ABSTRACT
Introduction and objectives: When using radial access established as the approach of choice to perform coronary angiographies it is important to avoid radial spasm as it is the leading cause of access failure. This study aims to determine whether a topical anesthetic cream reduces the rate of radial spasm, as well as the increased gain with the use of different vasodilators.
Methods: Randomized, double-blind, and single-center clinical trial. Patients will be randomized to receive the anesthetic cream vs placebo, and 4 types of different vasodilator cocktails will be used in each group. The presence—or not—of radial spam and caliper gain will be analyzed.
Conclusions: Demonstrating the efficacy of the anesthetic cream, and different vasodilators to reduce radial spam would have a significant clinical impact, and justify its systematic use when performing coronary angiographies.
Registered at The Spanish Agency of Medicines and Medical Devices (AEMPS) EudraCT number: 2017-000321-12.
Keywords: Radial spasm. Anesthetic cream. Vasodilators. Coronary angiography. Luminal diameter.
RESUMEN
Introducción y objetivos: Con el abordaje radial establecido como técnica de elección para la coronariografía, es importante evitar el espasmo radial como principal causa de fallo en el acceso intravascular. En este estudio se pretende demostrar si la anestesia tópica en crema disminuye la incidencia de espasmo radial, así como conocer la ganancia de calibre con el uso de diferentes vasodilatadores.
Métodos: Ensayo clínico aleatorizado doble ciego en un solo centro. Los pacientes se aleatorizarán para recibir crema anestésica o placebo, y se utilizarán 4 tipos de cócteles vasodilatadores en cada grupo. Se analizará la presencia o no de espasmo radial y la ganancia de calibre como objetivos primarios.
Conclusiones: La demostración de la eficacia de la crema anestésica y de los diferentes vasodilatadores en la disminución del espasmo radial tendría un impacto clínico importante y justificaría su uso sistemático en la coronariografía.
Registrado en la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) con n.º EudraCT: 2017-000321-12.
Palabras clave: Espasmo radial. Crema anestésica. Vasodilatadores. Coronariografía. Diámetro luminal.
Abbreviations MLD: mean luminal diameter. RS: radial spasm. TA: topical anesthesia.
INTRODUCTION
Radial approach for cardiac catheterizations has become the most widely used across the world. In Spain it represents up to 75% of all the procedures performed and, in some centers, up to 91.1%.1 Compared to traditional femoral approach, this access has clearly proven its superiority from the safety standpoint of the procedures.2
Arterial canalization failure is often due to radial spasm (RS), and it can occur in up to 10% of all attempts. Also, it is associated with feminine sex, young age, low weight3 or deficits of certain enzymes that act on the endothelium.4 The special histological characteristics of this artery—with a high density of alpha-adrenergic receptors and smooth muscle cells—make it more prone to spasm.5
On the other hand, pain during lumbar puncture contributes to arterial canalization failure due to a higher frequency of appearance of spasm, vasovagal reaction with hypotension and discomfort for patient and operator, and the patient’s possible hemodynamic instability. Similarly, several patients complain of discomfort. As a matter of fact, the arterial puncture is described by many patients as the main moment of discomfort.5
Former studies have reported on the greater success achieved with isolated punctures for arterial gas analysis in the radial artery with the use of anesthesia injected around the puncture site. Also, more comfort and less pain have been reported by the patients.6 However, for many professionals injected anesthesia is ill-advised due to the pain caused by the injection. Also, because there are times that pain leads discomfort, and eventually RS.7 Despite of all this, the use of injected anesthesia is a common thing in procedures performed via radial access.
On the other hand, in the pediatric population as well as in different anatomical locations or in skin surgery, the use of topical anesthesia (TA) in the form of gel, cream or ointment has proven to minimize the pain associated with venous or arterial punctures, and some procedures too.8 The use of this type of anesthetic agents has not been properly studied in the cardiac catheterization setting. However, it could minimize the rate of RS, reduce pain when using this access, and improve the patient’s perception.
