ABSTRACT
Introduction and objectives: Vascular complications remain a potential problem after transcatheter aortic valve implantation (TAVI). Although suture-based vascular closure devices are most often used for vascular closure purposes, alternative plug-based vascular closure devices like the MANTA (Essential Medical Inc., United States) stand as a bail-out option for patients with failed suture-based closure devices. Since knowing the exact vessel depth is essential to use this device correctly before inserting the large introducer, we aimed to validate 2 different measurement techniques including preoperative multidetector computer tomography (MDCT) plus an alternative technique with the Angio-Seal device (Terumo Medical Corporation, United States) compared to a vendor specific measuring tool.
Methods: In patients eligible for TAVI, the depth of the femoral artery was measured preoperatively using MDCT, and then perioperatively with the Angio-Seal device. Both measurements were associated with the actual depth after puncture using the vendor-specific tool of the MANTA device.
Results: In a total of 168 patients treated with transfemoral TAVI, the depth of the vessel was measured both pre and perioperatively. The measurements obtained from the preoperative MDCT revealed the existence of a moderate correlation compared to the preoperative measurements obtained (r = 0.64; P < .001). Measurements obtained with the Angio-Seal device revealed a high correlation with the measuring tool included (r = 0.99; P < .001). Overall, 10 patients required the bail-out option with the MANTA device due to failed suture-based vascular closure devices.
Conclusions: In case of a failed suture-based vascular closure device after TAVI, the plug-based MANTA device can be used as a bail-out strategy. However, the measurement of the vessel depth obtained from preoperative MDCTs is not accurate enough for safe MANTA insertions. Measurements with the Angio-Seal device before inserting the large TAVI sheath stand as a simple solution to obtain exact measurements facilitating the use of the bail-out MANTA in case of failed suture-based closure vascular devices after TAVI.
Keywords: TAVI. Transfemoral. Access site complication. Vascular closure.
RESUMEN
Introducción y objetivos: Entre las potenciales complicaciones del implante percutáneo de válvula aórtica (TAVI) se encuentran las complicaciones vasculares. Los dispositivos de sutura son los más utilizados para el cierre vascular, pero algunos sistemas de cierre con colágeno (MANTA, Essential Medical Inc., Estados Unidos) ofrecen una solución de rescate cuando los de sutura fallan. Para la correcta implantación de este dispositivo es necesario conocer la profundidad exacta de la arteria femoral antes de la inserción del introductor del TAVI. El objetivo de este estudio fue validar 2 técnicas diferentes de medida, la tomografía computarizada con multidetector (TCMD) y una técnica alternativa que emplea el dispositivo Angio-Seal (Terumo Medical Corporation, Estados Unidos), en comparación con el sistema específico de medida del dispositivo MANTA.
Métodos: En pacientes que recibieron TAVI, se midió la profundidad de la arteria femoral mediante TCMD antes y durante el procedimiento con un dispositivo Angio-Seal. Ambas medidas se correlacionaron con la real obtenida tras la punción mediante el medidor del dispositivo MANTA.
Resultados: En 168 pacientes a quienes se realizó TAVI transfemoral, se midió la profundidad de la arteria femoral antes y durante el procedimiento. La medida con TCMD previa al procedimiento mostró una correlación moderada con las medidas durante el procedimiento (r = 0,64; p < 0,001). La medida con el dispositivo Angio-Seal mostró una alta correlación con la herramienta de medición (r = 0,99; p < 0,001). En total, 10 pacientes necesitaron rescate con dispositivo MANTA por fracaso de los dispositivos de sutura.
Conclusiones: En caso de fracaso de los dispositivos de sutura tras TAVI, el dispositivo de tapón de colágeno MANTA puede actuar como técnica de rescate. Sin embargo, la medida antes del procedimiento obtenida con TCMD no es precisa para implantar correctamente el dispositivo MANTA. La medición con un dispositivo Angio-Seal antes de la inserción del introductor del TAVI puede ser una solución sencilla para conocer las medidas con exactitud y para la inserción de rescate de un dispositivo MANTA, cuando fracasan los dispositivos de cierre por sutura.
Palabras clave: TAVI. Transfemoral. Complicaciones en punto de acceso. Cierre vascular.
Abbreviations MDCT: multidetector computer tomography. TAVI: transcatheter aortic valve implantation.
