ABSTRACT
Introduction and objectives: After the positive pre-clinical and clinical results with Angiolite, a cobalt-chromium sirolimus-eluting stent, we decided to analyze its performance in a non-selected, real-world population: the RANGO registry.
Methods: We conducted an observational, prospective, multicenter registry of patients with different clinical indications. All consecutive patients treated with percutaneous coronary intervention with, at least, 1 Angiolite stent and who gave their informed consent were included. The registry primary endpoint was the occurrence of target lesion failure (TLF) at 6, 12, and 24 months defined as cardiovascular death, myocardial infarction (MI) related to target vessel, and clinically driven target lesion revascularization. The secondary endpoints were the individual components of the primary endpoint, major adverse cardiovascular events (MACE: all-cause mortality, any MI, or any revascularization), and stent thrombosis. We describe the 2-year clinical results of the RANGO study in the entire population, in those who only received Angiolite stents, and in 2 predefined subgroups: diabetics and patients with small-vessels (≤ 2.5mm).
Results: 646 patients (426 of them only received Angiolite stents) with a high-risk profile were recruited: prevalence of previous MI (18.4%), previous coronary revascularization (23.4%), clinical presentation as ST-segment elevation MI (23.1%), and multivessel disease (47.8%). At the 2-year follow-up, the rates of TLF, MACE, and stent thrombosis were 3.4%, 9.6%, and 0.9%, respectively. Similar results were observed among patients treated with Angiolite stents only: TLF, 3.1%; MACE, 8.0%; thrombosis, 0.7%. The rates were not significantly different for the diabetic (TLF, 3.0%; MACE, 14.1%; thrombosis, 1.0%), and small-vessel subgroups (TLF, 4.3%; MACE, 12.1%; thrombosis, 0%).
Conclusions: In conclusion, the results of this observational registry on the use of Angiolite in a real-world population, including a high-risk population, corroborate the excellent results observed in previous studies, up to a 2-year follow-up. An extended 5-year follow-up is planned to discard the occurrence of late events.
Keywords: Sirolimus-eluting-stent. Durable fluoropolymer. Observational study. Efficacy. Safety. Stent thrombosis.
RESUMEN
Introducción y objetivos: Para confirmar los resultados observados en análisis preclínicos y clínicos del stent liberador de sirolimus Angiolite se diseñó el registro observacional de vida real RANGO.
Métodos: El registro prospectivo multicéntrico incluyó pacientes con distintas indicaciones clínicas que recibieron al menos 1 stent Angiolite para tratar su enfermedad coronaria y que dieron su consentimiento informado. El objetivo primario fue la incidencia de fracaso del tratamiento de la lesión (FTL) a 6, 12 y 24 meses, definido como muerte de causa cardiaca, infarto de miocardio en relación con el vaso tratado o nueva revascularización de la lesión tratada. Los objetivos secundarios fueron los componentes individuales del objetivo primario y las incidencias de eventos cardiacos mayores (MACE) y de trombosis del stent. Se presentan los resultados del registro RANGO a 2 años en la población global, en los pacientes que recibieron stent Angiolite y en 2 subgrupos predefinidos de diabéticos y vasos pequeños (≤ 2,5 mm).
Resultados: Se seleccionaron 646 pacientes (426 solo recibieron stents Angiolite) con un perfil de riesgo elevado: infarto previo (18,4%), revascularización coronaria previa (23,4%), presentación clínica como infarto agudo con elevación del segmento ST (23,1%) y enfermedad multivaso (47,8%). A los 2 años, la incidencia de FTL en el grupo global fue del 3,4%, la de MACE fue del 9,6% y la de trombosis del stent fue del 0,9%. En el grupo tratado solo con stents Angiolite, los resultados fueron similares (FTL 3,1%, MACE 8,0% y trombosis 0,7%). Los resultados no fueron significativamente diferentes en los diabéticos (FTL 3,0%, MACE 14,1% y trombosis 1,0%) y en los pacientes con vasos pequeños (FTL 4,3%, MACE 12,1% y trombosis 0%).
Conclusiones: Los resultados del registro observacional RANGO a los 2 años en población de vida real con perfil de riesgo elevado confirman los excelentes resultados del stent Angiolite observados en estudios previos. Se plantea un seguimiento clínico a 5 años para descartar eventos muy tardíos.
Palabras clave: Stent liberador de sirolimus. Fluoropolimero estable. Estudio observacional. Eficacia. Seguridad. Trombosis del stent.
Abbreviations
DES: drug-eluting stents. MACE: major adverse cardiovascular events. PCI: percutaneous coronary intervention. TLF: target lesion failure. TLR: target lesion revascularization. TVR: target vessel revascularization.