Together with TA, the use of different vasodilator drug combinations with unfractionated heparin (the so-called «radial cocktail»)—after successful arterial access—has proven to reduce the rates or arterial spasm and radial occlusion after the procedure.9-12 In particular drugs like verapamil, nitroglycerin, nitroprusside, nicorandil, isosorbide dinitrate or phentolamine in different doses have been compared with one another and also with placebo with heterogeneous results with arterial spams having been reported in 4% to 12% of the cases. Verapamil in doses of 5 mg and nitroglycerin 200 µg have yielded the best results so far. However, to this date, no comparison studies between the 2 drugs at these doses have ever been drawn or randomized for this matter.13 Therefore, it has not been fully established which is the best drug combination to prevent spasm and radial occlusion.
At our center, the current radial puncture procedure includes the use of injected anesthesia around the puncture site plus a cocktail of 5000 IU of unfractionated heparin, and 2.5 mg of verapamil. The rate of RS in our cath lab is around 10% of all punctures performed. In some patients, other drugs commonly available in our setting are often used—at the operator’s criterion—like nitroprusside, nitroglycerin or high doses of verapamil.
The objective of this study is to demonstrate whether the administration of topical anesthesia reduces the rate of RS and improves the patient’s perception regardless of the vasodilator used. Also, to compare arterial caliber gain with different vasodilators.
METHODS
Study design
Double-blind randomized clinical trial conducted at a single center to analyze the rate of RS in patients treated with TA in cream with lidocaine 25 mg/g + prilocaine 25 mg/g (Emla) in topical solution compared to placebo, as well as the effect of vasodilators (table 1) (verapamil 2.5 mg or 5 mg, nitroglycerin 200 µg, nitroprusside 150 µg) in the arterial caliber while attempting vascular access to perform diagnostic transradial cardiac catheterization.
Table 1. Inclusion and exclusion criteria of the E-RADIAL study
| Composition of the radial cocktail | Type of dilution |
|---|---|
| Cocktail #1 (verapamil 2.5 mg): | 12.5 mg of verapamil are diluted in 95 mL of FSS at 0.9%. A total of 20 mL are loaded in the syringe and fully administered. |
| Cocktail #2 (verapamil 5 mg): | 25 mg of verapamil are diluted in 90 mL of FSS at 0.9%. A total of 20 mL are loaded in the syringe and fully administered. |
| Cocktail #3 (nitroglycerin 0.2 mg): | 5 mg of nitroglycerin are diluted in 95 mL of FSS at 0.9%. A total of 4 mL of this solution are loaded in a 20 mL-syringe that is completed with FSS at 0.9%. The entire load of the syringe is administered. |
| Cocktail #4 (nitroprusside 0.150 mg): | 50 mg are diluted in 10 mL of FSS at 0.9% followed by the extraction of 1 mL of this solution that is diluted again in 100 mL of FSS at 0.9%. A total of 3 mL of the latter solution are loaded in a 20 mL-syringe that is completed with FSS at 0.9%. The entire load of the syringe is administered. |
|
FSS, physiological saline solution. |
|
Study population
The study will be conducted entirely at Unidad de Hemodinámica y Cardiología Intervencionista of Complejo Hospitalario Universitario de Albacete, Spain. All consecutive patients treated with diagnostic cardiac catheterization via radial access from November 2020 until completing the sample estimated will be included. Patients will need to meet the inclusion criteria and none of the exclusion ones (table 2).
Table 2. Inclusion and exclusion criteria of the E-RADIAL study
| Inclusion criteria | Exclusion criteria |
|---|---|
| Age > 18 years | Allergy or intolerance to any of the drugs used in the study. |
| Informed consent signing | Baseline systolic arterial blood pressure < 90 mmHg. |
| Elective diagnostic cardiac catheterization with intended radial access |
Impossibility to understand the study or give the corresponding informed consent. |
| Introductor 5 French |
Ethical aspects
The study has been approved by the center ethics committee, and a favorable resolution was obtained. The study has been registered by Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) with registration No. EudraCT: 2017-000321-12. The study will observe the principles established in the Declaration of Helsinki. Also, written informed consent will be obtained from all the patients before joining the study.
Study endpoints
Primary endpoints
– Study the rate of RS using a topical anesthetic cream before radial puncture.
– Study radial artery caliber gain using different vasodilators.