INTRODUCTION
Despite growing experience and the development of new closure devices, the rates of vascular complications after transcatheter aortic valve implantation (TAVI) remain high (between 5% and 18%).1-6 Recently, a new collagen plug-based device was recently introduced. Favorable results have been reported regarding the rate of vascular complications associated with this new collagen plug-based MANTA vascular closure device (Essential Medical Inc., United States) compared to suture-based devices.7-9 However, due to several potential disadvantages (including major bleeding events with rates that go from 1% to 16%), limited data on future vessel accessibility, and significantly higher costs, the routine use of the new device has been put into question compared to suture-based devices.7,10,11 Since puncture sites can safely be closed using suture-based devices, the new generation of plug-based systems may, therefore, be a valuable alternative as a bail-out strategy in case of failed suture-based devices.
However, one drawback of the MANTA system as a bail-out device is that it requires to know exactly the distance between the skin incision and the vessel for safe deployment and functionality purposes before inserting the large introducer sheath. Unfortunately, the vendor-specific measuring tool is not wrapped separately. Therefore, we aimed to validate 2 alternative measuring techniques including the preoperative multidetector computer tomography (MDCT), and the Angio-Seal device before inserting the large introducer sheath to get significant information of the depth of the vessel without having to unwrap the device.
METHODS
Patient and procedural characteristics
Patients agreed to the data retrospective anonymized analysis. A total of 168 consecutive patients with severe aortic stenosis scheduled for TAVI were included. All patients were evaluated by interdisciplinary heart teams. As a standard procedure all patients received MDCT to plan TAVI. All procedures were performed under local anesthesia. In all the cases both femoral arteries were used. One side for the TAVI sheath and the other for the pigtail catheter for the angiography plus a 7-Fr arterial line for hemodynamic monitoring. Routine closure follows with a Proglide closure device and a 6-Fr Angio-Seal device for the TAVI side plus a 6-Fr Angio-Seal for the contralateral side.
Measuring the depth of the vessel
Preoperatively, the depth of the vessel was measured using preoperative MDCT. The depth of the vessel was measured on a split screen using the Picture Archiving and Communication System imaging modality. In all the patients the measurements were obtained perpendicularly at skin level towards the femoral artery at mid-femoral head level (figure 1).
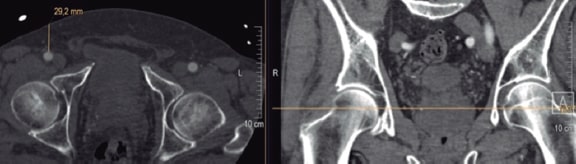
Figure 1. Measuring technique of the vessel depth at mid-hip head level (25 mm + 10 mm = 35 mm MANTA depth).
Perioperatively, measurements were obtained using the introducer sheath of a 6-Fr Angio-Seal device as follows. The introducer sheath reaches the vessel when bleeding through the indicator channel of the introducer sheath starts (figure 2A). In this position the letter or dot on the outside of the introducer sheath is noted and translated into distance using the schematic representation shown on figure 2B and table 1. According to the instructions for use of the MANTA vascular closure device, 1 cm had to be added. Then, the deployment depth of the MANTA device was noticed.12
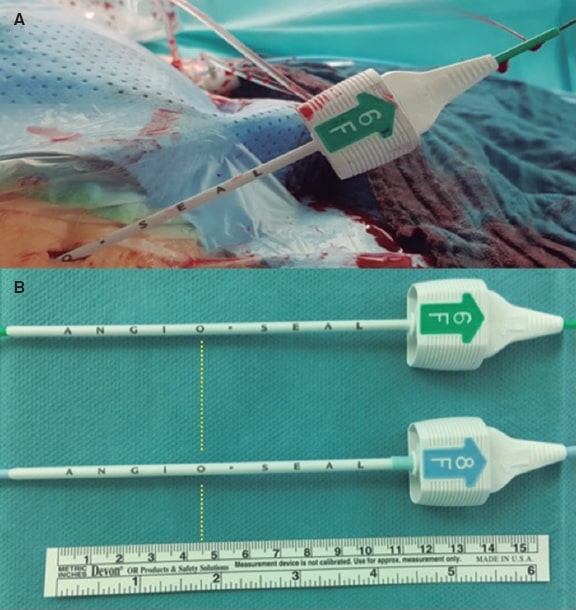
Figure 2. A: Measurement of the vessel depth equal to ‘O’. B: Translation into distance using the scheme shown on table 1 eventually ending in a MANTA depth of 5.5 cm.
