ABSTRACT
Introduction and objectives: Percutaneous coronary interventions (PCI) of chronic total occlusions (CTO) are long procedures where many patients suffer moderate-to-high level anxiety and pain. Virtual reality (VR) has proven capable of reducing procedural pain and anxiety in many medical procedures. The objective of this study is to demonstrate that the use of VR during CTO PCI reduces anxiety and pain compared to conventional routine clinical practice.
Methods: Randomized, controlled, open-label, superiority trial clinical trial with 2 parallel arms including 58 patients with a scheduled CTO PCI randomized on a 1:1 ratio to VR during the procedure or conventional management. In both arms, the administration of anxiolytic drugs will be left to the lead operator’s discretion and based on the degree of anxiety o pain perceived. The remaining actions for the management of pre- and perioperative anxiety will be identical in both arms. The primary endpoint will be the maximum level of anxiety perceived by the patient. Secondary endpoints will be the level of patient-perceived pain, the need for intraoperative anxiolytic drug therapy, dose of drug administered, and satisfaction with the VR goggles.
Results: The results of this study will add significant knowledge on the utility of VR regarding anxiety reduction in CTO PCIs.
Conclusions: The ReViCTO trial is the first randomized clinical trial to use VR during a PCI CTO. Its results will show the utility of this technology to reduce anxiety and pain in PCIs performed on CTOs.
Diseño del ensayo registrado en ClinicalTrials.gov (identificador: NCT05458999).
Keywords: Chronic total coronary occlusion Virtual reality Anxiety
RESUMEN
Introducción y objetivos: Las intervenciones coronarias percutáneas (ICP) sobre oclusiones totales crónicas (OTC) son procedimientos largos en los que muchos pacientes sufren ansiedad y dolor. La realidad virtual ha demostrado reducir el dolor y la ansiedad en muchos procedimientos médicos. Nuestro objetivo es demostrar que el uso de la realidad virtual durante la ICP de OTC reduce la ansiedad y el dolor en comparación con la práctica convencional.
Métodos: Ensayo clínico aleatorizado, controlado, abierto y de superioridad con 2 grupos paralelos en el que 58 pacientes con una ICP de OTC programada serán aleatorizados 1:1 al uso de realidad virtual frente al tratamiento convencional. La administración de fármacos ansiolíticos será a criterio del operador principal y en función del grado de ansiedad o dolor percibido. El resto de las acciones para el tratamiento de la ansiedad serán idénticas en ambos grupos. El objetivo primario será el nivel máximo de ansiedad percibido por el paciente. Los objetivos secundarios serán el nivel de dolor percibido por el paciente, la necesidad de tratamiento farmacológico ansiolítico, la dosis de fármaco administrada y la satisfacción con la realidad virtual.
Resultados: Los resultados de este estudio añadirán conocimientos importantes sobre la utilidad de la realidad virtual en la reducción de la ansiedad en los procedimientos de ICP de OTC.
Conclusiones: El ensayo ReViCTO es el primer ensayo clínico aleatorizado que utiliza la realidad virtual durante la ICP en OTC. Sus resultados mostrarán la utilidad de esta tecnología para reducir la ansiedad y el dolor en esta intervención.
Diseño del ensayo registrado en ClinicalTrials.gov (identificador: NCT05458999).
Palabras clave: Oclusión total crónica Realidad virtual Ansiedad
Abbreviations
CTO: chronic total coronary occlusion. PCI: percutaneous coronary intervention. VAS: visual analogue scale. VASA: visual analogue scale of anxiety. VASP: visual analogue scale of pain. VR: virtual reality.
INTRODUCTION
Chronic total coronary occlusions (CTO) are diagnosed in up to 15% of patients with coronary artery disease undergoing coronary angiography.1 Percutaneous coronary interventions (PCI) of CTOs are one of the greatest challenges we face in interventional cardiology due to the complexity of these procedures and the increased risk of complications.2 Over the past few decades, advances in techniques and devices have made it possible to obtain better results while reducing the associated complications.3-5 Anxiety and pain during these procedures are often treated with oral benzodiazepines plus opioids or IV benzodiazepines upon request during the procedure. The possibility of performing these procedures without anesthesia or sedation avoids the risks associated with these therapies. On the contrary, it submits the patient to pain and anxiety during the procedure. Several factors such as long procedures, patient immobility (especially in biradial access), and monotonous and hostile environments (operating rooms or cath labs) influence patient anxiety. Virtual reality (VR) has been successfully used in several clinical settings such as transcatheter aortic valve implantation6 or atrial fibrillation ablation7 to reduce intraoperative anxiety. There is no evidence on the use of VR reducing perioperative patient anxiety during PCI, specifically in CTO PCI. Compared to standard PCI, this procedure could benefit even further from VR due to its longer duration, use of double arterial access, and possibility of triggering ischemia and chest pain.