Secondary endpoints
– Study the rate of radial-radial, and radial-femoral crossing with each strategy.
– Study the rate of vasovagal reactions requiring treatment in each group.
– Study parameters associated with pain during radial artery canalization using pain assessment analogue scales.
– Subjective assessment of pain and comfort by the patient using pain assessment analogue scales, and dedicated tests.
– Subjective assessment of the difficulty involved in the puncture and perception of RS by the operator using dedicated tests.
Study development
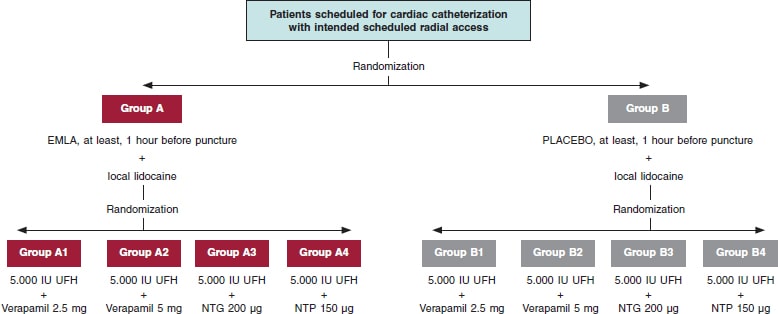
The administration of TA/placebo plus cocktail (table 1) will be fully randomized (figure 1). Both the patient and the treating interventional cardiology will be blind to the group they’ll be assigned to. If certain circumstances or complications occur, and if deemed necessary, the chain of secrecy can be broken only if investigators abide, and only under strict clinical judgement.
Figure 1. Flowchart of patients from the E-RADIAL study. NTG, nitroglycerin; NTP, nitroprusside; UFH, unfractionated heparin.
Placebo with cream of similar color, consistency, and characteristics to Emla will be prepared, and they both will be marked with letters A (Emla) and B (placebo). Both placebo and the TA will be prepared by personnel from the hospital pharmacy unit. The nursing team in charge of the patients while waiting for cardiac catheterization at the cath lab will randomize each patient, and the only blind element of the study. TA or placebo will be administered in both wrists and, at least, 1 hour before the procedure.
Prior to puncture, 25 mg of subcutaneous local anesthesia will be injected into the puncture area (mepivacaine at 2%). Another 1-2 minutes will need to pass before it starts to work.
Different cocktails (table 1) will be prepared at the dilution often used at Complejo Hospitalario Universitario de Albacete cath lab in 100 mL-jars of physiological saline solution (NaCl at 0.9%). Each jar will be marked with an alphanumeric code and its content will remain blind to everyone but the nursing team in charge of randomization.
Variable quantification during puncture
After monitoring the patient, arterial blood pressure will be determined invasively, as well as the baseline heart rate before administering the cocktail that will be used just after the introduction of hydrophilic guidewire (Radiofocus 5-Fr, Terumo, Japan). Similarly, arterial blood pressure will be recorded 2 minutes after the cocktail administration, as well as the maximum heart rate during puncture.
All vagal data that can occur and any other complications associated with access will be written down. The crossing rate to other accesses will also be studied prioritizing homolateral (cubital, distal radial) or contralateral access. Unless the operator specifies otherwise, femoral access will be set aside as the third go-to option.
Radial spasm determination and caliber gain quantification
RS will be defined as yes/no—both qualitative and dichotomically—and considered as sudden, transient, and abrupt narrowing of the radial artery during puncture. It will be clinically determined by, at least, 1 of the following events: loss of pulse during puncture, pain in the upper limb during catheter manipulation or entrapment. Its presence can also be determined through the angiography if spasm is seen during contrast injection.
Caliber gain will be determined through quantitative analysis of the radial artery luminogram. Therefore, an angiography will be immediately performed after the insertion of the introducer sheath plus another one 2 minutes after the injection of the antispasmodic cocktail. The radial artery caliber will be measured in the segment located between the tip of the arterial introducer sheath—2 cm away from it—and the location where it meets the humeral artery. Measurements will be acquired through computerized quantitative analysis (Xcelera, Philips, United States) after previous calibration of the arterial introducer sheath in the same segment before and after the cocktail injection to determine the mean luminal diameter (MLD).