Following these precautionary measures, 1 suture-based Proglide closure device was inserted and TAVI was performed as usual.
Table 1. Vessel depth measured using the Angio-Seal device. Eventually, 1 cm had to be added to be able to use the MANTA bail-out device
| Angio-Seal | Vessel depth (cm) |
|---|---|
| A | 0.5 |
| N | 1.5 |
| G | 2.5 |
| I | 3.5 |
| O | 4.5 |
| * | 5.5 |
| S | 6.5 |
| E | 7.5 |
| A | 8.5 |
| L | 9.5 |
Vascular closure
During closure, operators aimed for systolic blood pressures < 160 mmHg. Heparin, and protamine were used at the operator’s discretion. The delivery sheath was removed with a standard on-site wire while the access site was closed using the prepared Proglide system plus an additional 6-Fr Angio-Seal device. If the prepared suture-based system ruptures or in the presence of remaining severe bleeding following the insertion of the 8-Fr Angio-Seal introducer sheath, the introducer of the Angio-Seal was removed without implanting the plug, a MANTA vascular closure device was inserted, and then released based on the predefined vessel depth. The wire was removed after the final angiography to identify all possible access site-related complications. The contralateral side was closed using a 6-Fr Angio-Seal device.
A 300 mg clopidogrel loading dose was administered postoperatively, but not in patients already on clopidogrel. In patients on oral anticoagulants, therapy was interrupted prior to the procedure. All operators were familiar with all the vascular closure devices used.
Postoperative follow up
All patients received compression bandage at the puncture site for 6 hours and were monitored on an intermediate care unit for, at least, 24 hours. All the medical attention provided to the puncture site due to residual bleeding, and all postoperative imaging such as MDCT scan or duplex sonography were documented until hospital discharge.
Statistical analysis
The categorical variables were expressed as counts (percentages) while the continuous variables were expressed as median [interquartile range]. The correlation between measurements was estimated using Spearman’s rank correlation coefficient.
The data supporting this study findings are available from the corresponding author upon reasonable request.
RESULTS
A total of 168 patients treated with transfemoral TAVI were included. Patients were typical TAVI patients. The patients’ baseline characteristics are shown on table 2.
Table 2. Baseline characteristics
| Clinical characteristics | N = 168 |
|---|---|
| Age (years) | 83 [79.3-86.0] |
| Gender (male) (%) | 66 (39.0%) |
| Body mass index (kg/m2) | 26.9 [24.2-30.5] |
| NYHA ≥ III (%) | 144 (85.2) |
| Logistic EuroSCORE I (%) | 16.8 [12.2-22.8] |
| Arterial hypertension (%) | 152 (89.9%) |
| Coronary artery disease (%) | 113 (66.8%) |
| s/p PCI (%) | 62 (36.6%) |
| s/p CABG (%) | 17 (10.1) |
| Atrial fibrillation (%) | 67 (39.6) |
| Pulmonary hypertension (%) | 12 (7.1%) |
| Diabetes mellitus (%) | 59 (34.9%) |
| Peripheral artery disease (%) | 29 (17.1%) |
| COPD (%) | 34 (20.1%) |
| s/p stroke (%) | 17 (10.1%) |
CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; PCI, percutaneous coronary intervention; NYHA, New York Heart Association; s/p, status post. | |
Measurements from preoperative MDCTs were obtained from all patients revealing a moderate correlation compared to perioperative measurements obtained using the vendor-specific MANTA mea- suring tool (r = 0.64; P < .001; figure 3).
+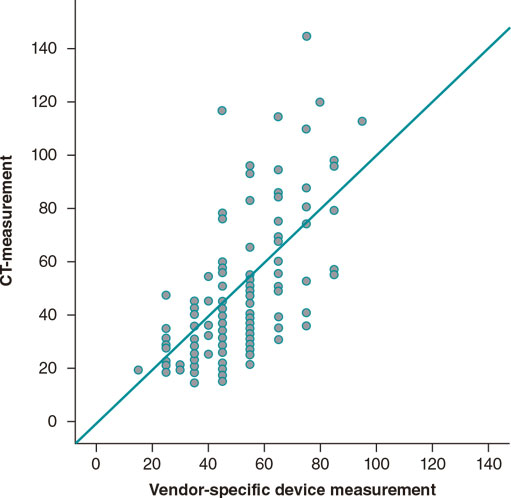
Figure 3. Correlation between the depth of the vessel measured on the previous multidetector computer tomography and on the vendor-specific measuring tool. CT, computed tomography.
