Abstract
Introduction and objectives: Patients with a low post-percutaneous coronary intervention (PCI) fractional flow reserve (FFR) are at a higher risk for future adverse cardiac events. The objective of the current study was to assess specific patient and procedural predictors of post-PCI FFR.
Methods: The FFR-SEARCH study is a prospective single-center registry of 1000 consecutive all-comer patients who underwent FFR measurements after an angiographically successful PCI with a dedicated microcatheter. Mixed effects models were used to search for independent predictors of post-PCI FFR.
Results: The mean post-PCI distal coronary pressure divided by the aortic pressure (Pd/Pa) was 0.96 ± 0.04 and the mean post-PCI FFR, 0.91 ± 0.07. After adjusting for the independent predictors of post-PCI FFR, the left anterior descending coronary artery as the measured vessel was the strongest predictor of post-PCI FFR (adjusted β = -0.063; 95%CI, -0.070 to -0.056; P < .0001) followed by the postprocedural minimum lumen diameter (adjusted β = 0.039; 95%CI, 0.015-0.065; P = .002). Additionally, male sex, in-stent restenosis, chronic total coronary occlusions, and pre- and post-dilatation were negatively associated with postprocedural FFR. Conversely, type A lesions, thrombus-containing lesions, postprocedural percent stenosis, and stent diameter were positively associated with postprocedural FFR. The R2 for the complete model was 53%.
Conclusions: Multiple independent patient and vessel related predictors of postprocedural FFR were identified, including sex, the left anterior descending coronary artery as the measured vessel, and postprocedural minimum lumen diameter.
Keywords: Percutaneous coronary intervention. Post-PCI FFR. Predictors.
RESUMEN
Introducción y objetivos: Los pacientes con una reserva fraccional de flujo (FFR) posintervención coronaria percutánea (ICP) baja tienen mayor riesgo de futuros eventos cardiacos adversos. El objetivo del presente estudio fue evaluar predictores específicos de pacientes y procedimientos de FFR tras una ICP.
Métodos: El estudio FFR-SEARCH es un registro prospectivo de un solo centro que incluyó 1.000 pacientes consecutivos que se sometieron a una evaluación de la FFR tras una ICP con éxito angiográfico utilizando un microcatéter específico. Se utilizaron modelos de efectos mixtos para buscar predictores independientes de FFR tras la ICP.
Resultados: La media de presión distal dividida entre la presión aórtica tras la ICP fue de 0,96 ± 0,04, y la media de la FFR tras la ICP fue de 0,91 ± 0,07. Tras ajustar por predictores independientes de FFR tras la ICP, la arteria descendente anterior izquierda como vaso medido fue el predictor más fuerte (β ajustado = −0,063; IC95%, −0,070 a −0,056; p < 0,0001), seguida del diámetro luminal mínimo posprocedimiento (β ajustado = 0,039; IC95%, 0,015 a 0,065; p = 0,002). Además, el sexo masculino, la reestenosis del stent, las oclusiones totales crónicas y la pre- y posdilatación se correlacionaron negativamente con la FFR posprocedimiento. Por el contrario, las lesiones de tipo A, las lesiones con trombos, el porcentaje de estenosis posprocedimiento y el diámetro del stent se correlacionaron positivamente con la FFR posprocedimiento. El R2 para el modelo completo fue del 53%.
Conclusiones: Se identificaron diversos predictores independientes relacionados con los pacientes y con los vasos para la FFR posprocedimiento, incluyendo el sexo, la arteria descendente anterior izquierda como vaso medido y el diámetro luminal mínimo posprocedimiento.
Palabras clave: Intervención coronaria percutánea. FFR post-ICP. Predictores.
Abbreviations:
FFR: fractional flow reserve. LAD: left anterior descending coronary artery. MLD: minimum luminal diameter. PCI: percutaneous coronary intervention.
INTRODUCTION
The limitations of an accurate assessment of the hemodynamic significance of coronary artery lesions through angiographic guidance alone are well-known.1 Instead, the fractional flow reserve (FFR) has proven to be a useful technique to address the coronary physiology and the hemodynamic significance of coronary segments before and after performing an intervention.2-4 Also, measuring FFR post-stenting has proven to be a strong and independent predictor of major adverse cardiovascular events at the 2-year follow-up.3-5
While FFR primarily takes into account the relative luminal narrowing and the amount of viable myocardium perfused by a specific vessel, several factors have been shown to impact the FFR values prior to performing a percutaneous coronary intervention (PCI). Therefore, longer lesion length, high syntax scores, calcifications, and tortuosity are associated with significantly lower FFR values. Conversely, the presence of microvascular dysfunction, chronic kidney disease and female gender have been associated with higher FFR values.6-11
At the present time, there is lack of data on independent predictors of post-PCI FFR. Therefore, the objective of the present study was to assess the patient and procedural characteristics associated with low post-PCI FFR in an all-comer patient population.