Caliber gain will be estimated in percentage according to the following formula:
Caliber gain = 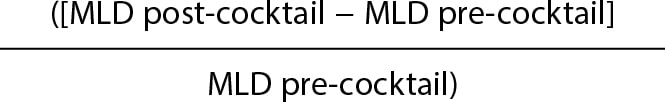 × 100
× 100
Postoperative patient assessment
The patient will be asked to give his opinion on the radial puncture through the pain qualitative analogue scale, and the comfort scale consisting of 4 questions (annex of the supplementary data).
Similarly, the interventional cardiologist will give his evaluation through a survey including 2 questions (annex of the supplementary data), the difficulties found while performing the puncture, and how the procedure was accomplished via the access used.
Statistical analysis
The analysis will be conducted using the SPSS statistical software package for Windows v 21.0.
In descriptive statistics frequencies and percentages will be used to express discrete variables while mean, median, mode, standard deviation, and ranges will be used to express continuous variables. The rate of spams and other study components will be described through frequencies and percentages. The statistical analysis of the main variables will be conducted by intention-to-treat analysis. The chi-square test will be used to study differences among proportions while the continuous variables will be analyzed using the Student t test if normally distributed or else non-parametric tests if not normally distributed. In the presence of non-homogeneous distribution of confounding variables between the groups that will be analyzed, a logistic regression analysis will be conducted that should collect those clinically significant and non-homogeneously distributed parameters.
It is our will to conduct an intermediate analysis after which the study will move on or not (existence of a significant difference in the primary endpoint of RS > 7,5% between both groups).
Estimate of the sample size
According to former studies, it is estimated that the proportion of patients who will have RS in the control group will be 10%3,5 being the criterion of clinical effectiveness the reduction of this percentage off by 50%, which is why it will be necessary to have a minimum sample of 668 patients.
This volume of patients will allow us to confirm the statistical significance of the variations described in radial artery vasodilation with different types of vasodilators.
DISCUSSION
Currently, the arterial approach via radial access is used in 91.1%1 of all diagnostic and therapeutic coronary angiographies performed. In particular, the rates of bleeding complications have dropped thus contributing to the patients’ comfort. This access has facilitated the implementation of safe coronary angiography and outpatient angioplasty programs even in complex settings.14-16
Hand in hand with this and assuming pain hypothesis and adrenergic discharge are caused by puncture and risk factors for RS, different strategies have come up to contribute to the proper administration of anesthesia promoting patients’ comfort, and looking to reduce the rate of RS. As it happens in other places, at our center the use of subcutaneously injected anesthesia is the common practice since the direct correlation between less RS and proper anesthetic release in the punction area has already been confirmed.5 This study paves the way for a possible change in the routine clinical practice that could be associated—or not—with TA in cream pharmaceutical form. The medical literature includes different and very heterogeneous studies that, whether randomized or not, have tried to assess the utility of this type of creams. However, all of them include small samples (usually less than 100 patients), which makes it difficult to extrapolate the results.
We have a few examples of injected anesthesia vs a composite of TA plus injected anesthesia with favorable results from the latter.17,18 As far as we know, the heterogeneity of designs, and the small sample sizes make us question studies like these.
Although subcutaneous anesthesia—often with lidocaine—has proven to improve pain at the puncture site and reduce the rate of RS compared to TA there is a huge controversy regarding the active principles and drug combination that should be used, the specific action times of these drugs or which are the best pharmaceutical forms. However, it seems that the cream/ointment formulation, and the lidocaine/prilocaine combination (Emla type) yield the best results of all.18
Assuming that this type of formulation is the most widely studied and looking to achieve an adequate design with a representative sample, the E-RADIAL trial (Effectiveness in preventing radial spasm of different vasodilators and topic local anesthesia during transradial cardiac catheterization) has just been started. Although it is not the first trial to propose this hypothesis, it is the first one indeed to confirm it on a double-blind randomized clinical trial and compare it to different radial cocktails and a wide sample size.