INTRODUCTION
Drug-eluting stents (DES) are one of the greatest advances in the percutaneous treatment of coronary artery disease. These devices have consistently shown lower rates of revascularization of the treated vessel in a wide range of clinical situations, and have become the treatment of choice.1 However, the risk of late and very late stent thrombosis arose with first-generation DES,2 and, to this date, it is still a matter of concern.3 This phenomenon has been associated with side effects to the drug (impairing the proliferation of new endothelial cells), the polymer, the stent platform or a combination of them on the vessel wall, leading to delayed or incomplete endothelialization, persistent inflammatory reactions, and the development of neo-atherosclerosis. New DES have been developed with superior efficacy in terms of abolishing the need for revascularization, but with the reassurance of much lower rates of stent thrombosis, the most dreadful clinical manifestation of suboptimal vessel healing. The Angiolite stent (iVascular, Spain) is a thin-strut cobalt-chromium sirolimus-eluting stent with biostable coating made of 3 layers: acrylate to ensure adhesion to the metal surface, fluoroacrylate loaded with sirolimus (1.4 µg/mm2), and a top layer of fluoroacrylate for drug release control (> 75% elution within the first month).
The Angiolite stent was initially tested in a pre-clinical model with very promising results,4 with an equivalent antiproliferative response, and a better healing pattern compared to the XIENCE stent (Abbott Vascular, United States). Subsequently, a first-in-human study5 (ANCHOR study) proved a powerful inhibition of neointimal hyperplasia as seen on the OCT: The Angiolite stent efficiently inhibited the proliferative response (vessel area stenosis, 4.4% ± 11.3%), in- stent late lumen loss at 6 months (0.07 mm ± 0.37 mm), and had a low rate of strut malapposition (1.1% ± 6.2%). Finally, the ANGIOLITE study,6 a randomized clinical trial, compared the Angiolite stent to the XIENCE stent in 223 patients (randomization with a 1:1 allocation ratio). In this study, the primary endpoint, the 6-month in-stent late lumen loss, was non-inferior in the Angiolite group (0.04 mm ± 0.39 mm) compared to the XIENCE group (0.08 mm ± 0.38 mm). The stent received the CE marking (Conformité Européenne) for its routine use. Therefore, we designed the present observational, prospective, registry to endorse the previous results in the routine clinical practice, with wider indications for use.
METHODS
Study design
The EPIC02-RANGO study was designed as a prospective, single-arm, multicenter, observational registry for the evaluation of the safety and efficacy profile of the Angiolite stent in unselected patients representative of the routine clinical practice. The study design was approved by all investigators and the sponsor as well. A reference ethics committee approved the protocol and the informed consent forms; local ethics committees were informed that this study would be conducted in their centers in compliance with the national legislation. The study was conducted and monitored by an independent contract research organization. The authors of this original manuscript independently conducted the data final analysis, interpreted the study results, and drafted/wrote this original manuscript. The sponsor was informed on the status of the study and the final results, but had no further participation.
Selection of the study population
To be enrolled in the study, subjects should met all the 3 following inclusion criteria: ≥ 18 years-old; treated with percutaneous coronary intervention (PCI) with at least 1 Angiolite stent; and have received proper information and signed the corresponding informed consent.
To guarantee a real-world population, non-stringent exclusion criteria were applied. Subjects were only excluded from the study if they met any of the following exclusion criteria: contraindication to dual antiplatelet therapy; established cardiogenic shock; unlikely to complete the scheduled follow-up; or formal refusal to participate in the study.
The PCI (predilatation, invasive imaging, postdilatation, planning, and final performance) was left at the discretion of the operator, and was indicative of the real-world use of the stents. Medical treatment during and after the procedure, including antiplatelet regime and duration, also followed the standard local practices; however, we suggested the investigators to follow the guidelines available on the management of these patients.1,7
Endpoints
The primary endpoint was target lesion failure (TLF) at 6, 12, and 24 months defined as cardiovascular death, target vessel myocardial infarction or clinically driven target lesion revascularization.
-
The secondary endpoints were:
-
– Target vessel failure defined as cardiovascular death, target vessel myocardial infarction or target vessel revascularization.
-
– Major adverse cardiovascular events (MACE) defined as all-cause mortality, any myocardial infarction or any target vessel revascularization.
-
– Stent thrombosis (definite or probable, as defined by the ARC criteria8).
In all cases, myocardial infarction refers to spontaneous infarction only. Two subgroups were predefined: patients with diabetes, and patients with Angiolite stents placed in small vessels (stent diameter ≤ 2.5 mm).
Sample size calculation
We conducted an exploratory analysis that rendered a population of 640 patients (with an estimated loss to follow-up of 10%). This sample size produces a 2-sided 95% confidence interval with a precision equal to 1.75% when the TLF rate is 4.86%. This value was obtained from the data published from different contemporary stents9-17 (table 1 of the supplementary data).