Measurements using the Angio-Seal device were also successfully obtained from all patients revealing a high correlation with the vendor-specific MANTA measuring tool (r=0.99; P < .001).
Successful vascular closure was achieved in 158 cases using as a standard closure including the suture-based Proglide and the 6-Fr Angio-Seal device. The bail-out MANTA device was required in 10 patients (5.9%) due to Proglide suture rupture (n = 5) or remaining severe bleeding after insertion of the Angio-Seal introducer sheath (n = 5). The MANTA closure device was successfully inserted thanks to the measurements obtained with the Angio-Seal device before large sheath insertion. A total of 40 patients (23%) needed medical attention on the puncture site including prolonged pressure bandage or CT scan or duplex sonography. A total of 7 patients (4.1%) required postoperative surgery at the puncture site. One patient already carried a previous MANTA bail-out device that eventually led to a complete vascular occlusion due to known arterial occlusive disease. A total of 4 patients suffered a pseudoaneurysm after the routine use of the Proglide and the Angio-Seal that could not be treated by thrombin injection.
DISCUSSION
Although the rate of access site-related vascular complications after TAVI has decreased over the last few years, these complications are still associated with higher mortality and morbidity rates.2,3,6,13 In this context, suture-based closure devices such as the Prostar or the Proglide are safe and effective widely used tools.14 Although the use of the Prostar is associated with a lower risk of vascular stenosis, the use of the Proglide device has led to lower rates of adverse events such as device malfunction or residual bleeding.2,13,15,16 However, both systems rely on a similar suture-based technique that demands careful preparation before the large delivery sheath can be inserted. The safe use of these closure devices after the insertion of large delivery sheaths is not possible anymore. Therefore, in case of closure device failure and severe bleeding, covered stent implantation using the cross-over technique or surgery to achieve hemostasis may be the only option left. Although the implantation of a covered stent graft is an effective treatment option for bleeding control, implanting covered stents using the cross-over technique can be challenging. Also, the external iliac and common femoral arteries are exposed to flexion of the hip joint, which may be associated with higher stent compression and fractures.17-19 Additionally, the costs of covered stents are high.
Surgery should be spared as the last resort option only as it often needs to be performed under general anesthesia and the loss of blood is high until the surgical cut-down is prepared. Furthermore, wound infection or lymphatic fistulae may occur, thus delaying the patient’s mobilization after TAVI, which may be associated with pneumonia or thrombosis.
Recently, a new plug-based closure device, the MANTA vascular closure device, has entered the clinical arena, and proved its efficacy and safety profile after TAVI. The first reports show rapid hemostasis and low rates of complications after implantation of the MANTA device, even lower compared to the Prostar and the Proglide vascular closure devices.9 In contrast with this, a recently published randomized clinical trial showed similar results regarding access site bleedings compared to the Proglide system. However, while suture-based closure required additional closure devices more often like the Proglide or the Angio-Seal, the MANTA closure device numerically required complex maneuvers more often like covered stents or surgical bail-out strategies. The reason behind this may be the crossing of the wire through the toggle, which cannot be re-accessed using additional devices like the Angio-Seal or the Proglide.20
The considerably higher costs involved, 4 times more expensive compared to the Proglide, the unknown influence on the femoral artery wall, and re-access after device implantation have delayed the quick market penetration of this device as well as its routine use.
However, when using the MANTA vascular closure device, it is of utmost importance to measure the distance between the skin and the vessel accurately to ensure the precise placement of the anchor. During a scheduled MANTA procedure, this measurement is routinely obtained before the insertion of the large delivery sheath using a dedicated 8-Fr device that comes together with the MANTA device in the sterile package. This measurement may be cumbersome and yield inaccurate values if performed after the large sheath has been inserted given the degree of device-related bleeding. Therefore, we evaluated 2 different techniques to obtain this important data before the insertion of a large introducer device without having to unwrap the device to be prepared for a potential bail-out use. Compared with previous data we proved that a moderate correlation exists in the measurements obtained from preoperative MDCT only that were not good enough to allow the use of the MANTA device safely.21 An inaccurate release of the system could lead to malapposition with persistent major bleeding especially in small or heavily calcified vessels or even to the vessel total occlusion. This inaccuracy may be explained by a smaller angle in the direction of the stitches compared to the perpendicular measurements obtained on the MDCT or to a different distribution or position of a skin flap in very obese patients during the MDCT and the procedure.