METHODS
The FFR-SEARCH study is a prospective single-center registry that assessed the routine distal pressure divided by the aortic pressure (Pd/Pa) and FFR values of all consecutive patients after an angiographically successful PCI. The primary endpoint was to study the impact of post-PCI FFR on the rate of major adverse cardiovascular event at the 2-year follow-up. Accordingly, no further actions were taken to improve post-PCI FFR. The study was performed in full compliance with the Declaration of Helsinki. The study protocol was approved by the local ethics committee. All patients gave their written informed consent to undergo the procedure. Also, anonymous datasets for research purposes were used in compliance with the Dutch Medical Research Act. A total of 1512 patients treated between March 2016 and May 2017 at the Erasmus Medical Center were eligible to enter our study. A total of 504 of these patients were excluded due to hemodynamic instability (156), a rather small distal outflow (129), the operator’s decision not to proceed with post-PCI hemodynamic assessment (148) or other reasons (79). A total of 1000 patients were included in the study. The microcatheter could not cross the treated lesion in 28 patients, technical issues with the catheter prevented post-PCI assessments in 11 patients, and in 2 patients the post-PCI FFR measurements had to be aborted prematurely due to adenosine intolerance. This left 959 patients whose post-PCI FFR values were measured in at least 1 angiographically successfully treated lesion.
Quantitative coronary angiography
The preprocedural lesion type was defined according to the ACC/AHA guidelines12 and divided into 4 categories: A, B1, B2, and C. Comprehensive quantitative coronary angiography analyses were performed pre- and post-stent implantation in all the treated lesions. An angiographic view with minimal foreshortening of the lesion and minimal overlapping with other vessels was selected. Similar angiographic views were used pre- and post-stent implantation. Measurements included pre- and postprocedural percent diameter stenosis, reference vessel diameter, lesion length, and minimum luminal diameter (MLD). In case of a total occlusion in patients presenting with ST-segment elevation myocardial infarction (STEMI) or chronic total coronary occlusion (CTO), the MLD was considered zero and the percent diameter stenosis, 100%. The reference vessel diameter and the lesion length were measured from the first angiographic view with restored flow. All measurements were taken using CAAS for Windows, version 2.11.2 (Pie Medical Imaging, The Netherlands).
Fractional flow reserve measurements
All FFR measurements were acquired using the Navvus RXi system (ACIST Medical Systems, United States), a dedicated FFR microcatheter with optical pressure sensor technology.13,14 Measurements were performed after an intracoronary bolus of nitrates (200 µg). The catheter was advanced while mounted over the previously used guidewire approximately 20 mm distal to the most distal border of the stent. The FFR was defined as the mean distal coronary artery pressure divided by the mean aortic pressure during maximum hyperemia achieved by the continuous IV infusion of adenosine at a rate of 140 µg/kg/min via the antecubital vein. In this study no vessels were assessed using intracoronary adenosine.
Statistical analysis
At baseline, the categorical variables were expressed as counts (percentage) and the continuous ones as mean ± standard deviation. To assess the independent predictors of post-PCI FFR, all the patient and vessel characteristics were primarily assessed through an univariate test using a mixed effects model (LME-model) with a random effect for the patients and a fixed effect for the post-PCI FFR. All variables were subsequently inserted in a multivariate LME-model using the enter method that resulted in all the significant independent predictors of post-PCI FFR values. A forest plot was developed to depict all variables with the corresponding 95% confidence intervals (95%CI). Beta (β) values show the average increase or decrease of the FFR values in the case of dichotomous variables or the increment per unit increase in the case of continuous variables. Statistical analyses were performed using the statistical software package R (version 3.5.1, packages: Hmisc, lme4 and nlme, RStudio Team, United States).
RESULTS
Demographic characteristics
The mean age was 64.6 ± 11.8 years and 72.5% were males. In 959 patients, at least, 1 lesion was measured with an overall 1165 successfully treated and measured lesions. The patient demographics and baseline characteristics are shown on table 1. Up to 70% of the patients presented with an acute coronary syndrome, and 18% had confirmed thrombus as seen on the angiography. Intravascular imaging modalities were used in 9.6% of the patients to guide the procedure. Overall, 1.4 ± 0.6 lesions were treated per patient and in 1.2 ± 0.5 lesions per patient the post-PCI FFR was successfully assessed. The average overall stent length per vessel was 29 mm ± 17 mm with an average stent diameter of 3.2 mm ± 0.5 mm.