Table 1. Baseline and clinical characteristics
| Total N = 646 | Angiolite only population N = 426 | |
|---|---|---|
| Age (years old) | 66.41 ± 11.93 | 65.72 ± 11.98 |
| Male sex | 495 (76.6%) | 320 (75.1%) |
| Cardiovascular risk factors & history | ||
| Hypertension | 402 (62.2%) | 254 (59.6%) |
| Dyslipidemia | 385 (59.6%) | 251 (58.9%) |
| Diabetes mellitus* | 199 (30.8%) | 119 (27.9%) |
| Current smoker | 182 (28.2%) | 127 (29.8%) |
| Chronic kidney disease | 46 (7.1%) | 25 (5.9%) |
| Peripheral vascular disease* | 44 (6.8%) | 23 (5.4%) |
| Previous stroke | 28 (4.3%) | 17 (4.0%) |
| Previous myocardial infarction | 119 (18.4%) | 73 (17.1%) |
| Previous coronary surgery | 20 (3.1%) | 13 (3.1%) |
| Previous PCI | 131 (20.3%) | 78 (18.3%) |
| Atrial fibrillation | 34 (5.3%) | 20 (4.7%) |
| Heart failure | 46 (7.1%) | 32 (7.5%) |
| Valvular heart disease ≥ grade III | 16 (2.5%) | 7 (1.6%) |
| PCI indication | ||
| NSTEMI | 220 (34.1%) | 141 (33.1%) |
| STEMI | 149 (23.1%) | 112 (26.3%) |
| Stable angina | 120 (18.6%) | 68 (16.0%) |
| Unstable angina (negative biomarkers) | 72 (11.1%) | 51 (12.0%) |
| Silent myocardial ischemia | 32 (5.0%) | 19 (4.5%) |
| Other | 53 (8.2%) | 35 (8.2%) |
|
NSTEMI, non-ST-elevation acute myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction. * Significant differences between patients with the Angiolite stent only vs patients with any stents in addition to the Angiolite, P < .05. Data are expressed as no. (%) or mean ± standard deviation. |
||
Population analysis
The primary safety and efficacy analysis considered all patients who received the Angiolite stent only except for those who withdrew their consent. The secondary analysis was performed on all patients included in the study who received, at least, 1 Angiolite stent plus another different stent except for those who withdrew their consent.
Clinical events committee
An independent data and safety monitoring board reviewed the cumulative safety data to safeguard the well-being of the participants. All events were remotely monitored by a contract research organization. The clinical events committee reviewed, adjudicated, and classified all adverse events. The 5 members of the clinical events committee were not affiliated to the centers that participated in the study.
A total of 90 random patient audits (14% of the global population) were conducted at 4 centers, including the top 3 recruiters. The result of these audits detected 9 unreported events, most of them corresponded to scheduled procedures that required admission (non-cardiac surgeries and 2 scheduled PCI cases). None of the events associated with these audits corresponded to events classified as primary or secondary endpoints.
Descriptive statistics
All continuous variables were summarized using the following descriptive statistics: n (based on the number of recorded data values for each parameter), mean, standard deviation, 95% confidence interval for the mean, median, interquartile range [Q1, Q3], maximum, and minimum. The frequency and percentages (based on the number of recorded data values for each parameter) of the observed values are reported for all categorical measures. In general, all data are listed, and sorted by study site, and subject.
Statistical methods
Regarding the continuous variables, results were expressed as mean ± standard deviation. Variables were compared using an independent t test or the Mann-Whitney test, when applicable. Categorical variables are expressed as counts and percentages and compared using the chi-square test or Fisher’s exact test. Variables were compared between patients with only the Angiolite stent versus patients with other stents in addition to the Angiolite one. The clinical variables at 6, 12, and 24 months were expressed as counts and percentages. Time-to-event hazard curves were expressed as Kaplan-Meier estimates.
These methods were applied for the entire cohort and the 2 predefined subgroups, when appropriate: patients with diabetes, and patients with small vessel lesions (stent diameter ≤ 2.5 mm).
The statistical software SAS Version 9.4 was used for all statistical analyses, listings, tabulations, and figures.
RESULTS
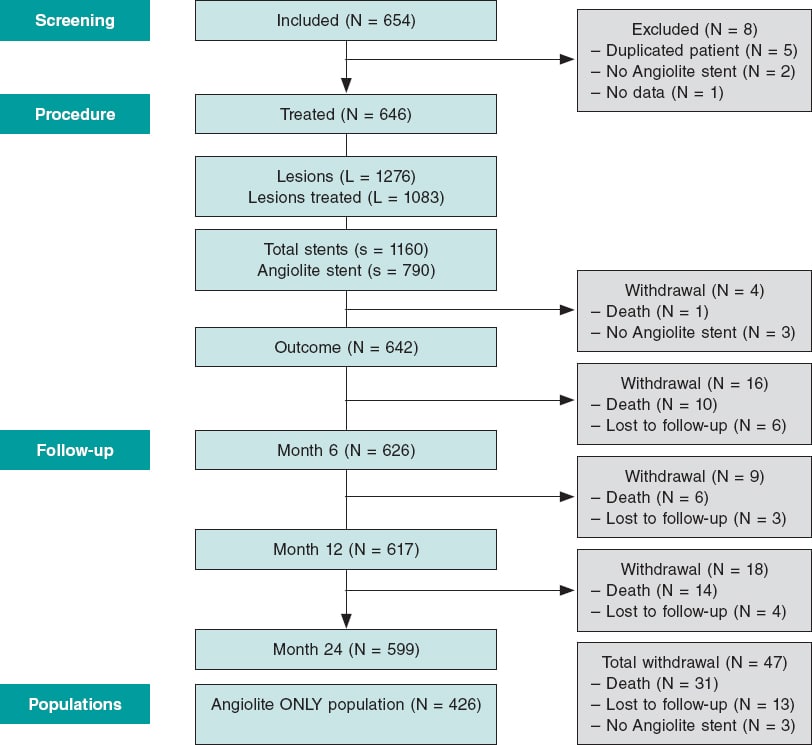
A total of 654 patients were recruited from 16 academic medical centers in Spain and Portugal from June 2017 through July 2018. A total of 8 patients were excluded for not meeting the selection criteria (2 in whom the Angiolite stent was not intented to be used, 5 duplicated patients with staged, planned, procedures, and 1 patient without any data available). Therefore, the population analyzed consisted of 646 patients (figure 1); a total of 426 patients were treated with Angiolite stents only (primary analysis).
