ABSTRACT
Introduction and objectives: Although drug-eluting stents are the main treatment in percutaneous coronary interventions (PCI), drug-coated balloons (DCB) represent an appealing alternative as they eliminate the risk of stent thrombosis and avoid leaving any metal structure in the vessel wall. However, limited evidence has been published to date on the vessel wall healing processes, plaque remodeling, plaque composition, and the impact on the coronary microcirculation after percutaneous coronary intervention with DCB (DCB-PCI).
Methods: This is investigator-initiated, single-center, single-arm, open-label, pilot study of 30 patients with native vessel disease undergoing DCB-PCI. Intravascular ultrasound and angiography-derived index of microvascular resistance (IMRangio) will be performed before and immediately after PCI, and at 3 months of follow-up.
Conclusions: The study aims to provide new evidence on the modification of atherosclerotic plaque in patients with de novo lesions undergoing PCI with DCB. This will be assessed by examining the change in the percentage of atheroma volume and late lumen enlargement using intravascular ultrasound and by evaluating changes in the microcirculation using IMRangio.
Registered at Clinicaltrials.gov (NCT06080919).
Keywords: Drug-coated balloon. Intravascular ultrasound. Angiography-derived index of microvascular resistance.
RESUMEN
Introducción y objetivos: Pese a que los stents farmacoactivos son el tratamiento principal en las angioplastias coronarias, los balones farmacoactivos representan una alternativa interesante dado que eliminan el riesgo de trombosis del stent sin dejar ningún tipo de estructura metálica en la pared del vaso. No obstante, la evidencia en cuanto a los procesos de cicatrización de la pared del vaso, el remodelado, los cambios en la composición de la placa ateroesclerótica y el impacto en la microcirculación coronaria tras el intervencionismo coronario percutáneo (ICP) con balón farmacoactivo aún no se ha esclarecido.
Métodos: Estudio piloto abierto, de un solo grupo, iniciado por el investigador, de 30 pacientes con enfermedad de vaso nativo sometidos a ICP con balón farmacoactivo. Se realizará ecografía intravascular y se determinará el índice de resistencia microvascular derivado de la angiografía (angio-IRM) antes, inmediatamente después y a los 3 meses de seguimiento de la angioplastia.
Conclusiones: Se aportará nueva evidencia sobre la modificación de la placa en pacientes con enfermedad de vaso nativo tratados con balón farmacoactivo, evaluando el cambio en el porcentaje del volumen de ateroma y el aumento luminal tardío, así como los cambios en la microcirculación mediante angio-IRM.
Registrado en Clinicaltrials.gov (NCT06080919).
Palabras clave: Balón farmacoactivo. Ecografía intravascular. Índice de resistencia microvascular derivado de la angiografía.
Abbreviations DCB: drug-coated balloon. EEM: external elastic membrane. IMRangio: angiography-derived index of microcirculatory resistance. IVUS: intravascular ultrasound. PCI: Percutaneous coronary intervention.
INTRODUCTION
Coronary artery disease is the leading single cause of mortality worldwide, accounting for more than 7 million deaths annually1 and its prevalence has been increasing in the last 20 years.2 Percutaneous coronary intervention (PCI) has been crucial in the treatment of coronary artery disease.3,4 The advent of drug-eluting stents (DES) has substantially reduced restenosis rates through the deposition of antiproliferative drugs in the vessel wall. DES have evolved over the years and have become the gold standard in PCI.5 Drug-coated balloons (DCB) represent an alternative in the setting of PCI. DCB consist of a balloon coated with antiproliferative agents encapsulated in a polymer matrix.6 Upon inflation, the balloon brings the antiproliferative drug into contact with the vessel wall. The main goal of DCB is to eliminate the risk of stent thrombosis and achieve lower restenosis rates by not leaving any type of metal structure in the treated segment.6
The safety and efficacy of DCB have been extensively studied in de novo coronary artery disease.6 In small vessel disease, DCB have demonstrated noninferiority to DES in several randomized clinical trials.7 A recent meta-analysis has shown that the use of DCB, compared with that of DES, is associated with a lower risk of vessel thrombosis and a trend toward a lower risk of acute myocardial infarction.8 In large vessel de novo lesions, current data do not support the widespread use of DCB over DES, although DCB appear to be safe and effective.9,10 Nevertheless, there is a need to elucidate the elution on the vessel wall, healing processes, plaque remodeling, plaque composition and the impact on the coronary microcirculation following PCI with DCB.