Table 1. Baseline patient and vessel characteristics
| Variable | Total FFR-SEARCH registry |
|---|---|
| Patient characteristics | (N = 1000) |
| Age | 64.6 ± 11.8 |
| Sex, male | 725 (73) |
| Hypertension | 515 (52) |
| Hypercholesterolemia | 451 (45) |
| Diabetes | 191 (19) |
| Smoking history | 499 (50) |
| Previous stroke | 77 (8) |
| Peripheral arterial disease | 76 (8) |
| Previous myocardial infarction | 203 (20) |
| Previous PCI | 264 (26) |
| Previous CABG | 57 (6) |
| Indication for PCI | |
| Stable angina | 304 (30) |
| NSTEMI | 367 (37) |
| STEMI | 329 (33) |
| Vessel characteristics | (N = 1165) |
| Lesion type | |
| A | 125 (11) |
| B1 | 233 (20) |
| B2 | 379 (33) |
| C | 428 (37) |
| LAD | 593 (51) |
| Bifurcation | 138 (12) |
| Calcified | 402 (35) |
| In-stent restenosis | 39 (3) |
| Thrombus | 214 (18) |
| Stent thrombosis | 14 (1) |
| Ostial | 97 (8) |
| CTO | 42 (4) |
| Stenosis pre procedural | 69 ± 22 |
| Reference diameter pre procedural (mm) | 2.6 ± 0.6 |
| Length pre procedural (cm) | 21 ± 11 |
| MLD pre (mm) | 0.9 ± 0.6 |
| Predilatation | 769 (66) |
| Postdilatation | 691 (59) |
| Stenosis post procedural | 44 ± 13 |
| Reference diameter post procedural (mm) | 2.7 ± 0.5 |
| Length post procedural (cm) | 24 ± 13 |
| MLD post procedural (mm) | 2.6 ± 0.5 |
| Number of stents | 1.4 ± 0.6 |
| Stent length (cm) | 29 ± 17 |
| Stent diameter (mm) | 3.2 ± 0.5 |
| Mean post-PCI Pd/Pa | 0.96 ± 0.04 |
| Mean post-PCI FFR | 0.91 ± 0.07 |
|
CABG, coronary artery bypass graft; CTO, chronic total coronary occlusion; FFR, fractional flow reserve; LAD, left anterior descending artery; MLD, minimum luminal diameter; NSTEMI, non-ST segment elevation acute myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; Pd/Pa, ratio of mean distal coronary artery pressure to mean aortic pressure; Values are expressed as mean ± standard deviation or no. (%). |
|
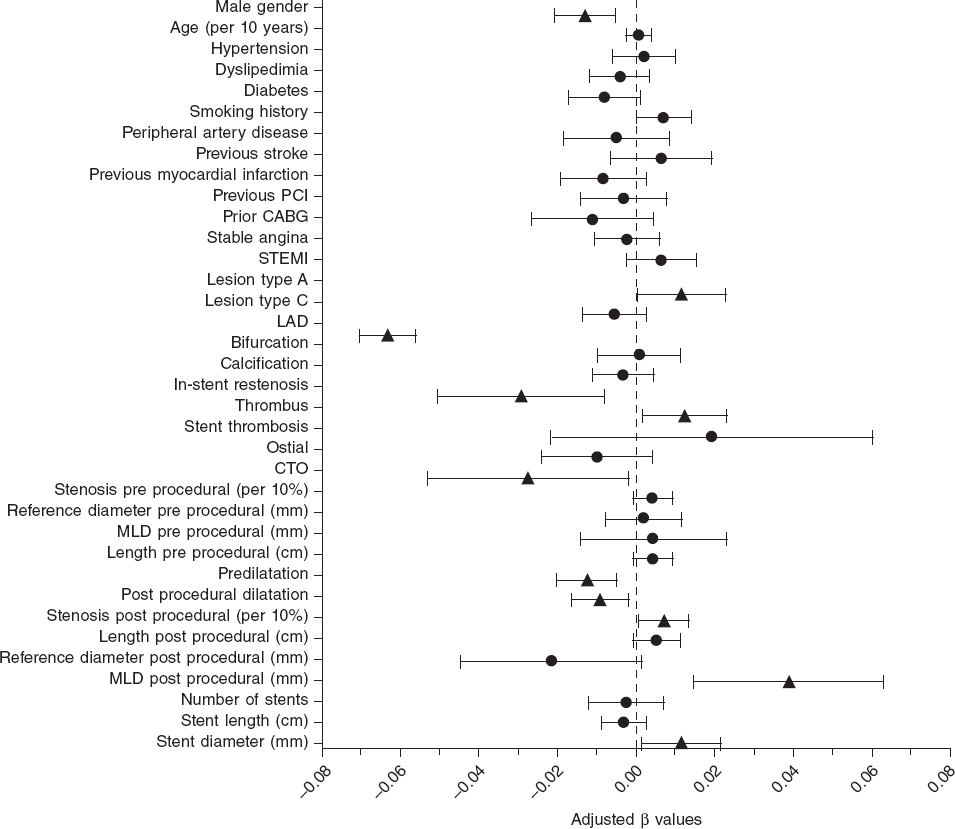
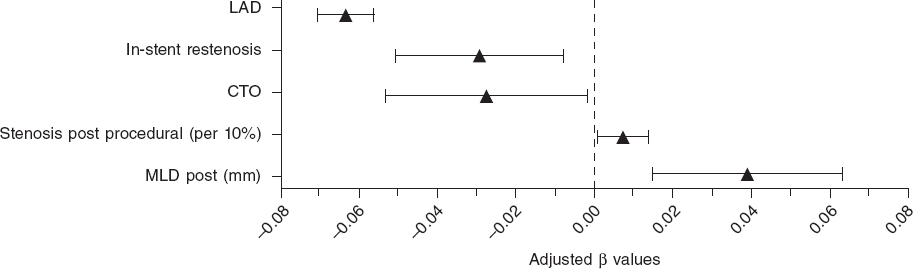
The mean post-PCI FFR was 0.91 ± 0.07 and 7.7% of vessels had a post-PCI FFR ≤ 0.80. In the LME-model and after adjusting for independent predictors of post-PCI FFR, the left anterior descending coronary artery (LAD) as the measured vessel was the strongest predictor of post-PCI FFR (adjusted β = -0.063; 95%CI, -0.070 to -0.056; P < .0001) followed by the postprocedural MLD (adjusted β = 0.039; 95%CI, 0.015-0.065]; P = .002). Additionally, male sex, in-stent restenosis, CTO, and pre- and post-dilatation were negatively correlated with postprocedural FFR. Conversely, type A lesions, thrombus-containing