Figure 1. Flow chart of the study.
The baseline characteristics and clinical data, as well as the angiographic and procedural features are shown on table 1 and table 2, respectively. Noteworthy, the population has a high-risk profile with a remarkable prevalence of previous myocardial infarction (18.4%), previous coronary revascularization (23.4%), clinical presentation as ST-segment elevation myocardial infarction (23.1%), and multivessel disease (47.8%).
Table 2. Angiographic and procedural features
| Total N = 646 | Angiolite only population N = 426 | |
|---|---|---|
| Coronary angiography | ||
| Radial approach | 585 (90.6%) | 396 (93.0%) |
| Extension of the disease | ||
| No. of diseased vessels* | ||
| 1 | 337 (52.2%) | 289 (67.8%) |
| 2 | 198 (30.7%) | 92 (21.6%) |
| 3 | 111 (17.1%) | 45 (10.6%) |
| Left main coronary artery* | 29 (4.5%) | 12 (2.8%) |
| Proximal LAD disease | 179 (27.7%) | 110 (25.8%) |
| Diffuse disease* | 128 (19.8%) | 63 (14.8%) |
| No. of lesions per patient* | 1.98 ± 1.24 | 1.51 ± 0.90 |
| No. of treated lesions per patient* | 1.68 ± 0.95 | 1.25 ± 0.53 |
| No. of stents per patient* | 1.80 ± 1.11 | 1.24 ± 0.55 |
| Index procedure | ||
| Revascularization | ||
| Complete | 489 (75.7%) | 331 (77.7%) |
| Functional | 84 (13.0%) | 51 (12.0%) |
| Intravascular imaging | ||
| IVUS | 15 (2.3%) | 5 (1.2%) |
| OCT | 12 (1.9%) | 7 (1.6%) |
| Staged revascularization* | 85 (13.2%) | 26 (6.1%) |
|
IVUS, intravascular ultrasound; LAD, left anterior descending coronary artery; OCT, optical coherence tomography. * Significant differences between patients with the Angiolite stent only vs patients with any stents in addition to the Angiolite, P < .05. Data are expressed as no. (%) or mean ± standard deviation. |
||
The mean ± standard deviation number of lesions per patient was 1.98 ± 1.2, the mean number of treated lesions per patient was 1.68 ± 0.9 with a mean number of stents per patient of 1.80 ± 1.1. These numbers were significantly lower among patients treated with the Angiolite stent and consistent with the different patient profile. Table 3 summarizes the characteristics and treatment of each individual lesion. Interestingly, Angiolite stents were more frequently used to treat the infarct-related artery compared to other stents in our population. Subsequently, lesions with thrombus were more common in the group treated with Angiolite stents only while severe calcification was more prevalent in the entire group. Procedural complications occurred in 10 patients, 7 of them associated with Angiolite stents: 1 uncrossable lesion, 1 guidewire-related distal perforation, 1 severe no-reflow phenomenon, and 4 cases of dissection, 2 of them treated with additional stents. The procedural and device success rates were 99.7% and 99.2%, respectively. In more complex anatomic scenarios, specifically lesions with moderate/severe calcification, the procedural and device success rates stayed high (99.6% and 99.3%, respectively). Those rates were 100% in the subgroup of lesions at bifurcations or at left main coronary artery level.