In contrast, the measurements obtained with the Angio-Seal device followed by an imaging-based predefinition of the corresponding MANTA implantation depth kept a close association with the measurements of the MANTA device. With this information, the femoral artery can be safely closed after a failed Proglide system. Unsolvable failed suture-based device with an additional Angio-Seal or Proglide due to severe bleeding or suture rupture occurred in 5.9% of the patients. Bail-out with MANTA insertion was successful in all patients. Only 1 patient required surgery due to a complete vascular occlusion (Thrombolysis in Myocardial Infarction grade-0 flow) associated with the MANTA device. In retrospect, a prior MDCT had revealed a very small vessel diameter and wall calcification at the puncture site. In these patients, a surgical cut-down would have been the access of choice.
The method presented here is also helpful in other clinical settings without prior MDCT in which large bore sheath are used such as delayed closures after emergency extracorporeal membrane oxygenation or Impella device placement (Abiomed Inc., United States). In these cases, a wire can be inserted through the arterial cannula or Impella CP introducer, and late vascular closure can then be safely achieved using a MANTA vascular closure device. To avoid the unpacking of the sterile device before the simple measuring method the use of an Angio-Seal is cost-effective, not time consuming, and provides information for future reference in case it is needed.
Limitations
The lack of randomization, and the small number of patients are obvious limitations of this study that should be taken into consideration when analyzing the data presented here.
CONCLUSIONS
Compared to MDCT measurements, the routine measurement of vessel depth using the Angio-Seal device stands as a simple option to obtain exact values to allow the bail-out use of the MANTA device in cases of failed suture-based closure device after TAVI. This method can also be used effectively in cases of delayed vascular closures of late explantations of the Impella device or emergency cannulations for venoarterial extracorporeal membrane oxygenation.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
J. Blumenstein, T. Maruskin, O. Husser, and H. Möllmann contributed to the design, analysis, and writing of this manuscript. D. Sötemann, C. Eckel, C. Grothusen, G. Dohmen, C. Tesche, and H. Al.Terki contributed to both the writing and supervision of the manuscript.
CONFLICTS OF INTEREST
Neither one of the authors have made any disclosures regarding this manuscript, and they have all met all the requirements defined by the International Committee of Medical Journal Editors regarding the criteria for authorship of scientific articles.
WHAT IS KNOWN ABOUT THE TOPIC?
- Vascular closure can often be performed safely using suture-based devices after TAVI. However, suture ruptures or insufficient closures can directly lead to major vascular complications. In cases like this, closure can be performed using a different plug-based device (Manta Device). However, one drawback of this device is that it requires to know exactly the distance between the skin incision and the vessel for safe deployment and functionality purposes before inserting a large introducer sheath.
WHAT DOES THIS STUDY ADD?
- This study proved that preoperative MDCT obtained inappropriate measurements of the vessel depth. However, a new measuring technique can be established using an Angio-Seal device before inserting the large introducer sheath. In case of failed suture-based closures, the exact depth of the vessel should be known to be able to use a Manta device for bail-out closures. In addition, this technique can also be effectively in case of delayed vascular closures of late explantations of Impella devices or in cases of emergency cannulations for venoarterial extracorporeal membrane oxygenation.
REFERENCES
1. Barbanti M, Binder RK, Freeman M, et al. Impact of low-profile sheaths on vascular complications during transfemoral transcatheter aortic valve replacement. EuroIntervention. 2013;9:929-935.
2. Barbash IM, Barbanti M, Webb J, et al. Comparison of vascular closure devices for access site closure after transfemoral aortic valve implantation. Eur Heart J. 2015;36:3370-3379.
3. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016;374:1609-1620.
4. Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients:a propensity score analysis. Lancet. 2016;387:2218-2225.
