To the Editor,
Critical, severe, and congenital aortic stenosis (AS) is challenging regarding the decision-making process, and has high mortality and morbidity rates,1 being percutaneous treatment the one more commonly used in most centers. The objective of this study was to assess the clinical and echocardiographic progression of congenital ASs treated with percutaneous valvuloplasty (PV), and the predictive factors of worse disease progression.2,3
RSevere (peak velocity > 4 m/s or mean gradient > 40 mmHg), and critical ASs (ductus-dependent systemic flow) were included retrospectively based on the first postnatal echocardiography diagnosed during the fetal stage and until the first month of life and then treated with PV in a tertiary center from 2009 through 2019. The criteria established by the Declaration of Helsinki were followed, and the patients’ informed consent was waived.
Left ventricular ejection fraction (LVEF), endomyocardial fibroelastosis, flows in ductus, foramen ovale, ascending aorta, aortic arch, mitral regurgitation, and hydrops were all analyzed. The size and shape of the aortic valve, the size, function, and ventricular fibroelastosis at birth, the immediate control after PV and at the follow-up, the hemodynamic gradients of PV, and complications were collected. PV was considered effective with peak residual hemodynamic gradients ≤ 35 mmHg or 50% decrease with normal LVEF. The need for Ross surgery, heart transplant, and death were regarded as unfavorable disease progression. Qualitative variables were expressed as percentages, and the quantitative ones as median and interquartile range [IQR]. The contrast of univariate hypothesis was conducted using the Mann-Whitney U test, and Fisher’s exact test. Confounding variables distinction was conducted through multivariate analysis using the linear regression inverse verisimilitude method. The significance level of the alpha risk was .05%.
A total of 23 patients were obtained, 6 of whom (26.09%) were women. Overall, 7 patients (30.44%) were associated with aortic coarctation, and 2 (11.39%) with moderate-to-severe mitral stenosis. A total of 6 critical ASs (26.09%) were found, 2 of which (11.39%) were unicuspid, 8 (34.78%) pure bicuspid, and 12 (52.17%) tricuspid; a total of 8 raphes were reported between the non-coronary and right coronary leaflets, and 2 between the right and left coronary leaflets.
Regarding the moment of diagnois, 6 ASs (26.09%) were diagnosed during the prenatal stage and 17 (73.91%) during the neonatal period. A total of 3 ASs (13.05) from the prenatal group had severe dysfunction with grade 4 friboelastosis and flow reversal in the ascending and transverse aortic arch. One of them had hydrops and the other one was treated with an elective fetal valvuloplasty that was performed on week 26.
A total of 6 patients (26.09%) presented with cardiogenic shock at birth. Only in critical ASs systo-diastolic dysfunction was reported with median LVEF of 42.50% [IQR, 40.00-57.25], which was lower compared to the severe ones (P = .011). A total of 10 patients (47.83%) had mitral regurgitation, 2 of them (8.70%) severe.
The PV was performed after a median 42 days of life [IQR, 12.25-56], 7 of which (30.43%) wre performed within the first week of life. The balloon used was the TYSHAK mini or II (NuMED Inc., United States). The balloon/valvular diameter ratio was 1.00 [IQR, 0.91-1.03], which was significantly higher in the unicuspid valve group. The vascular access was the femoral artery. The overall effectiveness rate was 78.26%, 83.33% (n = 5/6) in critical ASs, and 76.5% (n = 13/17) in the severe ones.
After a median follow-up of 4 years [IQR, 3,5-6,25] the survival rate was 92.31%. A total of 15 patients (65.22%) remained reintervention-free. Reinterventions (figure 1) occurred within the first year of life: 4 patients needed a new PV, 3 patients required Ross surgery, and 2 surgical valvulotomy (1 died due to postoperative cardiogenic shock), and 1 heart transplant due to heart failure and pulmonary hypertension (the patients died due to rejection, and pulmonary hypertension).