Table 3. Characteristics and treatment of each individual lesion
| Total L = 1083 (84.9% of all lesions) | Angiolite only population L = 531 (82.5% of all lesions) | |
|---|---|---|
| Vessel | ||
| Left anterior descending territory | 459 (42.4%) | 236 (44.4%) |
| Right coronary territory | 327 (30.2%) | 172 (32.4%) |
| Circumflex territory | 273 (24.9%) | 112 (21.2%) |
| Left main coronary artery | 19 (1.8%) | 5 (0.9%) |
| Other | 5 (0.7%) | 6 (1.1%) |
| AHA/ACC Classification* | ||
| A | 95 (8.8%) | 68 (12.8%) |
| B1 | 355 (32.8%) | 193 (36.3%) |
| B2 | 429 (39.6%) | 185 (34.8%) |
| C | 204 (18.8%) | 85 (16.0%) |
| Lesion characteristics | ||
| Thrombus* | 145 (13.4%) | 91 (17.1%) |
| Stent at the infarct-related artery* | 366 (33.8%) | 249 (46.9%) |
| Severe calcification* | 85 (7.8%) | 22 (4.1%) |
| Restenotic lesion treated | 37 (3.4%) | 22 (4.1%) |
| Chronic total coronary occlusion | 37 (3.4%) | 20 (3.8%) |
| Lesion at bifurcation | 108 (10.0%) | 47 (8.9%) |
| Severe tortuosity | 142 (13.1%) | 62 (11.7%) |
| Vessel diameter (mm) | 2.91 ± 0.55 | 2.91 ± 0.53 |
| Lesion length (mm)* | 19.47 ± 9.80 | 17.56 ± 8.26 |
| Pre-dilatation* | 786 (72.6%) | 363 (68.4%) |
| Scoring balloon | 45 (4.2%) | 11 (2.1%) |
| Cutting balloon | 28 (2.6%) | 8 (1.5%) |
| Rotational atherectomy | 27 (2.5%) | 9 (1.7%) |
| Thrombectomy* | 75 (6.9%) | 48 (9.0%) |
| Stents implanted | S = 1160 | S = 529 |
| No. of stents per lesion | 1.07 ± 0.45 | 1.00 ± 0.35 |
| Characteristics of the stent* | ||
| Type = Angiolite stent | 784 (67.6%) | 529 (100.0%) |
| Stent diameter (mm) | 2.99 ± 0.51 | 2.99 ± 0.46 |
| Stent length (mm) | 21.38 ± 8.51 | 20.34 ± 7.03 |
| Maximum pressure (atm) | 14.61 ± 2.48 | 14.69 ± 2.46 |
| Stent crossing the lesion at the 1st attempt | 1067 (98.5%) | 527 (99.2%) |
| Lesions at bifurcation | 104 (96.3%) | 45 (95.7%) |
| Moderate or severe calcification | 268 (97.1%) | 75 (97.4%) |
| Left main coronary artery | 19 (100%) | 5 (100%) |
| Postdilatation | 284 (26.2%) | 149 (28.1%) |
| Balloon diameter (mm) | 3.24 ± 0.62 | 3.25 ± 0.53 |
| Type of balloon, non-compliant | 186 (67.4%) | 112 (76.7%) |
|
ACC, American College of Cardiology; AHA, American Heart Association; L, lesions; S, stents. * Significant differences between patients with the Angiolite stent only vs patients with any stents in addition to the Angiolite, P < .05. Data are expressed as no. (%) or mean ± standard deviation. |
||
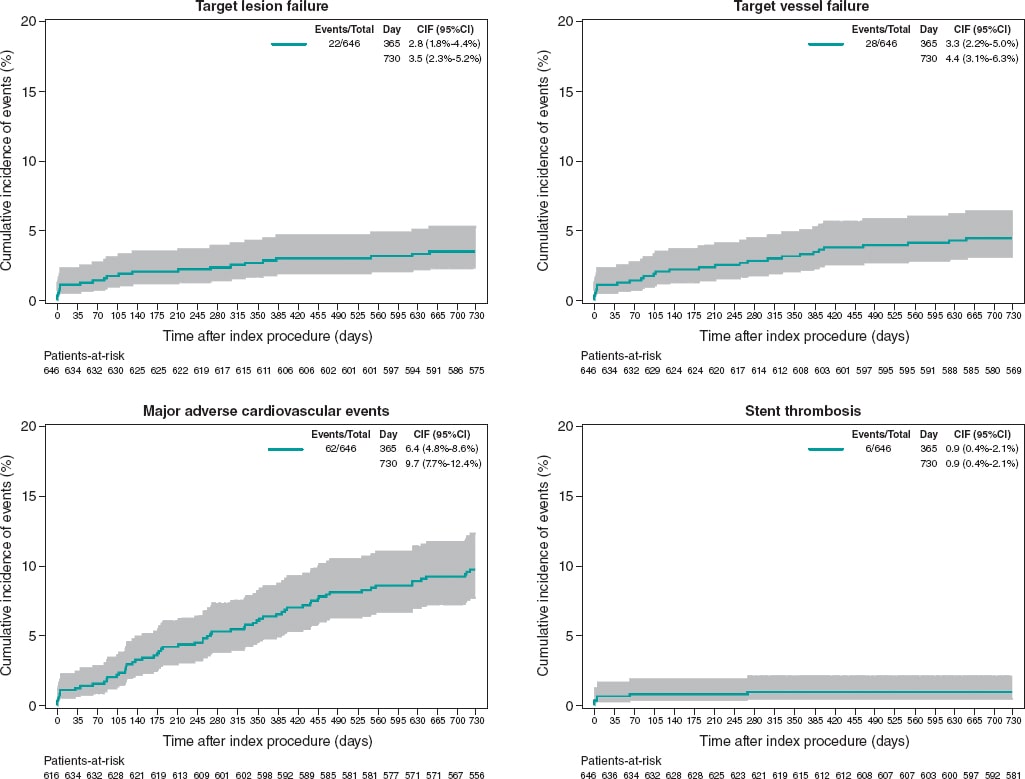
The 6-month and 1-year follow-ups were good, with only 9 (1.4%) and 12 (1.9%) patients lost to follow-up, respectively. At the 1-year follow-up, 368 patients (59.6%) were still on dual antiplatelet therapy; this rate dropped to a 15.5% at the 2-year follow-up. During the established follow-up period (2 years for all patients), only 13 patients (2%) were lost. In the global population, at 2 years, the rates of TLF, target vessel failure, and MACE were 3.4%, 4.3%, and 9.6%, respectively. Two of the 9 cases of TLF were not associated with Angiolite stents but with other stents implanted. The rate of definite/probable stent thrombosis was 0.9%; all patients were on dual antiplatelet therapy when the event occurred. Interestingly, 4 cases appeared during the first week of follow-up, 1 case within the first month, and only 1 case of stent thrombosis after 6 months (268 days). Table 4 and figure 2 summarize the individual event rate and timing.