lesions, postprocedural percent diameter stenosis, and stent diameter were positively correlated with postprocedural FFR. The R2 for the entire model was 53%. Figure 1 shows all significant and non-significant adjusted predictors included in the LME-model. Table 2 shows all adjusted and unadjusted predictors with corresponding β values and 95%CI. The most important predictors are shown on figure 2.
Figure 1. Forest plot of independent predictors of post-PCI FFR. Adjusted beta values with 95% confidence intervals. Triangles indicate significant predictors while circles are indicative of non-significant predictors in the multivariate generalized mixed model to predict post-PCI FFR. ACS, acute coronary syndrome; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; LAD, left anterior descending coronary artery; CTO, chronic total coronary occlusion; MLD, minimum lumen diameter.
Table 2. Predictors for post-PCI FFR
| Variable | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| P | β(95%CI) | P | β(95%CI) | |
| Patient characteristics | ||||
| Male sex | .214 | -0.006 (-0.015 – 0.003) | .001 | -0.013 (-0.021 – -0.005) |
| Age (per 10 years) | .976 | 0.000 (-0.03 – 0.03) | .724 | 0.001 (-0.002 – 0.003) |
| Hypertension | .013 | -0.010 (-0.018 – -0.002) | .610 | 0.002 (-0.006 – 0.010) |
| Hypercholesterolemia | < .001 | -0.019 (-0.027 – -0.011) | .287 | -0.004 (-0.012 – 0.004) |
| Diabetes | < .001 | 0.018 (0.008 – 0.042) | .081 | -0.008 (-0.017 – 0.001) |
| Smoking history | .007 | 0.020 (0.010 – 0.019) | .054 | 0.007 (-0.0001 – 0.014) |
| Previous stroke | .831 | -0.002 (-0.017 – 0.013) | .342 | 0.006 (-0.0007 – 0.019) |
| Peripheral arterial disease | .022 | -0.017 (-0.032 – -0.003) | .460 | -0.005 (-0.018 – 0.008) |
| Previous myocardial infarction | .002 | -0.016 (-0.026 – -0.006) | .137 | -0.008 (-0.019 – 0.003) |
| Previous PCI | < .001 | -0.016 (-0.025 – -0.007) | .569 | -0.032 (-0.014 – 0.008) |
| Previous CABG | .896 | -0.001 (-0.019 – 0.017) | .166 | -0.011 (-0.014 – 0.004) |
| Indication for PCI | ||||
| Stable angina | < .001 | -0.025 (-0.034 – -0.016) | .563 | -0.002 (-0.011 – 0.005) |
| STEMI | < .001 | 0.032 (0.025 – 0.041) | .171 | 0.006 (-0.003 – 0.015) |
| Vessel characteristics | ||||
| Lesion type | ||||
| A | <.001 | 0.022 (0.009 – 0.035) | .040 | 0.012 (0.0005 – 0.023) |
| C | .045 | -0.008 (-0.016 – -0.0002) | .172 | -0.006 (-0.014 – 0.002) |
| LAD | <.001 | -0.070 ( -0.077 – -0.064) | <.001 | -0.063 (-0.070 – -0.056) |
| Bifurcation | < .001 | -0.024 (-0.036 – - 0.012) | .883 | 0.001 (-0.010 – 0.011) |