The present report describes the design and rationale for a study of plaque modification and impact on the microcirculation after PCI with DCB (the PLAMI study).
METHODS
The study will be an investigator-initiated, single-center, single-arm, open-label, pilot study in patients undergoing PCI with DCB for de novo lesions. The study has been approved by the hospital ethics committee on research involving medical products. The study has been registered in ClinicalTrials.gov (NCT06080919).
Procedure
Eligible patients will be informed about the study and will be required to provide signed informed consent prior to inclusion. Patients will undergo DCB-PCI under intravascular ultrasound (IVUS) guidance. Angiography-derived coronary physiology will be assessed after the procedure using Angio Plus software (Pulse Medical Imaging Technology, China). The angiography images will be used to obtain the angiography-derived index of microcirculatory resistance (IMRangio) values, before and after DCB-PCI. All procedures will be performed according to current European guidelines5: the target lesion will be predilated with semicompliant balloons or noncompliant balloons, with a diameter equal to the reference vessel diameter and with an appropriate length. Multiple predilations will be accepted. The DCB will be the paclitaxel-coated balloon Pantera Lux (BIOTRONIK AG, Switzerland).
The lesion will then be treated with a DCB with a reference vessel diameter/balloon diameter ratio of 1:1. DCB length will be equal to lesion length + 5 mm. DCB inflation time will be set at 45 to 60 seconds to guarantee correct and complete drug elution. The prespecified reasons for DES implantation after DCB-PCI will be residual stenosis > 30%, dissections > type B and TIMI flow < 3.6 Angiographic follow-up with IVUS and IMRangio evaluation will be performed 3 months after the index procedure. The study timeline is summarized in figure 1.
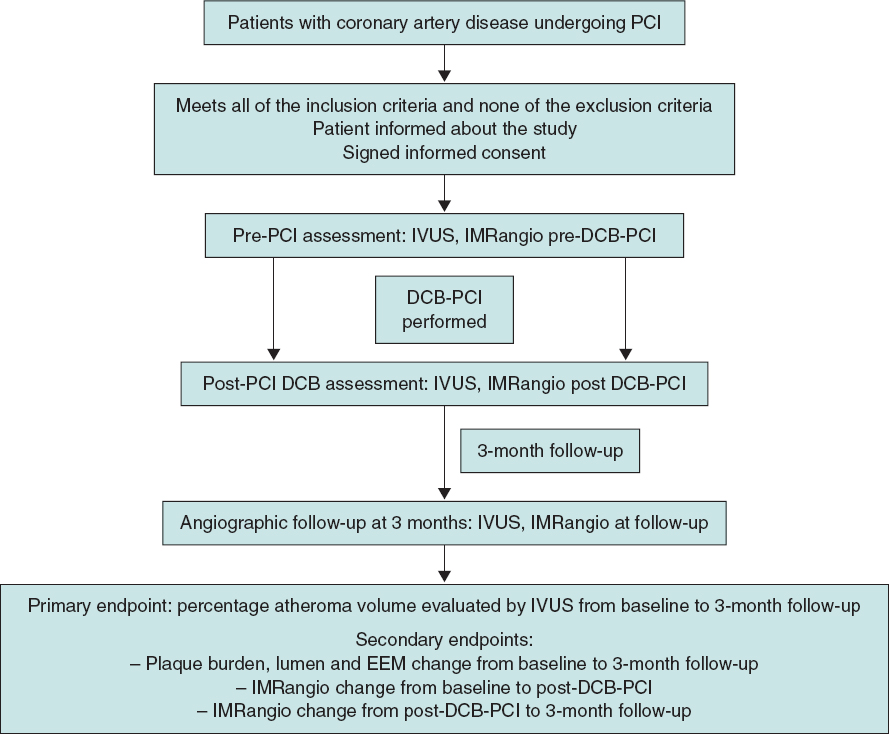
Figure 1. Timeline of the PLAMI study. DCB, drug-coated balloon; IMRangio, angiography-based index of microcirculatory resistance; IVUS, intravascular ultrasound; PCI, percutaneous coronary intervention.