Table 4. Outcomes in the global population
| Total population (N = 646) | 6-month follow-up | 1-year follow-up | 2-year follow-up |
|---|---|---|---|
| Death | 11 (1.7%) | 17 (2.6%) | 31 (4.8%) |
| Cardiovascular death | 6 (0.9%) | 8 (1.2%) | 11 (1.7%) |
| Myocardial infarction | 11 (1.7%) | 16 (2.5%) | 20 (3.1%) |
| Target vessel myocardial infarction | 6 (0.9%) | 8 (1.2%) | 8 (1.2%) |
| Definite/probable device thrombosis | 5 (0.8%) | 6 (0.9%) | 6 (0.9%) |
| Revascularization | 13 (2.0%) | 22 (3.4%) | 32 (5.0%) |
| Target lesion revascularization | 6 (0.9%) | 8 (1.2%) | 9 (1.4%) |
| Target vessel revascularization | 7 (1.1%) | 11 (1.7%) | 15 (2.3%) |
| Non-target vessel revascularization | 6 (0.9%) | 11 (1.7%) | 17 (2.6%) |
| Target lesion failurea | 13 (2.0%) | 18 (2.8%) | 22 (3.4%) |
| Target vessel failureb | 14 (2.2%) | 21 (3.3%) | 28 (4.3%) |
| MACEc | 25 (3.9%) | 41 (6.3%) | 62 (9.6%) |
|
MACE, major adverse cardiovascular events. a Target lesion failure defined as cardiovascular death, target vessel myocardial infarction, and clinically indicated target lesion revascularization. b Target vessel failure defined as cardiovascular death, target vessel myocardial infarction, and target vessel revascularization. c MACE defined as all-cause mortality, any myocardial infarction, any revascularization. |
|||
Figure 2. 2-year cumulative incidence of events in the entire population (N = 646).
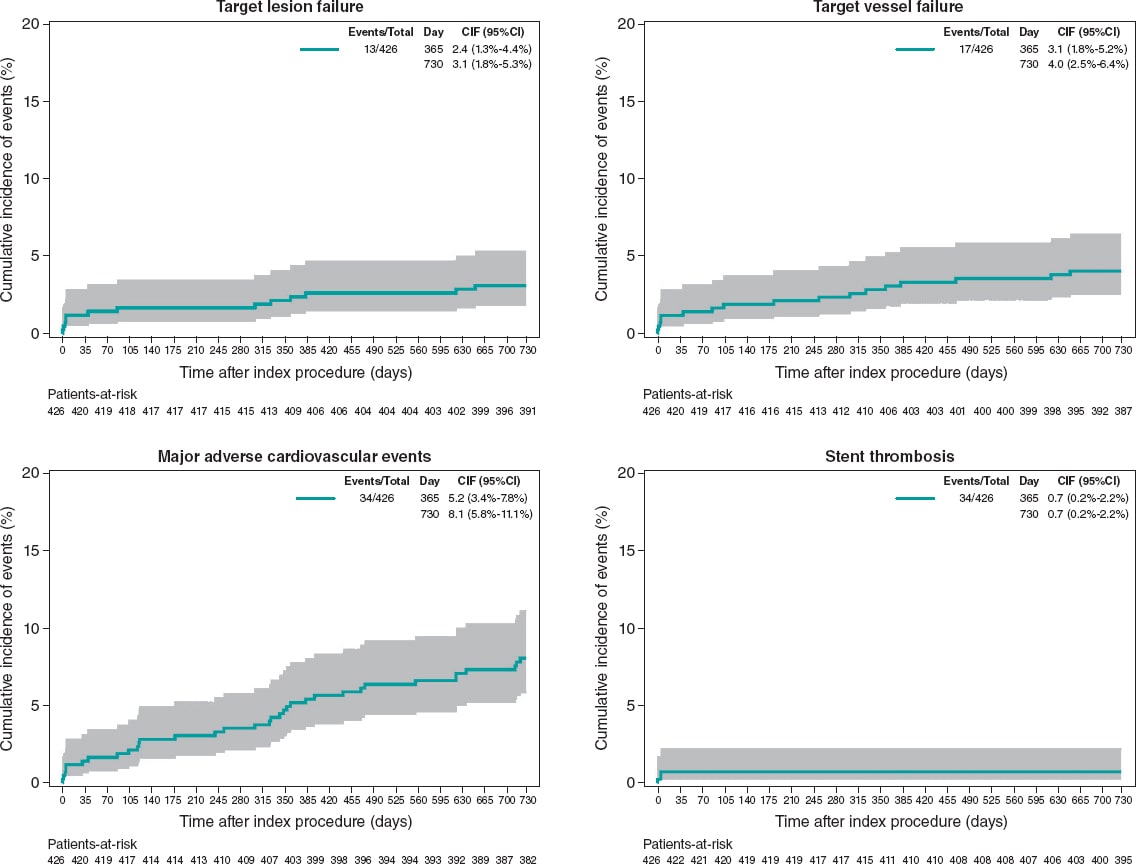
In the primary analysis population (patients treated with Angiolite stents only) at 2 years, the rates of TLF, target vessel failure, and MACE were 3.1%, 4.0%, and 8.0%, respectively. The rate of definite/probable stent thrombosis was 0.7%. No cases of stent thrombosis were found beyond the first 6 months. Table 5 and figure 3 summarize the individual event rate and timing.