| Calcified | < .001 | -0.025 (-0.033 – -0.017) | .409 | -0.003 (-0.011 – 0.005) |
| In-stent restenosis | .006 | -0.031 (-0.053 – -0.009) | .007 | -0.029 (-0.051 – -0.008) |
| Thrombus | < .001 | 0.031 (0.021 – 0.042) | .026 | 0.012 (-0.001 – 0.023) |
| Stent thrombosis | .920 | 0.002 (-0.034 – 0.038) | .362 | 0.019 (-0.022 – 0.060) |
| Ostial | .181 | -0.010 (-0.024 – 0.005) | .165 | -0.010 (-0.024 – 0.004) |
| CTO | .002 | -0.034 (-0.056 – -0.013) | .036 | -0.027 (-0.053 – -0.002) |
| Stenosis pre procedural (per 10%) | <.001 | 0.007 (0.005 – 0.009) | .105 | 0.004 (-0.0009 – 0.009) |
| Reference diameter pre procedural (mm) | <.001 | 0.030 (0.023 – 0.037) | .704 | 0.002 (-0.008 – 0.011) |
| Length pre procedural (cm) | .900 | -0.00002 (-0.004 – 0.003) | .101 | 0.004 (0.0008 – 0.009) |
| MLD pre procedural (mm) | <.001 | -0.015 (-0.022 – -0.008) | .638 | 0.004 (-0.014 – 0.023) |
| Predilatation | <.001 | -0.019 (-.027 – -0.011) | .002 | -0.012 (-0.020 – -0.005) |
| Postdilatation | <.001 | 0.027 (-0.035 – -0.019) | .015 | -0.009 (-0.016 – -0.002) |
| Stenosis post procedural (per 10%) | .077 | 0.003 (-0.0003 – 0.006) | .029 | 0.01 (0.0007 – 0.01) |
| Reference diameter post procedural (mm) | <.001 | 0.035 (0.027 – 0.042) | .067 | -0.022 (-0.045 – 0.002) |
| Length post procedural (cm) | .312 | -0.002 (-0.005 – 0.001) | .086 | 0.001 (-0.0007 – 0.001) |
| MLD post procedural (mm) | <.001 | 0.032 (0.024 – 0.040) | .002 | 0.039 (0.015 – 0.063) |
| Number of stents | <.001 | -0.012 (-0.018 – -0.006) | .620 | -0.002 (-0.012 – 0.007) |
| Stent length (cm) | <.001 | 0.019 (0.009 – 0.041) | .286 | -0.003 (-0.009 – 0.002) |
| Stent diameter (mm) | <.001 | 0.033 (0.025 – 0.042) | .026 | 0.012 (0.001 – 0.022) |
|
Beta (β) values are indicative of the average increase or decrease of the FFR values in cases of dichotomous variables or the increment per unit increase in cases of continuous variables. 95%CI, 95% confidence interval; CABG, coronary artery bypass graft; CTO, chronic total coronary occlusion; FFR, fractional flow reserve; LAD, left anterior descending coronary artery; MLD, minimum lumen diameter; STEMI, ST-segment elevation myocardial infarction. |
||||
Figure 2. Forest plot of most important predictors of post-PCI FFR. Adjusted beta values with 95% confidence intervals. The figure includes all significant predictors from the multivariate generalized mixed model predicting post-PCI FFR except for categorical variables with beta values < 0.02. LAD, left anterior descending coronary artery; CTO, chronic total coronary occlusion; MLD, minimum lumen diameter.