This vasolidator comparison is a particularly new approach of our trial. There is some controversy on the use, or not, of such drugs: although some centers in our country do not use vasodilators on a routine basis, it seems to be proven that, overall, its use promotes arterial dilatation and, therefore, the navigability of catheters with lower rates of spasm.9,13 Currently, no such thing as head-on comparisons of cocktails have been drawn in trials to assess their efficacy and safety profile.19 Therefore, we designed our study taking into consideration that a comparison can be drawn among these different drugs in quantitative terms using MLD gain.
Although not part of our study primary endpoints we assume that—with radial access clearly established in the routine clinical practice of cath labs—the operator’s experience, his learning curve or even the rotating fellow/resident’s learning curve can have an impact on the rate of success of puncture, RS, as well as on other complications. This can be an interesting aspect we could discuss. As far as we know both in the current medical literature and good practice recommendations regarding the radial access20—although with limitations depending on the study analyzed—it seems reasonable to assume that the threshold to overtake the learning curve would be at around 30-5021 cases for conventional diagnostic coronary angiography, and > 100-200 cases for complex coronary anatomies22,23 or even in the ST-segment elevation acute coronary syndrome setting. In the E-RADIAL study, all operators widely exceed the number of cases recommended for this curve in diagnostic coronary angiography. Even so, while collecting data for the E-RADIAL we’ll have the possibility to know the identity of the operator who will perform the puncture, his years of experience using radial access, and whether a resident or a novel interventional cardiology (< 2 years of experience) was involved. Also, we will try to know descriptively the rate of puncture success, and whether any RS differences or other complications occurred.
The design of this clinical trial used 4 types of radial cocktail (table 1) from the ones most widely used ones in today’s clinical practice. However, this is also a controversial issue. On the one hand, some centers don’t use vasodilators systematically after radial puncture. On the other hand, choosing one over the other at the cath labs where they’re used is often based on the good clinical results obtained empirically in the routine clinical practice. Unlike the use of heparin to prevent radial occlusion, evidence is scarce regarding benefits from vasodilators, and no homogeneous head-on comparisons have been drawn among different drug cocktails. Verapamil in doses of 5 mg, and nitroglycerin in doses of 200 µg have yielded the best results so far. However, to this date, they have never been compared to one another at these doses or in a randomized way.13 Certain clinical features of the patients can turn the use of these cocktails into a controversial issue. As an example of this, in patients with very severe left ventricular dysfunction or severe aortic stenosis the use of these drugs can trigger significant adverse reactions, mainly hypotension or significant hemodynamic changes. Although, in theory, overall, these drugs are contraindicated in these clinical settings, the dose used, slow infusion, and other factors like the patients’ clinical stability, the existence—or not—of associated heart failure or different comorbidities can turn the use of these drugs into a safe practice. In its design the E-RADIAL study includes a head-on comparison of cocktails and some of the aforementioned drugs and doses. Therefore, it is an opportunity to know what the clinical implication of these drugs really is regarding adverse events.
One of the possible weaknesses or aspects that should be discussed in this trial is pain assessment and quantification. A reproducible design was attempted while assuming the difficulties posed by individual subjectivity. Therefore, following in the footsteps of former studies and registries, we decided to use the most standardized method available to this date in the medical literature: analogue scales.
Another possible weakness or cofounding factor in the study design is the systematic use of sodium heparin via arterial access as standard prevention against radial occlusion.20 According to the drug label24 the heparin-induced cardiac tamponade solution is often an acid solution with a pH between 5.0 and 7.5. The mean arterial pH is between the traditional values of 7.35 and 7.45, and could be partially altered when in contact with heparin solutions thus favoring, through different mechanisms, the development of RS, something not clearly established to this date. To solve this possible bias, the IV—not intraarterial use—of heparin was selected. Although evidence is certainly scarce and heterogeneous the IV use of heparin does not seem to increase the rate of radial occlusion, which is more associated with the heparin dose used and factors like compression time, type of material or size of the radial introducer sheath used that are well established as predictors of radial occlusion.25,26
CONCLUSIONS
The E-RADIAL study is the first randomized clinical trial to assess, on the one hand, the implications of less RS due to topical anesthesia and, on the other, arterial caliber gain with the use of different vasodilators.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
J. J. Portero-Portaz: idea, methodology, validation, formal analysis, drafting of the original project; J. G. Córdoba-Soriano: idea, methodology, review and edition of the manuscript; A. Gutiérrez-Díez: idea, methodology, validation, formal analysis, review and edition of the manuscript; A. Gallardo-López, and D. Melehi El-Assali: idea, methodology, review and edition of the manuscript; L. Expósito-Calamardo, and A. Prieto-Lobato: research, review, and edition of the manuscript; E. García-Martínez, S. Ruiz-Sánchez, M. R. Ortiz Navarro, and E. Riquelme-Bravo: methodology, review, and edition of the manuscript; J. Jiménez-Mazuecos: idea, methodology, review and edition of the manuscript.