Table 5. Outcomes in the primary analysis population: patients treated with the Angiolite stent only
| Angiolite only population (N = 426) | 6-month follow-up | 1-year follow-up | 2-year follow-up |
|---|---|---|---|
| Death | 5 (1.2%) | 10 (2.3%) | 18 (4.2%) |
| Cardiovascular death | 3 (0.7%) | 5 (1.2%) | 7 (1.6%) |
| Myocardial infarction | 5 (1.2%) | 5 (1.2%) | 10 (2.3%) |
| Target vessel myocardial infarction | 4 (0.9%) | 4 (0.9%) | 4 (0.9%) |
| Definite/probable device thrombosis | 3 (0.7%) | 3 (0.7%) | 3 (0.7%) |
| Revascularization | 7 (1.6%) | 11 (2.7%) | 18 (4.2%) |
| Target lesion revascularization | 3 (0.7%) | 4 (0.9%) | 5 (1.2%) |
| Target vessel revascularization | 4 (0.9%) | 7 (1.6%) | 9 (2.1%) |
| Non-target vessel revascularization | 3 (0.7%) | 4 (0.9%) | 9 (2.1%) |
| Target lesion failurea | 7 (1.6%) | 10 (2.3%) | 13 (3.1%) |
| Target vessel failureb | 8 (1.9%) | 13 (3.1%) | 17 (4.0%) |
| MACEc | 13 (3.2%) | 22 (5.3%) | 34 (8.0%) |
|
MACE, major adverse cardiovascular events. a Target lesion failure defined as cardiovascular death, target vessel myocardial infarction, and clinically indicated target lesion revascularization. b Target vessel failure defined as cardiovascular death, target vessel myocardial infarction, and target vessel revascularization. c MACE defined as all-cause mortality, any myocardial infarction, any revascularization. |
|||
Figure 3. 2-year cumulative incidence of events in the primary analysis population of patients treated with the Angiolite stent only (N = 426).
The subgroup analysis rendered 2-year results that were slightly worse that those observed in the global population:
-
– The diabetic subgroup showed rates of TLF, target vessel failure, and MACE of 3.0%, 4.5%, and 14.1%, respectively. The rate of stent thrombosis was 1.0%: 2 cases among 199 diabetic patients; only 1 of these cases appeared in the primary analysis of patients treated with the Angiolite stent only. Supplementary data give a description of the event rate (table 2 of the supplementary data).
-
– The patients with stents placed in small vessels (≤ 2.5 mm) showed rates of TLF, target vessel failure, and MACE of 4.3%, 6.0%, and 12.1%, respectively. No stent thrombosis was found. Supplementary data give a description of the event rate (table 3 of the supplementary data).
DISCUSSION
The results of the current real-world registry of the Angiolite coronary stent show an outstanding safety and efficacy profile as the ANCHOR5–first-in-human study–and the ANGIOLITE6 randomized clinical trial comparison with the XIENCE stent showed. The clinical profile shows a relatively high-risk population with a prevalence of diabetes mellitus of 30.8%, 17.6% on anticoagulation with oral drugs, 18.4% of patients diagnosed with previous myocardial infarction, and 23.4% with previous coronary revascularization. Also, a high rate of complex coronary artery disease was found in the recruited population: significant multivessel disease was diagnosed in 47.8%, compromised left main coronary artery in 4.5%, and diffuse coronary artery disease in 19.8% of the patients. Therefore, the mean number of significant lesions (1.98 ± 1.24), treated lesions (1.68 ± 0.95), and stents implanted per patient (1.8 ± 1.11) was relatively high. The ST-segment elevation myocardial infarction clinical setting of the PCI in around a quarter of the cases also shows the all-comer, real-world nature of the study.