DISCUSSION
This study is the largest report to this day of predictors of post-PCI FFR. Based on data derived from the FFR-SEARCH registry, we could identify several patient and procedural predictors of post-PCI FFR. These predictors will bring more in-depth interpretations of post-PCI FFR values to be able to identify correctly which vessels are prone to future events. At first, male gender appeared to be negatively correlated with postprocedural FFR. This finding is consistent with the findings of former studies that focused on the impact of gender on pre-PCI FFR measurements.6,11,15,16 Compared to females, males are known to have a lower prevalence of microvascular dysfunction.8,17 The concept of FFR is based on drug-induced maximal hyperemia to minimize microvascular resistance. Microvascular dysfunction may hamper this vasodilator response and consequently result in a dampened flow response and high FFR.15 Subsequently, on average, males have larger myocardial masses and myocardial perfusion territories compared to females.18,19 The importance of the latter is illustrated by the second and strongest predictor of post-PCI FFR in this study, the FFR measurements in the LAD. FFR values are associated with the myocardial mass and the outflow territory of the measured vessel. As such, the LAD—the vessel with the largest perfusion area—has previously been associated with lower pre- and postprocedural FFR values.20-22
The diameters of the stents implanted in the RCA are larger, on average, but the outflow territory of the LAD is even larger.23 This discrepancy between luminal dimensions and myocardial mass may explain why the optimal improvement of the FFR measurements in the LAD is difficult to achieve.23
Thirdly, larger stent diameters and larger post-PCI MLDs were associated with higher post-PCI FFR values. However, higher postprocedural percent stenosis was also associated with higher post-PCI FFR values. While these findings may seem contradictory, post procedural percent stenosis was not associated with post-PCI physiology in the DEFINE PCI study either.24
In the intravascular ultrasound substudy of the FFR-SEARCH registry, van Zandvoort et al. showed that evident signs of residual luminal narrowing including focal lesions, underexpansion, and malapposition were present in a significant amount of vessels with post-PCI FFR values ≤ 0.85. These findings were not readily apparent on the comprehensive quantitative coronary angiography.25 Percent diameter stenosis was 20% in the cohort of patients with post-PCI FFR values ≤ 0.85 and > 0.85.26
Together with the latter predictors of post-PCI FFR we identified several others. A dedicated analysis of 26 CTOs recently showed that postprocedural FFR values are typically low initially; however they seem to increase at the 4-month follow-up. The initially low post-PCI FFR values is thought to be due to the microvascular dysfunction of the recently opened vessel, a phenomenon that improves after several months.27 In-stent restenosis and pre- and postdilatation were associated with lower post-PCI FFR values. A finding that is consistent with former studies that showed that, in general, complex lesions are associated with lower post-PCI FFR values.20,21,26,28
Also, it was interesting to see the impact of clinical presentation on post-PCI FFR values in the study population in which most patients presented with acute coronary syndrome. Contrary to former studies that questioned the validity of invasive hyperemic physiological indices in patients with acute coronary syndrome, we could not confirm the impact of clinical presentation on post-PCI FFR values. However, the identification of a thrombus, that often occurs after a ruptured plaque in patients with acute coronary syndrome, was associated with significantly higher FFR values. Despite the restoration of epicardial flow by the PCI, a relatively large number of patients with STEMI have abnormal myocardial perfusion at the end of the procedure.29 This phenomenon is thought to be related to microvascular obstruction due to distal embolization (reperfusion injury) and tissue inflammation due to myocyte necrosis.30,31 The latter may explain the significantly higher post-PCI FFR values reported in patients presenting with thrombus-containing lesions compared to those without such lesions. Conversely, our findings also show that in patients without thrombus-containing lesions the post-PCI FFR may be a valuable diagnostic tool for the identification of patients at a high risk of future adverse cardiac events.
Limitations
This study was conducted with the Navvus microcatheter, a dedicated rapid exchange microcatheter with a mean diameter of 0.022 in that proved its utility in a slight but significant underestimation of the FFR compared to conventional 0.014 in pressure guidewires.32 That is why we cannot directly extrapolate the current findings to wire-based FFR devices.14 Based on the study protocol, no further action was taken in the presence of low post-PCI FFR values. The Target FFR and FFR REACT studies (NCT03259815 and NTR6711) will provide further information on post-PCI FFR and the potential of further actions to improve post-PCI FFR and clinical outcomes.33,34 These studies should also focus on the trade-off of potential benefits and harm when performing additional interventions in order to improve the final FFR values.
CONCLUSIONS
In this substudy of the FFR-SEARCH registry, the largest real-world post-PCI FFR registry conducted to this day, we identified sex, LAD vessels, postprocedural MLD, and several other independent predictors of postprocedural FFR.
FUNDING
The FFR SEARCH study was conducted with institutional support from ACIST Medical Inc.