The objective of this study is to determine whether the use of a VR system in PCIs on CTOs decreases the level of anxiety and pain during CTO procedures compared to conventional management.
METHODS
Overall study design
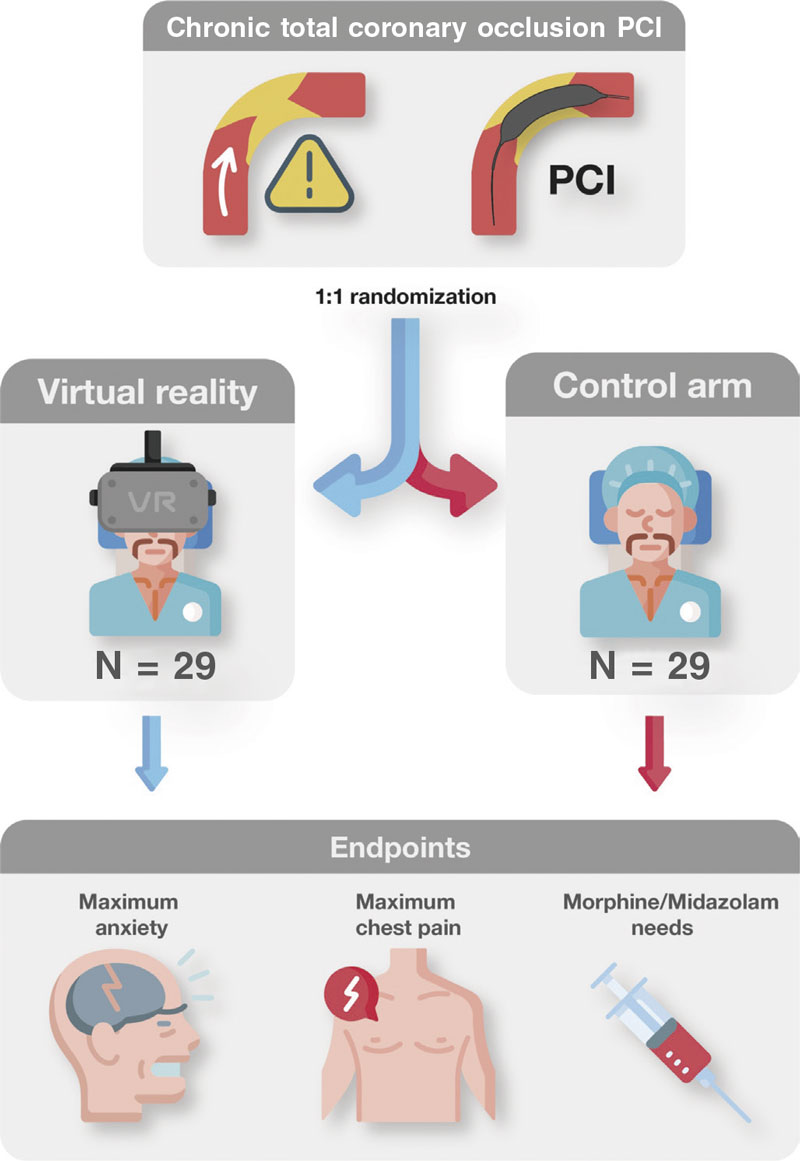
The Decreasing patient anxiety during revascularization of chronic total coronary occlusions using virtual reality glasses (ReViCTO) trial (ClinicalTrials.gov Identifier: NCT05458999) was designed as a randomized, controlled, open-label, superiority clinical trial with 2 parallel arms (procedural use of VR goggles vs conventional management) with a primary endpoint of maximum level of anxiety perceived by the patient measured through the visual analogue scale of anxiety (VASA).
The study will be conducted in full compliance with the principles set forth in the Declaration of Helsinki (1996) and the International Conference on Harmonization Good Clinical Practice Guideline. The study protocol was approved by the Clinical Research Ethics Committee (CREC) of Hospital Clínico Universitario de Valencia, Spain. All patients signed an informed consent form. The study is registered at clinicaltrials.gov (NCT05458999). The World Health Organization minimum standard list of items for clinical trials are listed in table 1 of the supplementary data. This protocol follows the SPIRIT 2013 Statement: Defining Standard Protocol Items for Clinical Trials.8
Study setting and eligibility criteria
The trial will be conducted at Hospital Clínico Universitario de Valencia, Spain, a reference teaching hospital on interventional cardiology that treats nearly 800 000 patients both from rural and metropolitan areas. Since this is a preliminary study it is designed as a single-center trial. All procedures will be performed by a team of 2 interventional cardiologists experienced in CTO revascularization. Patient enrolment started back in December 2021. On Dec. 25th 2022, 25 patients had already been enrolled in the study (43% of the target population). Patients with visual impairment, dementia, language barriers or any situations that would prevent the use of VR glasses will be excluded. Inclusion and exclusion criteria are listed in table 1. All patients must meet all the inclusion criteria and none of the exclusion criteria.