CONFLICTS OF INTEREST
Authors declared having no affiliation or participation in any organization or entity with any financial or non-financial interest in the topic at stake or in the materials discussed in this manuscript.
ACKNOWLEDGEMENTS
We wish to thank the nursing personnel of our unit for their work, dedication, and availability during the entire study.
WHAT IS KNOWN ABOUT THE TOPIC?
- RS is the leading cause of access failure in diagnostic or therapeutic coronary angiographies.
- The use of injected local anesthesia is standardized and reduces the rate of RS.
- There is no consensus on the use or non-use of vasodilators, which depends on the characteristics and routine clinical practice of each center.
WHAT DOES THIS STUDY ADD?
- The E-RADIAL study can pave the way to systematization in the use of other type of anesthesia.
- It will provide relevant information on the effectiveness of different vasodilators through head-on comparisons of the most widely used agents.
REFERENCES
1. Romaguera R, Ojeda S, Cruz-González I, Moreno R. Registro Español de Hemodinámica y Cardiología Intervencionista. XXX Informe Oficial de la Asociación de Cardiología Intervencionista de la Sociedad Española de Cardiología (1990-2020) en el año de la pandemia de la COVID-19. Rev. Esp Cardiol. 2021;74:1095-1105.
2. Rao SV, Turi ZG, Wong SC, Brener SJ, Stone GW. Radial versus femoral access. J Am Coll Cardiol. 2013;62(17 Suppl):S11-20.
3. Dandekar VK, Vidovich MI, Shroff AR. Complications of transradial catheterization. Cardiovasc Revasc Med. 2012;13:39-50.
4. Kocayigit I, Cakar MA, Kahyaog˘lu B, Aksoy MNM, Tatli E, Akdemir R. The relationship between serum asymmetric dimethylarginine levels and radial artery spasm. Anatol J Cardiol. 2020;23:228-232.
5. Ho HH, Jafary FH, Ong PJ. Radial artery spasm during transradial cardiac catheterization and percutaneous coronary intervention: incidence, predisposing factors, prevention, and management. Cardiovasc Revasc Med. 2012;
13:193-195.
6. Hudson TL, Dukes SF, Reilly K. Use of local anesthesia for arterial punctures. Am J Crit Care. 2006;15:595-599.
7. France JE, Beech FJ, Jakeman N, Benger JR. Anaesthesia for arterial puncture in the emergency department: a randomized trial of subcutaneous lidocaine, ethyl chloride or nothing. Eur J Emerg Med. 2008;15:218-220.
8. Tran NQ, Pretto JJ, Worsnop CJ. A randomized controlled trial of the effectiveness of topical amethocaine in reducing pain during arterial puncture. Chest. 2002;122:1357-1360.
9. Boyer N, Beyer A, Gupta V, et al. The effects of intra-arterial vasodilators on radial artery size and spasm: implications for contemporary use of trans-radial access for coronary angiography and percutaneous coronary intervention. Cardiovasc Revasc Med. 2013;14:321-324.
10. Ruiz-Salmerón RJ, Mora R, Vélez-Gimón M, et al. Espasmo radial en el cateterismo cardíaco transradial. Análisis de los factores asociados con su aparición y de sus consecuencias tras el procedimiento. Rev Esp Cardiol. 2005;58:504-511.