IVUS images will be taken before the DCB-PCI, immediately after, and at 3 months of follow-up using the Opticross HD 60 MHz (Boston Scientific Corp, United States) system. All IVUS studies will be performed after intracoronary administration of 200 μg of nitroglycerin. The IVUS images will be acquired at 30 frames per second with an automatic transducer pull back (at 0.5 mm/second) to the proximal reference vessel lesion. As there will be no stents to take as a reference, the proximal and distal side branches adjacent to the treated lesion will serve as references, matching the coronary angiographic images (figure 2). All IVUS images will be analyzed by an independent core lab.
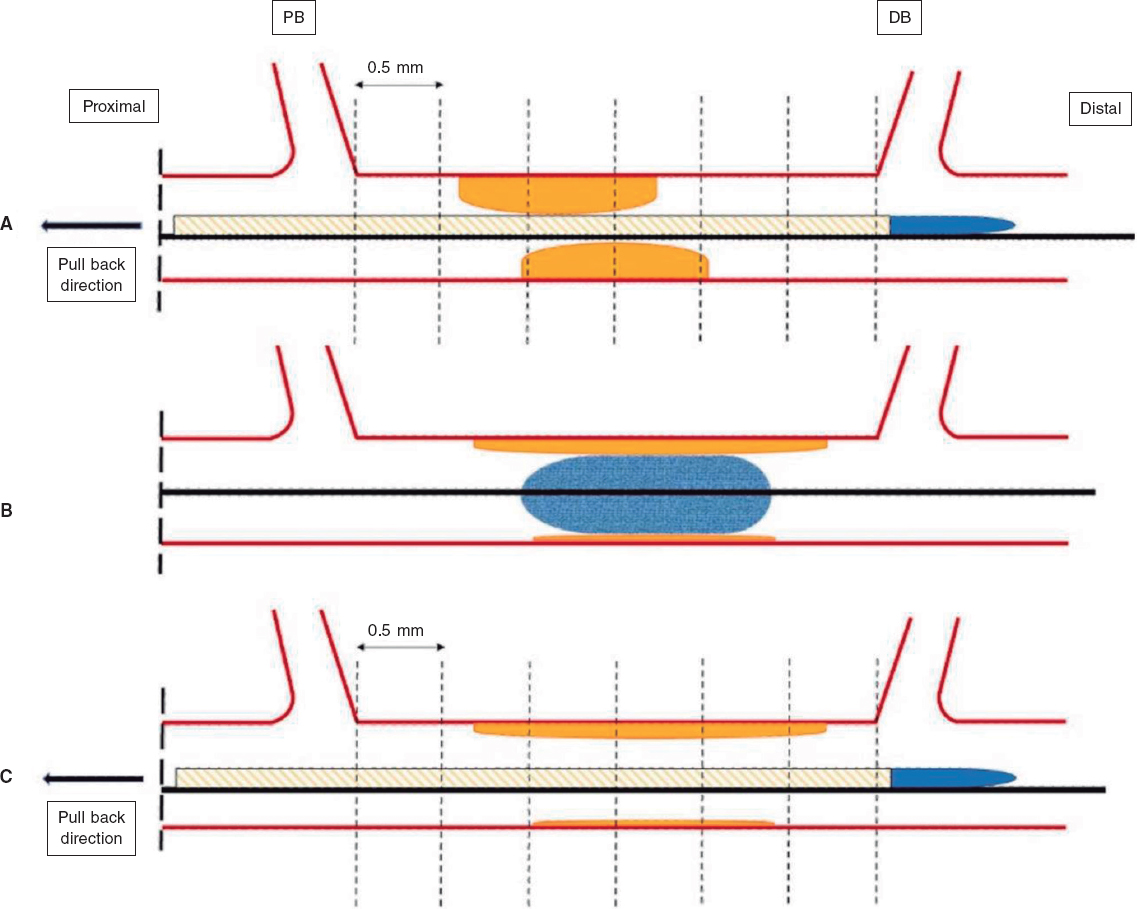
Figure 2. Schematic representation of IVUS acquisition. The IVUS images will be acquired before (A) and after (C) DCB-PCI (B) at 30 frames per second with an automatic transducer pull back (at 0.5 mm/second) to the proximal reference vessel lesion. The same anatomic slice will be analyzed before, after, and at 3 months of follow-up after the PCI by using reproducible landmarks (side branches). The first frame analyzed will be the distal point of the treated vessel before the exit of the DB (represented by the rightmost dotted line), and the last frame analyzed will be the proximal point of the vessel before the split of the proximal branch. DB, distal branch; DCB-PCI, drug-coated balloon percutaneous coronary intervention; IVUS, intravascular ultrasound; PB, proximal branch.