Table 1. Inclusion and exclusion criteria
| Inclusion criteria |
| Age > 18 years |
| Elective percutaneous coronary intervention on chronic total coronary occlusion |
| Physical and mental ability to wear virtual reality glasses |
| Exclusion criteria |
| Unable to or unwilling to give informed consent |
| Visual impairment |
| Dementia |
| Language barrier (unable to communicate fluently in Spanish or English) |
| Any other situations that would prevent the use of virtual reality glasses |
Assignment of interventions
Each patient will be randomized on a 1:1 ratio to the intervention (use of VR goggles during the CTO procedure) or the control arm (routine clinical practice). Given the small sample size estimated, permuted block randomization was used to guarantee an equal number of participants per arm.9 Random sequence was computer generated using blocks with a size unknown to the investigators until the end of recruitment. Patient enrolment and arm assignment will be performed by the investigators. Allocation concealment will be ensured using a web application that assigns a unique identification number and the assigned arm once the patient has been recruited for the trial. This system prevents changes to the identification number or arm deleting patients after randomization. Because of the nature of the trial no masking or blinding will be applied at any level.
Participant timeline
Since there is no follow-up, this study has a very simple timeline. Upon arrival to the cath lab, all patients scheduled for elective CTO PCI will be screened and checked to see if they meet all the inclusion criteria and none of the exclusion criteria. If they don’t meet these criteria, they will be considered a screening failure and will not participate in the study. If all criteria are met by the patients, the investigators will need to obtain their written informed consent right before patients arrive at the cath lab. The functioning of the trial will be explained orally and reading of the informed consent will be offered allowing enough time, if necessary. Patients will be randomized to wear VR goggles or to the control arm. During the PCI, all measures regarding anxiety will be applied regardless of the allocation arm. Also, all drug therapies administered will be registered by the study nurse. After the procedure, the patient’s perceived anxiety and pain will be assessed by the study nurse, this being the end of the trial for the patient (figure 1).
Figure 1. Central illustration. Trial flowchart.
Data collection
Demographics, the past medical history, preoperative (indication for revascularization, blood tests, left ventricular ejection fraction…), and perioperative variables [arterial access, radiation dose, maximum level of anxiety (VASA), and visual analogue scale of pain (VASP) perceived by the patient measured through visual analogue scale, nausea, and dizziness during the procedure] will be collected (table 2 of the supplementary data).
Clinical variables will be collected from the local and regional electronic clinical data system and asked directly to the patient when lacking. Blood test results will be collected from the local laboratory system using the last available determination. Echocardiographic and magnetic resonance imaging data will be collected from the local electronic clinical data system. The Seattle Angina Questionnaire, VASA, VASP, the presence of nausea or dizziness, overall satisfaction with the procedure, and overall satisfaction will be assessed by the study nurse and included in a dedicated form (table 3 of the supplementary data). All these data will be transferred to a dedicated database in 1 single local computer. This database is designed with range check for numerical variables to prevent erroneous data entry. Also, the database will check for duplicates when entering the hospital identification number. All data will be stored in a database kept in a dedicated computer with no Internet connection to avoid unwanted leaks or stole information. The investigators will have access to this database only.
Trial intervention
Eligible patients will be randomized to the intervention (VR goggles) or the control arm (routine clinical practice).
Virtual reality goggles
A commercial Oculus Quest 2 VR goggle system (Meta Platforms, Inc., United States) will be used. The viewing consisted of using the capabilities of the VR goggles to recreate a 2D playback that simulates the size of a large-format movie screen. Using Netflix video streaming system (Netflix Inc. United States), the documentary series “Our Planet” [Silverback Films, United Kingdom]10 will be played for all patients starting with chapter 1, and sequentially and automatically playing the following chapters. Before the procedure, the patient will be informed on the VR goggle system-based operation, possible side effects (nausea or dizziness), and the possibility to remove it at any time. Before the arterial puncture, the VR goggle system will be put on and checked for proper functioning. It will be removed before removing arterial introducers, when the patient wishes to do so or if serious complications occur. During the procedure, the patient’s general condition will be checked every 30 min.