AUTHORS' CONTRIBUTION
Conception and design: L.J.C. van Zandvoort, N.M. van Mieghem, and J. Daemen. Data aquisition: L.J.C. van Zandvoort, K. Masdjedi, J. Wilschut, W. Den Dekker, R. Diletti, F. Zijlstra, N.M. van Mieghem, and J. Daemen. Statistical analysis and manuscript writing: L.J.C. van Zandvoort and J. Daemen. Providing criticial feedback to the manuscript and approving the final content: L.J.C. van Zandvoort, K. Masdjedi, T. Neleman, M.N Tovar Forero, J. Wilschut, W. Den Dekker, R. Diletti, F. Zijlstra, N.M. van Mieghem, and J. Daemen.
CONFLICTS OF INTEREST
L.J.C. van Zandvoort received institutional research support from Acist medical Inc. J. Daemen received institutional research support from Pie Medical, ACIST Medical Inc., PulseCath, Medtronic, Boston Scientific, Abbott Vascular, Pie Medical and speaker and consultancy fees from PulseCath, Medtronic, ReCor Medical, ACIST Medical Inc. and Pie Medical. The remaining authors declared no conflicts of interest.
WHAT IS KNOWN ABOUT THE TOPIC?
- FFR has proven to be a useful technique to address coronary physiology and the hemodynamic significance of coronary segments pre- and post-intervention.
- Also, the FFR post-stenting has proven to be a strong and independent predictor of major adverse cardiovascular events at the 2-year follow-up.
- Unfortunately, at present, there is lack of data on independent predictors of post PCI FFR.
WHAT DOES THIS STUDY ADD?
- This study is the largest report to this day on predictors of post-PCI FFR.
- Based on data from the FFR-SEARCH registry, we could identify several patient and procedural predictors of post-PCI FFR.
- The main predictors included sex, LAD vessels, and postprocedural lumen dimensions. These predictors will help us interpret post-PCI FFR values and identify correctly the vessels that are prone to future events.
REFERENCES
1. Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation. 1995;92:2333-2342.
2. De Bruyne B, Fearon WF, Pijls NHJ, et al. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med. 2014;1533-4406.
3. Wolfrum M, Fahrni G, de Maria GL, et al. Impact of impaired fractional flow reserve after coronary interventions on outcomes:a systematic review and meta-analysis. BMC Cardiovasc Disord. 2016;16:177.
4. Rimac G, Fearon WF, De Bruyne B, et al. Clinical value of post-percutaneous coronary intervention fractional flow reserve value:A systematic review and meta-analysis. Am Heart J. 2017;183:1-9.
5. Kasula S, Agarwal SK, Hacioglu Y, et al. Clinical and prognostic value of poststenting fractional flow reserve in acute coronary syndromes. Heart. 2016;102:1988-1994.
6. Sareen N, Baber U, Kezbor S, et al. Clinical and angiographic predictors of haemodynamically significant angiographic lesions:development and validation of a risk score to predict positive fractional flow reserve. EuroIntervention. 2017;12:e2228-e2235.
7. Baranauskas A, Peace A, Kibarskis A, et al. FFR result post PCI is suboptimal in long diffuse coronary artery disease. EuroIntervention. 2016;12:1473-1480.
8. Crystal GJ, Klein LW. Fractional flow reserve:physiological basis, advantages and limitations, and potential gender differences. Curr Cardiol Rev. 2015;11:209-219.
9. Ahmadi A, Leipsic J, Ovrehus KA, et al. Lesion-Specific and Vessel-Related Determinants of Fractional Flow Reserve Beyond Coronary Artery Stenosis. JACC Cardiovasc Imaging. 2018;11:521-530.
10. Tebaldi M, Biscaglia S, Fineschi M, et al. Fractional Flow Reserve Evaluation and Chronic Kidney Disease:Analysis From a Multicenter Italian Registry (the FREAK Study). Catheter Cardiovasc Interv. 2016;88:555-562.
11. Fineschi M, Guerrieri G, Orphal D, et al. The impact of gender on fractional flow reserve measurements. EuroIntervention. 2013;9:360-366.
12. Ryan TJ, Faxon DP, Gunnar RM, et al. Guidelines for percutaneous transluminal coronary angioplasty. A report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee on Percutaneous Transluminal Coronary Angioplasty). Circulation. 1988;78:486-502.
13. Diletti R, Van Mieghem NM, Valgimigli M, et al. Rapid exchange ultra-thin microcatheter using fibre-optic sensing technology for measurement of intracoronary fractional flow reserve. EuroIntervention. 2015;11:428-432.
14. Menon M, Jaffe W, Watson T, Webster M. Assessment of coronary fractional flow reserve using a monorail pressure catheter:the first-in-human ACCESS-NZ trial. EuroIntervention. 2015;11:257-263.