11. Majure DT, Hallaux M, Yeghiazarians Y, Boyle AJ. Topical nitroglycerin and lidocaine locally vasodilate the radial artery without affecting systemic blood pressure: a dose-finding phase I study. J Crit Care. 2012;27:532.e9-13.
12. Beyer AT, Ng R, Singh A, et al. Topical nitroglycerin and lidocaine to dilate the radial artery prior to transradial cardiac catheterization: a randomized, placebo-controlled, double-blind clinical trial: the PRE-DILATE Study. Int J Cardiol. 2013;168:2575-2578.
13. Kwok CS, Rashid M, Fraser D, Nolan J, Mamas M. Intra-arterial vasodilators to prevent radial artery spasm: a systematic review and pooled analysis of clinical studies. Cardiovasc Revasc Med. 2015;16:484-490.
14. Córdoba-Soriano JG, Rivera-Juárez A, Gutiérrez-Díez A, et al. The Feasibility and Safety of Ambulatory Percutaneous Coronary Interventions in Complex Lesions. Cardiovasc Revasc Med. 2019;20:875-882.
15. Córdoba-Soriano JG, Jiménez-Mazuecos J, Rivera Juárez A, et al. Safety and Feasibility of Outpatient Percutaneous Coronary Intervention in Selected Patients: A Spanish Multicenter Registry. Rev Esp Cardiol. 2017;70:535-542.
16. Gallego-Sánchez G, Gallardo-López A, Córdoba-Soriano JG, et al. Safety of transradial diagnostic cardiac catheterization in patients under oral anticoagulant therapy. J Cardiol. 2017;69:561-564.
17. Tatlı E, Adem Yılmaztepe M, Gökhan Vural M, et al. Cutaneous analgesia before transradial access for coronary intervention to prevent radial artery spasm. Perfusion. 2018;33:110-114.
18. Youn YJ, Kim WT, Lee JW, et al. Eutectic mixture of local anesthesia cream can reduce both the radial pain and sympathetic response during transradial coronary angiography. Korean Circ J. 2011;41:726-732.
19. Shehab A, Bhagavathula AS, Kaes AA, et al. Effect of Vasodilatory Medications on Blood Pressure in Patients Undergoing Transradial Coronary Angiography: A Comparative Study. Heart Views. 2020;21:75-79.
20. Shroff AR, Gulati R, Drachman DE, et al. SCAI expert consensus statement update on best practices for transradial angiography and intervention. Catheter Cardiovasc Interv. 2020;95:245-252.
21. Hess CN, Peterson ED, Neely ML, et al. The learning curve for transradial percutaneous coronary intervention among operators in the United States: a study from the National Cardiovascular Data Registry. Circulation. 2014;
129:2277-2286.
22. Azzalini L, Ly HQ. Letter by Azzalini and Ly regarding article, “The learning curve for transradial percutaneous coronary intervention among operators in the United States: a study from the National Cardiovascular Data Registry”. Circulation. 2015;131:e357.
23. Hamon M, Pristipino C, Di Mario C, et al. European Association of Percutaneous Cardiovascular Interventions; Working Group on Acute Cardiac Care of the European Society of Cardiology; Working Group on Thrombosis on the European Society of Cardiology. Consensus document on the radial approach in percutaneous cardiovascular interventions: position paper by the European Association of Percutaneous Cardiovascular Interventions and Working Groups on Acute Cardiac Care** and Thrombosis of the European Society of Cardiology.EuroIntervention. 2013; 8:1242–1251.
24. Ficha técnica de la heparina sódica. Agencia Española de Medicamentos y Productos Sanitarios. Available online: https://cima.aemps.es/cima/dochtml/ft/56029/FT_56029.html. Accessed 20 Nov 2021.
25. Pancholy SB. Comparison of the effect of intra-arterial versus intravenous heparin on radial artery occlusion after transradial catheterization. Am J Cardiol. 2009;104:1083-1085.
26. Rashid M, Kwok CS, Pancholy S, et al. Radial Artery Occlusion After Transradial Interventions: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 201625;5:e002686.
* Corresponding author:
E-mail address: juanjose.porteroportaz@gmail.com (J.J. Portero-Portaz).