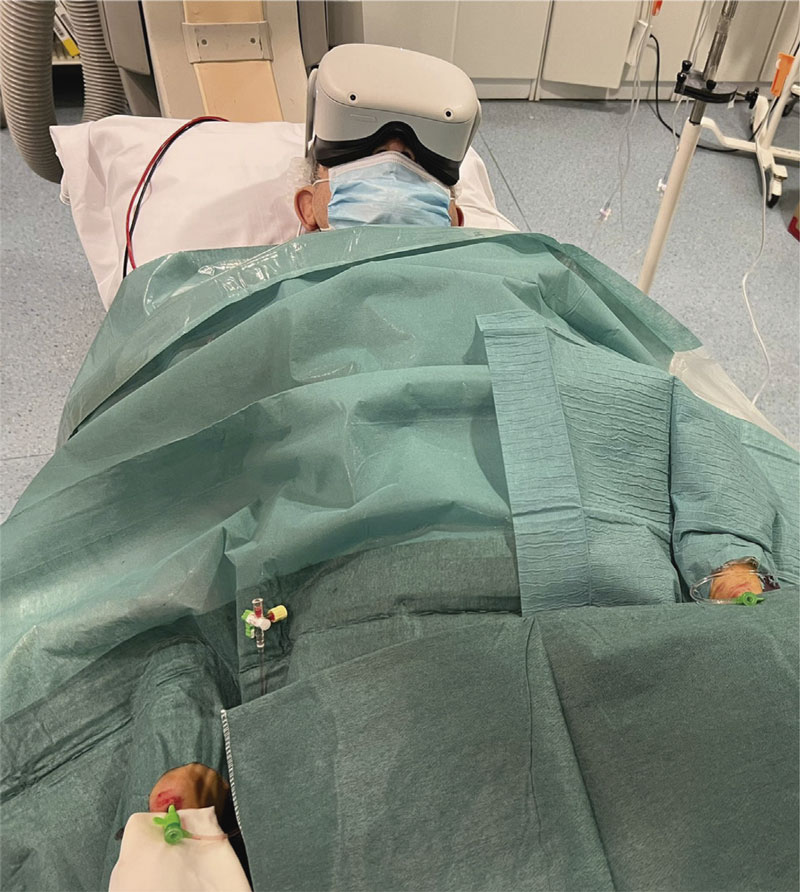
The system will be prepared following these steps: 1) drawing the security perimeter with the controller; 2) starting the Netflix application; 3) selecting the “Void Theater” option; 4) searching for the documentary series “Our Planet” and playing the first episode; 5) adjusting the screen size with the controller; 6) selecting travel mode; 7) putting the VR goggles on the patient (figure 2); 8) asking the patient if he can watch and hear correctly. If not, the VR goggles should be repositioned.
Figure 2. Real patient wearing virtual reality goggles during a chronic total coronary occlusion revascularization procedure using double radial artery access.
Control arm
The comparator chosen is the current clinical practice with no VR goggles. A possible comparator using a VR goggle with no content was discarded because of the high chances of claustrophobia or mental discomfort.
Both arms will receive drugs upon request to reduce perceived pain and anxiety. In both arms, anxiolytic drugs (morphine chloride or midazolam at 1 mg boluses) will be administered by the circulating nurse if the patient explicitly expresses the need for such treatment or if external signs of anxiety or pain (agitation, complaints...) are observed. The last decision on treatment administration will be left to the lead operator. The remaining actions for the management of pre- and perioperative anxiety will be identical in both arms. Preoperative anxiolytic treatment was not routinely administered to all patients, only upon the patient’s request.
Endpoints
The primary endpoint will be to assess changes to the maximum level of anxiety perceived by the patient during the procedure. Secondary endpoints will be to assess a) changes to the maximum level of pain perceived by the patient during the procedure; b) differences in the need for intraoperative anxiolytic drug therapy or doses of anxiolytic drugs (midazolam or morphine chloride) administered during the procedure; and c) the overall satisfaction experienced with the VR goggles.
Both the primary (anxiety) and secondary endpoints of pain will be measured through the VASA11 and pain (VASP)12 that go from 1 to 10. Both VASA and VASP will be collected by a specialized nurse right after the end of the procedure before leaving the room through a specific survey on the maximum level of anxiety or pain perceived during the procedure (table 3 of the supplementary data).
The VAS will be used to quantify the patients’ responses objectively. The VAS eliminates the examiner influence or bias that often comes with verbal questioning and is a more appealing method of evaluation for participants. Although this method is not perfect, it remains a common way to assess anxiety and pain.13,14 The patient will also be asked if he’d like VR to be used in other similar settings. Total doses of benzodiazepines (midazolam) or opioids (morphine) administered during the procedure will be registered in total milligrams.
Sample size estimate
The sample size was estimated based on the primary endpoint. In former studies that assessed anxiety during catheterization the standard deviation of VASA was 2.715,16 (σ). VASA > 2 (μ1 – μ2) was descriptive of clinically significant differences. To detect differences ≥ 2 in VASA assuming a normal distribution, alpha and beta risks of 0.05 (α) and 0.2 (β) in bilateral contrast in a sample size of 58 patients (29 in each arm) were estimated.