Angiography-derived assessment of coronary physiology will be performed with Angio Plus software (Pulse Medical Imaging Technology, China). For the evaluation of each lesion, at least 2 projections with a difference of > 25° will be selected. The operator will manually mark the points proximal and distal to the lesion, and the system automatically outlines the contours of the detected vessel. If the traced vessel trajectory deviates from the normal lumen, the necessary manual modifications will be performed. The artificial intelligence-assisted software combines the intravascular imaging information with the estimated vessel flow to obtain the IMRangio. All the angiography images will be analyzed by an independent core lab to obtain the IMRangio.
Study population and enrolment criteria
Patients will be screened to ensure they meet the inclusion criteria and none of the exclusion criteria prior to study enrolment. Inclusion criteria consist of an indication to undergo PCI for a de novo lesion according to current guidelines (with no restrictions regarding vessel size).5 Inclusion and exclusion criteria are summarized in table 1.
Table 1. Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Patient with CAD undergoing PCI with DCB with no limitation to vessel size | Age < 18 years |
| Cardiogenic shock | |
| ST-segment elevation myocardial infarction | |
| Use of mechanical circulatory support | |
| Complex coronary lesions* including chronic total occlusions, bifurcation lesions, left main coronary artery disease, severe calcified lesions, graft interventions and in-stent restenosis | |
| Inability to provide informed consent | |
| Unable to understand and follow study-related instructions or unable to comply with study protocol | |
| Currently participating in another trial | |
| Pregnant women | |
CAD, coronary artery disease; DCB, drug-coated balloon; PCI, percutaneous coronary intervention. | |
Sample size
Because of the exploratory nature of this study, no formal sample size calculation is required. Based on previous pilot studies with similar designs,12 a sample of 30 lesions is planned to evaluate the impact of DCB on coronary healing and the microcirculatory territory.
Study endpoints
The primary endpoint is the change in percentage atheroma volume evaluated by IVUS from baseline to 3 months of follow-up. Secondary endpoints will include a) lumen change from baseline to 3 months of follow-up (minimum, maximum, average areas), b) the percentage of progressors and percentage of regressors, c) external elastic membrane (EEM) change from baseline to post DCB-PCI (average), d) EEM change post-DCB-PCI to the 3-month follow-up (average), e) the percentage of remodeling types (neutral, negative, and positive), f) IMRangio change from baseline to post- DCB-PCI, g) IMRangio change from post-DCB-PCI to the 3-month follow-up.
An independent clinical event committee, consisting of cardiologists not participating in the trial, will review and adjudicate all major adverse cardiac events according to the study protocol.
Considering the luminal area as the area delimited by the luminal border, the minimal luminal area is defined as the smallest lumen area within the length of the treated lesion.13,14 The atheroma or plaque burden is defined as the ratio of atheroma area to the vessel EEM and is calculated by dividing the sum of plaque and media cross-sectional area (CSA) by the EEM CSA.13,14 As the atheroma area can be calculated in each frame, the total atheroma volume is obtained by taking the sum of the differences between the EEM CSA area and the luminal CSA for all available images.15 The percent of the volume of the EEM occupied by atheroma is called the percentage atheroma volume.15,16
Serial arterial remodeling types will be classified as usual: neutral if there is no change in EEM, negative if there is a decrease in the EEM and positive if vice versa.
Statistical considerations
Continuous variables will be described as mean ± standard deviation or median [interquartile range]. Categorical variables will be described as percentages. The paired t-test will be used to compare continuous variables measured before and after treatment in the same patient, and differences in proportions will be tested with the chi-square or Fisher exact test. A P value less than .05 (typically ≤ .05) will be considered statistically significant. Statistical analyses will be performed using Stata software version 13.1 (StataCorp LP, United States).