15. van de Hoef TP, Meuwissen M, Escaned J, et al. Fractional flow reserve as a surrogate for inducible myocardial ischaemia. Nat Rev Cardiol. 2013;10:439-452.
16. Kim HS, Tonino PA, De Bruyne B, et al. The impact of sex differences on fractional flow reserve-guided percutaneous coronary intervention:a FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) substudy. JACC Cardiovasc Interv. 2012;5:1037-1042.
17. Reis SE, Holubkov R, Lee JS, et al. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. Results from the pilot phase of the Women's Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol. 1999;33:1469-1475.
18. Iqbal MB, Shah N, Khan M, Wallis W. Reduction in myocardial perfusion territory and its effect on the physiological severity of a coronary stenosis. Circ Cardiovasc Interv. 2010;3:89-90.
19. Lin FY, Devereux RB, Roman MJ, et al. Cardiac chamber volumes, function, and mass as determined by 64-multidetector row computed tomography:mean values among healthy adults free of hypertension and obesity. JACC Cardiovasc Imaging. 2008;1:782-786.
20. Nam CW, Hur SH, Cho YK, et al. Relation of fractional flow reserve after drug-eluting stent implantation to one-year outcomes. Am J Cardiol. 2011;107:1763-1767.
21. Doh JH, Nam CW, Koo BK, et al. Clinical Relevance of Poststent Fractional Flow Reserve After Drug-Eluting Stent Implantation. J Invasive Cardiol. 2015;27:346-351.
22. Agarwal SK, Kasula S, Hacioglu Y, Ahmed Z, Uretsky BF, Hakeem A. Utilizing Post-Intervention Fractional Flow Reserve to Optimize Acute Results and the Relationship to Long-Term Outcomes. JACC Cardiovasc Interv. 2016;9:1022-1031.
23. Kimura Y, Tanaka N, Okura H, et al. Characterization of real-world patients with low fractional flow reserve immediately after drug-eluting stents implantation. Cardiovasc Interv Ther. 2016;31:29-37.
24. Jeremias A, Davies JE, Maehara A, et al. Blinded Physiological Assessment of Residual Ischemia After Successful Angiographic Percutaneous Coronary Intervention:The DEFINE PCI Study. JACC:Cardiovasc Interv. 2019;12:1991-2001.
25. van Zandvoort LJC, Masdjedi K, Witberg K, et al. Explanation of Postprocedural Fractional Flow Reserve Below 0.85. Circ Cardiovasc Interv. 2019;12:e007030.
26. van Zandvoort LJC, Witberg K, Ligthart J, et al. Explanation of post procedural fractional flow reserve below 0.85:a comprehensive ultrasound analysis of the FFR Search registry. In Cardiovascular Research Technologies (CRT) Conference 2018 March 3-6;Washingtong DC, United States. 2018.
27. Karamasis GV, Kalogeropoulos AS, Mohdnazri SR, et al. Serial Fractional Flow Reserve Measurements Post Coronary Chronic Total Occlusion Percutaneous Coronary Intervention. Circ Cardiovasc Interv. 2018;11:e006941.
28. Pijls NH, Klauss V, Siebert U, et al. Coronary pressure measurement after stenting predicts adverse events at follow-up:a multicenter registry. Circulation. 2002;105:2950-2954.
29. Stone GW, Webb J, Cox DA, et al. Distal microcirculatory protection during percutaneous coronary intervention in acute ST-segment elevation myocardial infarction:a randomized controlled trial. JAMA. 2005;293:1063-1072.
30. Shah NR, Al-Lamee R, Davies J. Fractional flow reserve in acute coronary syndromes:A review. Int J Cardiol Heart Vasc. 2014;5:20-25.
31. Cuculi F, De Maria GL, Meier P, et al. Impact of microvascular obstruction on the assessment of coronary flow reserve, index of microcirculatory resistance, and fractional flow reserve after ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2014;64:1894-904.
32. Pouillot C, Fournier S, Glasenapp J, et al. Pressure wire versus microcatheter for FFR measurement:a head-to-head comparison. EuroIntervention. 2018;13:e1850-e1856.
33. van Zandvoort LJC, Masdjedi K, Tovar Forero MN, et al. Fractional flow reserve guided percutaneous coronary intervention optimization directed by high-definition intravascular ultrasound versus standard of care:Rationale and study design of the prospective randomized FFR-REACT trial. Am Heart J. 2019;213:66-72.
34. Collison D, McClure JD, Berry C, Oldroyd KG. A randomized controlled trial of a physiology-guided percutaneous coronary intervention optimization strategy:Rationale and design of the TARGET FFR study. Clin Cardiol. 2020;43:414-422.