Statistical analysis
Quantitative variables will be expressed as mean ± standard deviation when they follow a normal distribution and as median [interquartile range] if they don’t. Qualitative ones will be expressed as percentages (absolute value). Fisher’s exact test or the chi-square test will be used to compare qualitative variables. Also, the Student t test or the Mann-Whitney U test will be used if quantitative variables don’t follow a normal distribution.
The primary endpoint (VASA), VASP, and dosage of drugs will be compared in both arms using the Student t test. The use or non-use of drugs during the procedure, the presence or absence of dizziness or nausea will be compared using Fisher’s exact test or the chi-square test. Subgroup analyses will be performed based on sex, age, and previous experience with new technologies.
All statistical tests will be bilateral and considered significant if P < .05. Statistical analyses will be performed with R Core Team (2020) statistical software package (R Foundation for Statistical Computing, Austria).
DISCUSSION
CTOs are present in up to 20% of the patients with coronary artery disease. These numbers increase parallel to age (up to 40% in diabetics or patients with heart failure).17,18 In the past, most of these patients were referred for revascularization surgery due to poorly successful PCIs in this kind of lesions. Over the past few decades, several advances have been made regarding devices and technical materials, organization, and concentration of complex procedures in reference centers. Also, increased operator experience has led to a high success rate of 90%, and a very low rate of severe complications19,20 with the corresponding increase in the number indications for PCI CTO.
Although PCIs are a common and relatively low risk procedure, many patients undergoing these treatments experience anxiety (up to 37% in some populations).21-23 Anxiety involves feelings of fear, tension or panic or the prospect that something unpleasant is about to happen. State anxiety may be more clinically relevant for patients undergoing PCI because it is transient in nature and amenable to clinical procedures. Patients undergoing PCI have multiple sources of anxiety including their own concerns. These concerns can include fear of discomfort, uncertainty, and fear associated with survival that can be more distressing than chest pain itself.24
PCI CTO creates more anxiety for the patients compared to other interventional procedures for several reasons. In the first place, double access with high-calibre sheaths is frequently used, even biradial. Repeated access punctures with consequently an increased pain and limited patient mobility contributes to more discomfort and higher anxiety levels. Secondly, the duration of the procedure is long, and can be up to 3 to 4 hours or more in some special scenarios. Being exposed to immobility in a monotone and hostile scenario for such a long time is a reasonable cause for anxiety. Thirdly, cath labs are often strange environments for the patient with machinery and equipment that may be frightening for him at first. Furthermore, discussion with the treating team, the use of terms unfamiliar to the patient or the existence of beeps and alarms can make the patient think that something bad might happen to him, thus increasing the levels of anxiety. Fourthly, patients undergoing elective PCI CTO usually undergo, at best, at least, 1 invasive coronary angiography, and commonly up to several coronary interventions including failed CTO revascularization attempts. Previous procedures could be remembered as painful or stressful and anticipation anxiety could appear. Stress and anxiety associated with needle-related procedures may lead to needle phobia,25 which could also contribute to a high level of anxiety. Fifthly, chest pain is an important factor of procedural anxiety during CTO PCI, and it occurs in a large number of patients. For example, with retrograde approaches, the flow of collateral branches on which the CTO-related myocardial territory is completely dependent is interrupted due to their occupation by the guidewire or microcatheter, thus causing ischemia and pain). Therefore, there is a potential high anxiety level in patients undergoing CTO PCI that depends on multiple factors and mechanisms that feed from one another.
At the end of the 20th century, it was noted that both behavioral and pharmaceutical interventions should be used to manage pain during medical procedures.26 Distraction techniques may be effective reducing the patients’ pain during various invasive procedures because pain involves both physical stimuli and emotional responses. Studies have shown that various distraction techniques like music, massage, breathing exercises, and behavioral therapy can effectively reduce the feeling of pain and stress symptoms during painful procedures.27,28
VR is a computer-generated simulation of the physical world that allows people to experience it in a realistic way. VR goggles achieve visual and auditory semi-isolation that, together with the images projected, evade the patient while act on environmental and emotional factors of anxiety. VR has proven superior to other distraction methods such as television, listening to music or playing games.29,30 VR has been used to relieve anxiety and pain in patients undergoing several kinds of procedures as needle-related interventions,31 burn wound debridement,32,33 physical therapy,34 dental procedures,35 colonoscopy,36 minor surgical procedures,37 nasal endoscopy38 or chemotherapy.39 A total of 4 randomized clinical trials have been conducted to study the level of anxiety experienced by adults undergoing different medical procedures like hysteroscopy,40 labor,41 and colonoscopy.42 The studies used various measurement tools such as the VAS scale from 0 to 10, the 5-point Likert scale 0-5, and the State-Trait Anxiety Inventor.