DISCUSSION
Although the use of DES remains predominant in the performance of PCI, complications such as stent thrombosis and in-stent restenosis led to the development of DCB. DCB have the theoretical benefit of not leaving metallic material in the vascular lumen, thereby reducing the possibility of mechanical complications such as malapposition, stent fracture, and stent thrombosis. This could potentially reduce neointimal proliferation and shorten the duration of dual antiplatelet therapy.6 Current guidelines assign a level IA recommendation to the treatment of in-stent restenosis.5 While the use of DCB in de novo lesions seems promising, it is not yet widespread. In addition, PCI is not without risks, as it involves a certain degree of injury to the artery wall from balloon inflations and stent struts.17,19 The vascular response to endothelial cell and smooth muscle cell injury represents a complex network of biochemical responses that involve the immune system. All these factors regulate the processes of neointimal hyperplasia, vascular remodeling, and normal reendothelialization of the arterial wall.17
The pathophysiology of restenosis and lumen loss after angioplasty is a complex process involving various factors and is not limited to neointimal hyperplasia.19 Acutely, plain old balloon angioplasty (POBA) generates an increase in luminal area that is mainly due to an expansion of the EEM, mainly attributed to the elastic properties of the vessel rather than to plaque compression or removal.20 Subsequently, within the first few minutes after PCI, there is an “acute recoil” due to the elastic properties of the arterial wall. In the chronic phase, IVUS data indicate that luminal loss is mainly due to a progressive reduction in EEM rather than an increase in atherosclerotic plaque volume. Unlike the acute phase where loss of area is solely due to elastic properties, “chronic recoil” leading to the loss of area also involves a combination factors such as fibrosis, apoptosis, and changes in the extracellular matrix.19,21 Interestingly, not all patients show negative remodeling with a decrease in EEM; around 25% show a persistent increase in EEM, which is correlated with a reduced restenosis rate. Consequently, restenosis appears to be primarily due to the direction and magnitude of changes in arterial remodeling,19 although neointimal hyperplasia also plays a role .
Nevertheless, the existing evidence is based on analysis after the use of traditional balloons. With DCB-PCI, late lumen enlargement has been observed compared with POBA.20 Although this finding has been partly attributed to the inhibition of neointimal proliferation by antiproliferative drugs,22 the role of plaque modification or vessel healing phenomena in influencing this process cannot be excluded. A previous study showed that late lumen enlargement was higher in areas with the highest plaque burden; however, that study was a retrospective assessment and used a quantitative coronary angiography protocol.23 It could be hypothesized that, by inducing controlled damage to the artery wall, together with the antiproliferative effect of DCB, positive vessel remodeling might be achieved, reducing restenosis rates without the need for DES. Therefore, with DCB-PCI, we are able to treat coronary stenosis not only from a mechanical point of view, but can also change the natural history of the disease and restenosis. In this regard, IVUS analysis will be essential to evaluate the reasons behind the gain or loss of luminal area. The dynamic changes produced after PCI are depicted in figure 3.
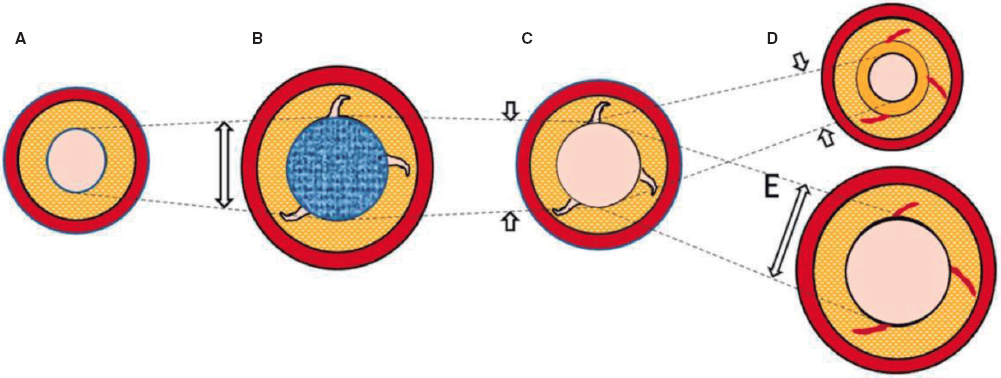
Figure 3. Central illustration. Schematic representation of timeline of DCB-PCI and lumen variation. A: pre-DCB-PCI de novo lesion. B: DCB-PCI (blue), generating injury to the vessel wall and an increase in lumen and EEM CSA. C: acute recoil. D, chronic recoil with decrease in EEM and neointimal hyperplasia. E. LLE due to maintenance of EEM area and no neointimal hyperplasia. The dotted lines represent the variations of the luminal area throughout the process. The image exemplifies how changes in luminal area, as well as plaque burden, are mainly due to variations in EEM rather than plaque compression. CSA, cross-sectional area; DCB-PCI, drug-coated balloon percutaneous coronary intervention; EEM, external elastic membrane; LLE, late lumen enlargement.