5. Toggweiler S, Leipsic J, Binder RK, et al. Management of vascular access in transcatheter aortic valve replacement:part 2:Vascular complications. JACC Cardiovasc Interv. 2013;6:767-776.
6. Van Mieghem NM, Tchetche D, Chieffo A, et al. Incidence, predictors, and implications of access site complications with transfemoral transcatheter aortic valve implantation. Am J Cardiol. 2012;110:1361-1367.
7. Biancari F, Romppanen H, Savontaus M, et al. MANTA versus ProGlide vascular closure devices in transfemoral transcatheter aortic valve implantation. Int J Cardiol. 2018;263:29-31.
8. Gheorghe L, Brouwer J, Mathijssen H, et al. Early Outcomes After Percutaneous Closure of Access Site in Transfemoral Transcatheter Valve Implantation Using the Novel Vascular Closure Device Collagen Plug-Based MANTA. Am J Cardiol. 2019;124:1265-1271.
9. Moriyama N, Lindstrom L, Laine M. Propensity-matched comparison of vascular closure devices after transcatheter aortic valve replacement using MANTA versus ProGlide. EuroIntervention. 2019;14:e1558-e1565.
10. Moccetti F, Brinkert M, Seelos R, et al. Insights From a Multidisciplinary Introduction of the MANTA Vascular Closure Device. JACC Cardiovasc Interv. 2019;12:1730-1736.
11. Hoffmann P, Al-Ani A, von Lueder T, et al. Access site complications after transfemoral aortic valve implantation - a comparison of MANTA and ProGlide. CVIR Endovasc. 2018;1:20.
12. Van Mieghem NM, Latib A, van der Heyden J, et al. Percutaneous Plug-Based Arteriotomy Closure Device for Large-Bore Access:A Multicenter Prospective Study. JACC Cardiovasc Interv. 2017;10:613-619.
13. Barbanti M, Capranzano P, Ohno Y, et al. Comparison of suture-based vascular closure devices in transfemoral transcatheter aortic valve implantation. EuroIntervention. 2015;11:690-697.
14. Toggweiler S, Leipsic J, Binder RK, et al. Management of vascular access in transcatheter aortic valve replacement:part 1:basic anatomy, imaging, sheaths, wires, and access routes. JACC Cardiovasc Interv. 2013;6:643-653.
15. Dimitriadis Z, Scholtz W, Borgermann J, Wiemer M, Piper C, Vlachojannis M, Gummert J, Horstkotte D, Ensminger S, Faber L, Scholtz S. Impact of closure devices on vascular complication and mortality rates in TAVI procedures. Int J Cardiol. 2017;241:133-137.
16. Giordano A, Corcione N, Ferraro P, et al. Comparison of ProGlide vs. Prostar in patients undergoing transcatheter aortic valve implantation. Minerva Cardioangiol. 2019;67:443-449.
17. Calligaro KD, Balraj P, Moudgill N, Rao A, Dougherty MJ, Eisenberg J. Results of polytetrafluoroethylene-covered nitinol stents crossing the inguinal ligament. J Vasc Surg.2013;57:421-426.
18. De Backer O, Arnous S, Sandholt B, et al. Safety and efficacy of using the Viabahn endoprosthesis for percutaneous treatment of vascular access complications after transfemoral aortic valve implantation. Am J Cardiol. 2015;115:1123-1129.
19. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, Rutherford RB, Group TIW. Inter-society consensus for the management of peripheral arterial disease. Int Angiol. 2007;26:81-157.
20. van Wiechen MP, Tchetche D, Ooms JF, Hokken TW, Kroon H, Ziviello F, Ghattas A, Siddiqui S, Laperche C, Spitzer E, Daemen J, de Jaegere PP, Dumonteil N, Van Mieghem NM. Suture- or Plug-Based Large-Bore Arteriotomy Closure:A Pilot Randomized Controlled Trial. JACC Cardiovasc Interv.2020.
21. Hassan MF, Lawrence M, Lee D, Velazco J, Martin C, Reddy R. Simplified percutaneous VA ECMO decannulation using the MANTA vascular closure device:Initial US experience. J Card Surg. 2020;35:217-221.
* Corresponding author: Department of Internal Medicine I, St.-Johannes-Hospital. Johannesstraße 9-13, 44139 Dortmund, Germany.
E-mail address: johannes.blumenstein@joho-dortmund.de (J. Blumenstein).