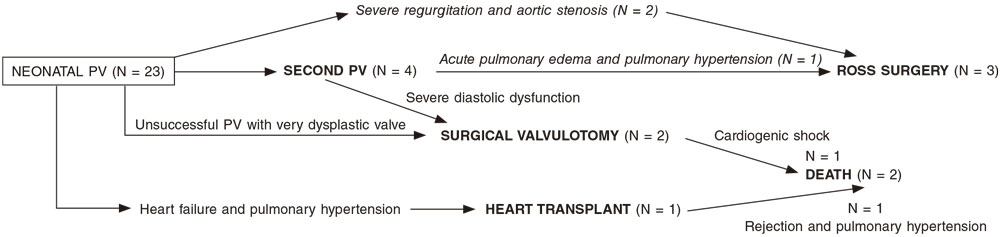
Figure 1. Need for new procedures in individuals after percutaneous valvuloplasty (PV).
During the patient’s disease progression, a 69.57% rate of aortic regurgitation was reported, 33% moderate and 8% severe (figure 2). No statistically significant differences were seen regarding disease progression into moderate-to-severe aortic regurgitation based on the balloon-to-annulus ratio (P = .435) or on the rate of procedural success (P = .446).
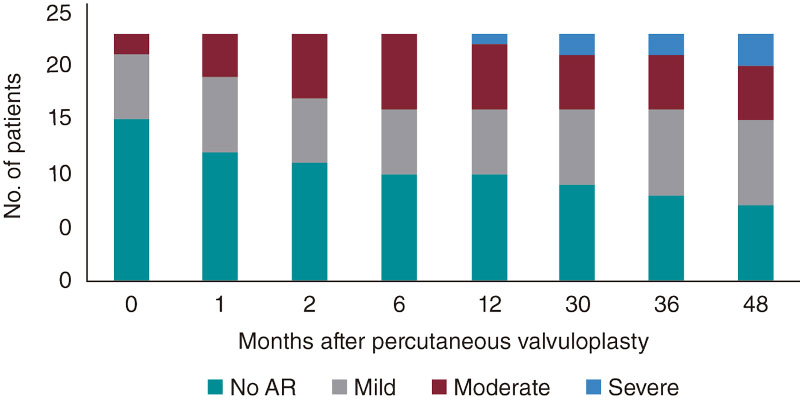
Figure 2. Evolution of aortic regurgitation (AR).
Statistically significant differences were reported in the univariate contrast for the risk factors of unfavorable progression in critical ASs (P = .021), greater systolic transvalvular gradient at birth (P = .027), shorter diameter of aortic annulus (P = .017), ductus-dependent systemic flow (P = .003), mechanical ventilation (P = .009), lower weight at birth (P = .001), younger age during the first PV (P = .013), lower LVEF (P = .021), and smaller diameter of the aortic annulus after PV (P = .009). No differences were seen based on valvular morphology (P = 1).
In the multivariate analysis (table 1) of previously significant and clinically relevant variables statistical significance was obtained for a smaller diameter of the aortic annulus (hazard ratio, 2.82; P = .016).
Table 1. Predictors of unfavorable disease progression
| Fisher’s exact test or Mann Whitney U test | Multivariate test: inverse verisimilitude method of a bivariate analysis | ||
| Variables | P | HR (95% confidence interval) | P |
| Critical AS | .021* | ||
| Prenatal diagnosis | .275 | ||
| Unicuspid valve | .462 | ||
| Affected by aortic coarctation | .369 | ||
| Affected by Shone syndrome | .146 | ||
| LVEF at birth | .101 | ||
| LV S-wave at birth | .222 | ||
| Diastolic dysfunction at birth | .131 | ||
| Mean gradient of AS at birth | .143 | ||
| Systolic gradient of AS at birth | .027* | 0.983 (0.906-1.066) | .676 |
| Aortic regurgitation at birth | .481 | ||
| Aortic annulus diameter at birth | .039* | ||
| Mitral stenosis at birth | .481 | ||
| Mitral regurgitation at birth | .069 | ||
| Z-score of mitral valve annulus at birth | .268 | ||
| Z-score of aortic valve annulus at birth | .017* | 1.234 (0.436-3.4494) | .692 |
| Z-score of LV end-diastolic diameter | 1.000 | ||
| LV end-diastolic gradient before the PV | .841 | ||
| Inotropic score at birth | .500 | ||
| Infusion of prostaglandins | .003* | ||
| Mechanical ventilation | .009* | ||
| Lower weight at birth | .001* | ||
| Age at cardiac catheterization | .013* | 0.896 (0.569-1.344) | .596 |
| Aortic systolic hemodynamic pressure before the PV | .687 | ||
| Mean aortic hemodynamic pressure before the PV | .622 | ||
| Aortic diastolic hemodynamic pressure before the PV | .107 | ||
| Mean LV hemodynamic pressure before the PV | .154 | ||
| LV systolic hemodynamic pressure before the PV | .424 | ||
| Aortic systolic hemodynamic pressure after the PV | .398 | ||
| LVEF after the PV | .021* | 1.007 (0.896-1.131) | .910 |
| LV S-wave on echocardiography after the PV | .106 | ||
| Echocardiographic aortic regurgitation after the PV | .609 | ||
| Z-score of the aortic annulus on the echocardiography after the PV | .009* | 0.355 (0.153-0.826) | .016* |
| Monitorization of mitral regurgitation after the PV | .635 | ||