Experiences with VR in interventional cardiology during procedures are scarce. Back In 2020, Bruno et al.6 used a randomized clinical trial to prove the that the use of a VR-based system was safe and feasible during TAVI and that VAS score was reduced by 3 points with the use of VR without impacting nausea or vomiting. Almost all patients said they would use this technology in a similar setting. It is remarkable that this study population was an old population (mean age, 83 years) without previous experience with VR and limited experience with new technologies. This shows that even in a population not used to new technologies that could be expected to reject or not tolerate VR goggles, its use was tolerated and effective. Moreover, this study found that it was important not only that patients accepted the new technology, but also that interventional cardiologists approved it. At first, their reaction went from full support to slight rejection. Those who hesitated to use this new approach thought that their interaction with the patient during the procedure might be limited. However, over time, when they saw that this was not the case, acceptance increased. Similarly, Roxburgh et al.7 tested the utility of VR in patients undergoing atrial fibrillation ablation in an observational study of 48 patients. They showed that VR reduced perceived pain during the procedure and that VR can be easily incorporated into the standard procedure workflow. As far as we know, no studies have ever been conducted on the use of VR during PCI CTO.
Most studies on VR technology have been observational and not standardized, which complicates comparing results and drawing solid conclusions. Additionally, currently, no guidelines or consensus have ever been published on how to incorporate VR technology to cardiac procedures.43 To address these challenges, an expert taskforce from the international scientific community may be useful to identify evidence gaps, set priorities, standardize research protocols, and create guidelines to implement VR technology in heart procedures.
Limitations
Some limitations should be taken in account in this clinical trial. First, the open-label nature of the trial could lead to bias favorable to the RV group due to the lack of blinding and potential for patients and investigators to influence the outcomes. The entire staff will be warned on this possible bias and advised to prevent it before each procedure. Second, some uncontrolled confounding factors may play a role in the differences seen in the administration of drug therapy between RV and the control groups. We will try to prevent or, at least, mitigate this treatment only if the patient explicitly wishes to do so or if outward signs of anxiety or pain are observed. Third, the results of this trial should be interpreted and applied with caution to other scenarios due to the single-center nature of this trial. Fourth, the primary and secondary endpoints of anxiolytic treatment needed could be closely correlated. Our objective is to determine whether the use of VR goggles decreases anxiety. However, it could happen that both groups have similar levels of anxiety and a greater need for anxiolytic treatment (which is a surrogate of increased anxiety, on the one hand, that could also expose patients to a higher risk of adverse effects, on the other). The choice of the primary and secondary endpoints of anxiety and need for anxiolytic treatment allows us to explore this possibility. Finally, the drug therapy of anxiety was not protocolized but left to the operator’s discretion.
CONCLUSIONS
The ReViCTO trial is the first randomized clinical trial ever designed to evaluate the use of VR during CTO PCI. Results will show the utility of this technology reducing anxiety and pain in PCI CTO.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
A. Fernández-Cisnal, and G. Miñana: study idea or design, data curation, analysis, and interpretation, drafting, and final approval of the version submitted for publication. B. Silla, J.M. Ramón, E. Valero, and S. García-Blas: data curation, analysis, and interpretation, revision of the manuscript regarding significant intellectual content, and final approval of the version submitted for publication. J. Núñez, V. Bodí, and J. Sanchis: data analysis and interpretation, revision of the manuscript regarding significant intellectual content, and final approval of the version submitted for publication. A. Fernández-Cisnal, and G. Miñana agree to take full responsibility for all aspects of the manuscript, and investigate and resolve all questions regarding the accuracy and truthfulness of the study as a whole.
CONFLICTS OF INTEREST
J. Núñez received fees for participating in the advisory boards and educational activities from Astra Zeneca, Boehringer-Ingelheim, NovoNordisk, Bayer, and Novartis. J. Sanchis received speaker fees from Abbott Vascular, and Prosmedica. G. Miñana received speaker fees from Abbott Vascular, and Teleflex, and support for attending meetings from Medtronic and World Medical. The remaining authors declared no other conflicts of interest whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- CTO PCI is one of the greatest challenges for interventional cardiology due to the complexity of these procedures and the increased risk of complications.
- Several factors like long procedures, patient immobility, and the presence of a monotonous and hostile environment influence patient anxiety, which is usually treated with benzodiazepines and opioids upon request during the procedure.
- Virtual reality has been successfully used in several clinical settings reducing intraoperative anxiety. There is no evidence that the use of VR reduces perioperative patient anxiety during CTO PCI.
WHAT DOES THIS STUDY ADD?
- The ReViCTO trial is the first randomized clinical trial ever conducted to use VR during PCI CTO. Its results will show the utility of this technology reducing anxiety and pain in PCI CTO.