The DCB that will be used in our study, paclitaxel, has been extensively analyzed as a balloon-coating drug due to its lipophilic properties and its ability to elute into the vessel wall.24 Moreover, the available paclitaxel-DCB have shown good results in patients undergoing PCI for native vessel disease.25 In contrast, because of the hydrophobic characteristics of sirolimus, maintaining an adequate percentage in the wall over the mid-term poses technical challenges. However, advances in the formulation of the new generation of sirolimus DCB are anticipated to address this issue by facilitating adequate drug release into the vessel wall.24
As previously mentioned, the coronary microcirculation is closely related to proper coronary functioning and the pathophysiology of coronary artery disease. While it is believed that the performance of PCI, as well as the injury and healing of the coronary artery, may affect the coronary microcirculation, the evidence regarding DCB-PCI is scarce. Moreover, the plaque rupture, intimal dissections and thrombus formation that occur during balloon angioplasty are a potential source of embolism to the microvascular bed.
Since direct visualization of the microcirculation is not feasible in clinical practice,26 its assessment relies on parameters reflecting its functional status, usually coronary flow reserve and the IMR. Coronary flow reserve is defined as the ratio between hyperemic flow in response to nonendothelial vasodilation and resting blood flow. It is crucial to exclude epicardial stenosis before using coronary flow reserve, as it provides an integrated measurement of both epicardial and coronary microcirculation.26 IMR is calculated as the product of distal coronary pressure at maximal hyperemia multiplied by the hyperemic mean transit time.
In our study, we will perform a noninvasive, nonhyperemic assessment of the coronary microcirculation using IMRangio. This approach aims to characterize the baseline status of the microcirculation and assess the microvasculature changes induced by PCI and their variation over a 3-month period.
By monitoring IMRangio before and after treating the stenotic epicardial lesion, we will be able to assess the effects of acute fracture of the atherosclerotic plaque and injury to the arterial wall in the microvascular bed. We also aim to investigate whether these collateral harmful changes provoked during angioplasty remain consistent or vary significantly at 3 months of follow-up. In this same context, the analysis of IVUS during follow-up will allow us to correlate the changes in the arterial wall and atherosclerotic plaque after DCB-PCI with microcirculation physiology. To date, no insights into the anatomical and physiological process of healing of the injured arterial wall after DCB-PCI have been available in the published literature.
CONCLUSIONS
The PLAMI study is a first-in-man pilot study that aims to provide new information on the modification of atherosclerotic plaque assessed by intracoronary imaging in patients with de novo lesions undergoing PCI with DCB.
FUNDING
None reported.
ETHICAL CONSIDERATIONS
The study has been approved by the hospital ethics committee on research involving medical products. Eligible patients will be informed about the study and must provide written informed consent prior to inclusion in the study. Possible gender/sex biases have been considered.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence has been used in the preparation of this article.
AUTHORS’ CONTRIBUTIONS
J.A Sorolla Romero, A.Teira Calderón, J. Sanz Sánchez and H.M. Garcia-Garcia contributed to the conception, design, drafting and revision of the article. J.P. Vílchez Tschischke, P. Aguar Carrascosa, F.Ten Morro, L. Andrés Lalaguna, L. Martínez Dolz and J.L. Díez Gil contributed to the critical revision of the intellectual content.
CONFLICTS OF INTEREST
None declared.
WHAT IS KNOWN ABOUT THE TOPIC?
- DCB have proven clinical effectiveness in cases of in-stent restenosis and de novo lesions involving small vessel coronary artery disease.
- Several studies in small vessel coronary artery disease have shown a benefit of DCB in the vessel wall, with late lumen enlargement during follow-up.
- However, there is little evidence of their use in larger vessels.
- In addition, the impact of DCB on the coronary microcirculation has not been evaluated to date.
WHAT DOES THIS STUDY ADD?
- The PLAMI study aims to characterize vessel healing using IVUS after DCB-PCI in patients with native vessel disease and to correlate these findings with the impact on microcirculation.
REFERENCES
1. Ralapanawa U, Sivakanesan R. Epidemiology and the Magnitude of Coronary Artery Disease and Acute Coronary Syndrome:A Narrative Review. J Epidemiol Glob Health. 2021;11:169-177.
2. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease:A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646.
3. Canfield J, Totary-Jain H. 40 Years of Percutaneous Coronary Intervention:History and Future Directions. J Pers Med. 2018;8:33.