| Monitorization of mitral stenosis after the PV | .100 | ||
95%CI, 95% confidence interval; AS, aortic stenosis; HR, hazard ratio; LV, left ventricle; LVEF, left ventricular ejection fraction; PV, percutaneous aortic valvuloplasty. * Statistically significant variables. | |||
This study—that shows the complexity and heterogeneity of AS—found that after a neonatal aortic PV has occurred some type of new intervention was required in a third of the patients with similar results to those reported by other series.4 Due to the complex decision-making process it is important to know which characteristics are associated with grimmer prognosis.5,6 It has been reported that worse prognosis is associated with lower weight, ductus-dependent systemic flow, and mechanical ventilation, critical AS, and a greater systolic aortic transvalvular gradient at birth, smaller annular sizes, and worse LVEF after the PV. Also, when the latter is performed earlier. These factors are often concomitant. However, only the diameter of the annulus has statistical significance in a multivariate analysis. A larger sample would have probably resulted in more significant variables. Even so, it seems obvious that the group of patients with poor clinical progression often share the same characteristics, indicative that they make up a totally different spectrum of the disease with different therapeutic needs while requiring multidisciplinary and individualized assessments.
FUNDING
The authors declare that no funding whatsoever was received to conduct this study.
AUTHORS’ CONTRIBUTIONS
Freixa-Benavente had the study idea, curated data, analyzed, drafted, and submitted the manuscript. P. Betrián-Blasco supervised the study, recruited patients and data, and analyzed them; also, he reviewed the manuscript final version and gave his approval. F. Rosés-Noguer, and G. Giralt-García reviewed the manuscript, and made significant remarks. Q. Ferrer-Menduiña supervised the study, recruited the patients, data, and drafted the manuscript; also, she reviewed the manuscript final version and gave her approval.
CONFLICTS OF INTEREST
The authors declared no conflicts of interest whatsoever.
REFERENCES
1. Freud LR, Mc Elhinney DB, Marshanll AC, et al. Fetal aortic valvuloplasty for evolving hypoplastic left heart syndrome: postnatal outcomes of the first 100 patients. Circulation. 2014;130:638-645.
2. Zaban NB, Herrmann JL, Hoyer MH, et al. Short- and intermediate-term results of balloon aortic valvuloplasty and surgical aortic valvotomy in neonates. Cardiol Young. 2020;30:489-492.
3. Feltes TF, Bacha E, Beekman RH , et al. Indications for Cardiac Catheterization and Intervention in Pediatric Cardiac Disease. A Scientific Statement from the American Heart Association. Circulation. 2011;123:2607-2652.
4. Brown DW, Dipilato AE, Chong ED, et al. Aortic Valve Reinterventions After Balloon Aortic Valvuloplasty for Congenital Aortic Stenosis. J Am Coll Cardiol. 2010;56:1740-1749.
5. Siddiqui J, Brizard CP, Galati JC, et al. Surgical Valvotomy and Repair for Neonatal and Infant Congenital Aortic Stenosis Achieves Better Results Than Interventional Catheterization. J Am Coll Cardiol. 2013;62:2134-2140.
6. Mc Lean KM, Lorts A, Pearl JM. Current treatment for congenital aortic stenosis. Curr Opin Cardiol. 2006;21:200-204.
* Corresponding author.
E-mail addresses: afreixbe11@gmail.com; afreixa@vhebron.net (A. Freixa-Benavente).