- Primary endpoint will be to assess changes to the maximum level of anxiety perceived by the patient.
- Secondary endpoints will be a) changes to the maximum level of pain during the procedure; b) differences in the need for intraoperative anxiolytic drug therapy; and c) overall satisfaction with the VR goggles.
REFERENCES
1. Tajti P, Burke MN, Karmpaliotis D, et al. Update in the Percutaneous Management of Coronary Chronic Total Occlusions. JACC Cardiovasc Interv. 2018;11:615-625.
2. Tajti P, Brilakis ES. Chronic Total Occlusion Percutaneous Coronary Intervention: Evidence and Controversies. J Am Heart Assoc. 2018;7:e006732.
3. Patel VG, Brayton KM, Tamayo A, et al. Angiographic success and procedural complications in patients undergoing percutaneous coronary chronic total occlusion interventions: a weighted meta-analysis of 18,061 patients from 65 studies. JACC Cardiovasc Interv. 2013;6:128-136.
4. Brilakis ES, Banerjee S, Karmpaliotis D, et al. Procedural outcomes of chronic total occlusion percutaneous coronary intervention: a report from the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc Interv. 2015;8:245-253.
5. Azzalini L, Karmpaliotis D, Santiago R, et al. Contemporary Issues in Chronic Total Occlusion Percutaneous Coronary Intervention. JACC Cardiovasc Interv. 2022;15:1-21.
6. Bruno RR, Lin Y, Wolff G, et al. Virtual reality-assisted conscious sedation during transcatheter aortic valve implantation: a randomised pilot study. EuroIntervention. 2020;16:e1014-e1020.
7. Roxburgh T, Li A, Guenancia C, et al. Virtual Reality for Sedation During Atrial Fibrillation Ablation in Clinical Practice: Observational Study. J Med Internet Res. 2021;23:e26349.
8. Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 Statement: Defining Standard Protocol Items for Clinical Trials. Ann Intern Med. 2013;158:200.
9. Schulz KF, Grimes DA. Unequal group sizes in randomised trials: guarding against guessing. Lancet. 2002;359:966-970.
10. Our Planet. Silverback Films, UK. Available at: https://www.netflix.com/es/title/80049832. Accessed 02 Feb 2023.
11. Williams VS, Morlock RJ, Feltner D. Psychometric evaluation of a visual analog scale for the assessment of anxiety. Health Qual Life Out. 2010;8:57.
12. Carlsson AM. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain. 1983;16:87-101.
13. Aubrun F, Paqueron X, Langeron O, Coriat P, Riou B. What pain scales do nurses use in the postanaesthesia care unit? Eur J Anaesth. 2003;20:745-749.
14. Kindler CH, Harms C, Amsler F, Ihde-Scholl T, Scheidegger D. The Visual Analog Scale Allows Effective Measurement of Preoperative Anxiety and Detection of Patients’ Anesthetic Concerns. Anesth Analg. 2000;90:706-712.
15. Delewi R, Vlastra W, Rohling WJ, et al. Anxiety levels of patients undergoing coronary procedures in the catheterization laboratory. Int J Cardiol. 2017;228:926-930.
16. Vlastra W, Delewi R, Rohling WJ, et al. Premedication to reduce anxiety in patients undergoing coronary angiography and percutaneous coronary intervention. Open Hear. 2018;5:e000833.
17. Damluji AA, Pomenti SF, Ramireddy A, et al. Influence of Total Coronary Occlusion on Clinical Outcomes (from the Bypass Angioplasty Revascularization Investigation 2 DiabetesTrial). Am J Cardiol. 2016;117:1031-1038.
18. Tajstra M, Pyka Ł, Gorol J, et al. Impact of Chronic Total Occlusion of the Coronary Artery on Long-Term Prognosis in Patients With Ischemic Systolic Heart Failure Insights From the COMMIT-HF Registry. JACC Cardiovasc Interv. 2016;9:1790-1797.
19. Christopoulos G, Karmpaliotis D, Alaswad K, et al. Application and outcomes of a hybrid approach to chronic total occlusion percutaneous coronary intervention in a contemporary multicenter US registry. Int J Cardiol. 2015;198:222-228.
20. Amat-Santos IJ, Martin-Yuste V, Fernández-Díaz JA, et al. Resultados inmediatos e impacto funcional y pronóstico tras la recanalización de oclusiones coronarias crónicas. Resultados del Registro Ibérico. Rev Esp Cardiol. 2019;72:373-382.
21. Astin F, Jones K, Thompson DR. Prevalence and patterns of anxiety and depression in patients undergoing elective percutaneous transluminal coronary angioplasty. Hear Lung J Acute Critical Care. 2005;34:393-401.
