J Cardiol. 2022;353:46-48.
12. Greenbaum AB, Babaliaros VC, Chen MY, et al. Transcaval Access and Closure for Transcatheter Aortic Valve Replacement: A Prospective Investigation. J Am Coll Cardiol. 2017;69:511-521.
13. Giacchi G, Freixa X, Hernández-Enríquez M, et al. Minimally Invasive Transradial Percutaneous Closure of Aortic Paravalvular Leaks: Following the Steps of Percutaneous Coronary Intervention. Can J Cardiol. 2016;32:1575.e17-e19.
14. Estévez-Loureiro R, Benito-González T, Gualis J, et al. Percutaneous paravalvular leak closure after CoreValve transcatheter aortic valve implantation using an arterio-arterial loop. J Thorac Dis. 2017;9:E103-E108.
15. Singh HS, Benson LN, Osten M, Horlick E. Cardiac Catheterization in Adult Congenital Heart Disease. En: Gatzoulis MA, Webb Piers GD, Daubeney EF. Management of Adult Congenital Heart Disease. 3rd edition. Elsevier; 2018. ISBN: 978-0-7020-6929-1.
16. Salinas P, Sánchez-Recalde A, Galeote G, et al. Intervencionismo percutáneo sobre coartación aórtica en el paciente adulto. En: Martín-Moreiras J, Cruz-González I. Manual de Hemodinámica e Intervencionismo Cardiaco. Marbán; 2014. ISBN: 978-84-7101-904-0.
17. Ebeid MR. Transhepatic vascular access for diagnostic and interven- tional procedures: Techniques, outcome and complications. Catheter Cardiovasc Interv. 2007;69:594–606.
18. National Institute for Health and Care Excellence. Guidance on the use of ultrasound locating devices for placing central venous catheters. NICE. 2002. Available online https://www.nice.org.uk/guidance/ta49/resources/guidance-on-the-use-of-ultrasound-locating-devices-for-placing-central-venous-catheters-pdf-2294585518021. Accessed 1 Jun 2022.
19. Privitera D, Mazzone A, Pierotti F, et al. Ultrasound-guided peripheral intravenous catheters insertion in patient with difficult vascular access: Short axis/out-of-plane versus long axis/in-plane, a randomized controlled trial. J Vasc Access. 2021. https://doi.org/10.1177/11297298211006996.
20. Wyss CA, Anliker O, Gämperli O, et al. Closure of Large Percutaneous Femoral Venous Access Using a Modified “Figure-of-Eight” Suture. Innovations (Phila). 2018 Mar/Apr;13(2):147-151
21. Coto HA. Closure of the femoral vein puncture site after transcatheter procedures using Angio-Seal. Catheter Cardiovasc Interv. 2002;55:16-19.
22. Geis NA, Pleger ST, Chorianopoulos E, et al. Feasibility and clinical benefit of a suture-mediated closure device for femoral vein access after percutaneous edge-to-edge mitral valve repair. EuroIntervention. 2015;10:1346-1353.
23. Yap J, Chen S, Smith TWR, at al. Transjugular mitral valve repair with the MitraClip: A step-by-step guide. Catheter Cardiovasc Interv. 2020;96:699-705.
24. Asmarats L, Puri R, Latib A, Navia JL, Rodés-Cabau J. Transcatheter Tricuspid Valve Interventions: Landscape, Challenges, and Future Directions. J Am Coll Cardiol. 2018;71:2935-2956.
25. Moñivas V, Li P, Sanchis R et al. Tratamiento percutáneo de la insuficiencia tricuspídea. Procedimiento detallado guiado por imagen con MitraClip. REC Interv Cardiol. 2020;2:118-128.
26. Romaguera R, Ojeda S, Cruz-González I, Moreno R. Spanish Cardiac Catheterization and Coronary Intervention Registry. 30th Official Report of the Interventional Cardiology Association of the Spanish Society of Cardiology (1990-2020) in the year of the COVID-19 pandemic. Rev Esp Cardiol. 2021;74:1095-1105.
27. Qintar M, Villablanca P, Lee J, et al. Patent foramen ovale closure with vena cava thrombus: You need an arm and a neck! Clin Case Rep. 2021;9:e03884.