4. Stefanini GG, Alfonso F, Barbato E, et al. Management of myocardial revascularisation failure:an expert consensus document of the EAPCI. EuroIntervention. 2020;16:e875-e890.
5. Neumann F, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
6. Jeger RV, Eccleshall S, Wan Ahmad WA, et al. Drug-Coated Balloons for Coronary Artery Disease:Third Report of the International DCB Consensus Group. JACC Cardiovasc Interv. 2020;13:1391-1402.
7. Tang Y, Qiao S, Su X, et al. Drug-Coated Balloon Versus Drug-Eluting Stent for Small-Vessel Disease:The RESTORE SVD China Randomized Trial. JACC Cardiovasc Interv. 2018;11:2381-2392.
8. Sanz Sánchez J, Chiarito M, Cortese B, et al. Drug-Coated balloons vs drug-eluting stents for the treatment of small coronary artery disease:A meta-analysis of randomized trials. Catheter Cardiovasc Interv. 2021;98:66-75.
9. Yerasi C, Case BC, Forrestal BJ, et al. Drug-Coated Balloon for de Novo Coronary Artery Disease:JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:1061-1073.
10. Nishiyama N, Komatsu T, Kuroyanagi T, et al. Clinical value of drug-coated balloon angioplasty for de novo lesions in patients with coronary artery disease. Int J Cardiol. 2016;222:113-118.
11. Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization. J Am Coll Cardiol. 2022;79:e21-e129.
12. Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans:delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193-202.
13. Xu J, Lo S. Fundamentals and role of intravascular ultrasound in percutaneous coronary intervention. Cardiovasc Diagn Ther. 2020;10:1358-1370.
14. Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37:1478-1492.
15. Gogas BD, Farooq V, Serruys PW, Garcia-Garcia HM. Assessment of coronary atherosclerosis by IVUS and IVUS-based imaging modalities:progression and regression studies, tissue composition and beyond. Int J Cardiovasc Imaging. 2011;27:225-237.
16. Tobis JM, Perlowski A. Atheroma Volume by Intravascular Ultrasound as a Surrogate for Clinical End Points. J Am Coll Cardiol. 2009;53:1116-1118.
17. Feinberg MW. Healing the injured vessel wall using microRNA-facilitated gene delivery. J Clin Invest. 2014;124:3694-3697.
18. Inoue T, Croce K, Morooka T, Sakuma M, Node K, Simon DI. Vascular Inflammation and Repair:Implications for Reendothelialization, Restenosis, and Stent Thrombosis. JACC Cardiovasc Interv. 2011;4:1057-1066.
19. Mintz GS, Popma JJ, Pichard AD, et al. Arterial Remodeling After Coronary Angioplasty. Circulation. 1996;94:35-43.
20. Her AY, Ann SH, Singh GB, et al. Comparison of Paclitaxel-Coated Balloon Treatment and Plain Old Balloon Angioplasty for De Novo Coronary Lesions. Yonsei Med J. 2016;57:337-341.
21. Geary RL, Nikkari ST, Wagner WD, Williams JK, Adams MR, Dean RH. Wound healing:A paradigm for lumen narrowing after arterial reconstruction. J Vasc Surg. 1998;27:96-108.
22. Sogabe K, Koide M, Fukui K, et al. Optical coherence tomography analysis of late lumen enlargement after paclitaxel-coated balloon angioplasty for de-novo coronary artery disease. Catheter Cardiovasc Interv. 2021;98:E35-E42.
23. Kleber FX, Schulz A, Waliszewski M, et al. Local paclitaxel induces late lumen enlargement in coronary arteries after balloon angioplasty. Clin Res Cardiol. 2015;104:217-225.
24. Yerasi C, Case BC, Forrestal BJ, et al. Drug-Coated Balloon for De Novo Coronary Artery Disease:JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:1061-1073.
25. Venetsanos D, Omerovic E, Sarno G, et al. Long term outcome after treatment of de novo coronary artery lesions using three different drug coated balloons. Int J Cardiol. 2021;325:30-36.
26. Kunadian V, Chieffo A, Camici PG, et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology &Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J. 2020;41:3504-3520.
* Corresponding authors.
E-mail addresses: sjorge4@gmx.com (J. Sanz Sánchez); hector.m.garciagarcia@medstar.net; hect2701@gmail.com (H.M. Garcia-Garcia).