22. Lenzen MJ, Gamel CJ, Immink AW. Anxiety and Well-Being in First-Time Coronary Angioplasty Patients and Repeaters. Eur J Cardiovasc Nur. 2002;1:195-201.
23. Trotter R, Gallagher R, Donoghue J. Anxiety in patients undergoing percutaneous coronary interventions. Hear Lung J Acute Critical Care. 2011;40:185-192.
24. Heikkilä J, Paunonen M, Virtanen V, Laippala P. Fear of patients related to coronary arteriography. J Adv Nurs. 1998;28:54-62.
25. McLenon J, Rogers MAM. The fear of needles: A systematic review and meta-analysis. J Adv Nurs. 2019;75:30-42.
26. Zeltzer LK, Altman A, Cohen D, LeBaron S, Munuksela EL, Schechter NL. American Academy of Pediatrics Report of the Subcommittee on the Management of Pain Associated with Procedures in Children with Cancer. Pediatrics. 1990;86(5 Pt 2):826-831.
27. Manne SL, Bakeman R, Jacobsen PB, Gorfinkle K, Redd WH. An analysis of a behavioral intervention for children undergoing venipuncture. Health Psychol. 1994;13:556-566.
28. Burns-Nader S, Joe L, Pinion K. Computer tablet distraction reduces pain and anxiety in pediatric burn patients undergoing hydrotherapy: A randomized trial. Burns. 2017;43:1203-1211.
29. Hoffman HG, Chambers GT, Meyer WJ, et al. Virtual Reality as an Adjunctive Non-pharmacologic Analgesic for Acute Burn Pain During Medical Procedures. Ann Behav Med. 2011;41:183-191.
30. Malloy KM, Milling LS. The effectiveness of virtual reality distraction for pain reduction: A systematic review. Clin Psychol Rev. 2010;30:1011-1018.
31. Wang Y, Guo L, Xiong X. Effects of Virtual Reality-Based Distraction of Pain, Fear, and Anxiety During Needle-Related Procedures in Children and Adolescents. Front Psychol. 2022;13:842847.
32. Kipping B, Rodger S, Miller K, Kimble RM. Virtual reality for acute pain reduction in adolescents undergoing burn wound care: A prospective randomized controlled trial. Burns. 2012;38:650-657.
33. Khadra C, Ballard A, Déry J, et al. Projector-based virtual reality dome environment for procedural pain and anxiety in young children with burn injuries: a pilot study. J Pain Res. 2018;11:343-353.
34. Soltani M, Drever SA, Hoffman HG, et al. Virtual Reality Analgesia for Burn Joint Flexibility: A Randomized Controlled Trial. Rehabil Psychol. 2018;63:487-494.
35. Lahti S, Suominen A, Freeman R, Lähteenoja T, Humphris G. Virtual Reality Relaxation to Decrease Dental Anxiety: Immediate Effect Randomized Clinical Trial. Jdr Clin Transl Res. 2020;5:312-318.
36. Veldhuijzen G, Klaassen NJM, Wezel RJAV, Drenth JPH, Esch AAV. Virtual reality distraction for patients to relieve pain and discomfort during colonoscopy. Endosc Int Open. 2020;08:E959-E966.
37. Clerc PGB, Arneja JS, Zwimpfer CM, Behboudi A, Goldman RD. A Randomized Controlled Trial of Virtual Reality in Awake Minor Pediatric Plastic Surgery Procedures. Plast Reconstr Surg. 2021;148:400-408.
38. Liu KY, Ninan SJ, Laitman BM, Goldrich DY, Iloreta AM, Londino AV. Virtual Reality as Distraction Analgesia and Anxiolysis for Pediatric Otolaryngology Procedures. Laryngoscope. 2021;131:E1714-E1721.
39. Fabi A, Fotia L, Giuseppini F, et al. The immersive experience of virtual reality during chemotherapy in patients with early breast and ovarian cancers: The patient’s dream study. Frontiers Oncol. 2022;12:960387.
40. Deo N, Khan K, Mak J, et al. Virtual reality for acute pain in outpatient hysteroscopy: a randomised controlled trial. Bjog Int J Obstetrics Gynaecol. 2021;128:87-95
41. Xu N, Chen S, Liu Y, Jing Y, Gu P. The Effects of Virtual Reality in Maternal Delivery: Systematic Review and Meta-analysis. Jmir Serious Games. 2022;10:e36695.
42. Cakir SK, Evirgen S. The Effect of Virtual Reality on Pain and Anxiety During Colonoscopy: A Randomized Controlled Trial. Turk J Gastroenterol. 2021;32:451-547.
43. Mahtab EAF, Egorova AD. Current and future applications of virtual reality technology for cardiac interventions. Nat Rev Cardiol. 2022;19:779-780.