The registry was designed to include all the patients in whom an Angiolite stent was intended to be used. Therefore, we may distinguish 2 different populations: those in whom ONLY the Angiolite stent was intended (primary analysis) and those who received different stents to treat other lesions on top of the Angiolite stent (secondary analysis). These populations have some significant differences: Angiolite ONLY-patients were more prone to have single vessel disease, few significant lesions, few treated lesions, and few stents implanted. Reasonably, this population with lower atherosclerotic burden showed less diffuse disease and fewer staged procedures. However, not all the characteristics of this group were so favorable since the presence of thrombus and the target lesion as the infarct-related artery were more common in the Angiolite ONLY stent group.
The primary endpoint, TLF at 1-year was consistently low both in the Angiolite ONLY population (primary analysis), 2.3%, and in the entire population (secondary analysis), 2.8%. Target vessel failure, a wider safety variable, was also noticeably low (3.1% and 3.3%, respectively). To confirm these results, MACE (including all-cause mortality too), a clinically oriented variable, was also very low (5.3% and 6.3%, respectively). An overview of the TLF results of different stents tested in registries and RCTs is shown on table 1 of the supplementary data. In these studies, the TLF mean value at 1-year is 5.4%, higher that the rate seen in this study.
The 2-year follow-up confirmed the very low rate of unfavorable cardiac events seen at the early 1-year period. The rate of new cardiac events, both device- and patient-oriented, within the second follow-up year was about half of the observed rate during the first year.
Both the ANCHOR FIH5 and the ANGIOLITE RCT6 pointed out an extraordinary antiproliferative efficacy of the Angiolite stent, with a mean late luminal loss < 0.05 mm. Consequently, we thought it was mandatory to assess the safety of this stent through the rate of stent thrombosis. The real-world use of the Angiolite stent is associated with a low rate of such a catastrophic complication (0.7% in primary analysis, 0.9% in secondary analysis), which guarantees the safe use of this powerful DES. The studies published showed a mean rate of stent thrombosis from 0.4% to 4.9% at the 2-year follow up (table 1 of the supplementary data). Also, the very low rate of definite/probable stent thrombosis beyond the first week (only 2 cases, 1 within the first month and the other 268 days later) restates this safety profile. We should mention that the use of dual antiplatelet therapy was high in this population (59.6% at the 1-year follow-up), which is indicative of the prevalence of acute coronary syndrome as the patients’ clinical presentation (68.3% of the patients).
The predefined subgroup analysis rendered interesting results. Diabetic patients showed TLF and stent thrombosis rates at 2 years, similar to the overall rate (3.0% vs 3.4%, and 1.0% vs 0.9%, respectively), while the rate of MACE was higher (14.1% vs 9.6%). This finding may show the worse clinical prognosis of diabetic patients, not necessarily associated with the lesion treated but with the remaining coronary artery disease. Our results are consistent with previous data published on the EVOLVE II substudy on diabetes13 that showed a 2-year TLF rate of 11.2% and a definite/probable stent thrombosis of 1.1%.
As expected, the subgroup of small vessel disease (≤ 2.5 mm) showed slightly higher rates of 2-year TLF and MACE (4.3% and 12.1%, respectively) than the global population (3.4% and 9.6%, respectively). The lack of definite/probable stent thrombosis cases could be indicative of detection bias as the thrombosis of these vessels may have a milder clinical expression. The results of this subgroup are usually hard to compare with other data as the definition of small vessel is highly arbitrary, from 2.25 mm to 3.0 mm. However, the results of our study are consistent with those reported in the Basket Small18 trial.
Limitations
The limitations of this study are the well-known issues of real-world observational registries: potential selection bias, reporting biases, and losses to follow-up (not in this case though, with a 98% of the follow-up period completed). However, the results are similar to previously reported data and are consistent with the results of previous studies with this stent. In the global population (patients who received other stents besides Angiolite stents), endpoints like probable stent thrombosis or cardiovascular death cannot be clearly attributed to a certain stent.
To minimize potential errors and reinforce the safety message, the steering committee has decided to extend the follow-up period up to 5 years.
CONCLUSIONS
In conclusion, the results of this observational registry on the use of the Angiolite DES in a real-world population confirm the excellent efficacy and safety profile seen in previous studies at the 2-year follow-up. An extended 5-year follow-up is planned to discard late events.
FUNDING
This study was conducted with financial support from Cardiva S.L. Data management and analysis were performed by an independent CRO. The final draft and the manuscript were wrote by investigators without any participation from the sponsors.
AUTHORS’ CONTRIBUTIONS
Idea and design: A. Pérez de Prado, F. Lozano Ruiz-Poveda, J. Moreu Burgos, B. García del Blanco, E. Pinar, V. Peral, J.R. Rumoroso, and R. Trillo Nouche. Data acquisition: A. Pérez de Prado, R. Ocaranza-Sánchez, F. Lozano Ruiz-Poveda, J. Moreu Burgos, R. Álvarez Ramos, A. Rodrigues, L. Fernández González, P. Aguar, B. García del Blanco, E. Pinar, V. Peral, F. Sainz Laso, J.R. Rumoroso, A. Torres, M. Sabaté, and R. Trillo Nouche. Statistical analysis and manuscript writing: A. Pérez de Prado, F. Lozano Ruiz-Poveda, J.R. Rumoroso, and R. Trillo Nouche. Provision of critical feedback to the manuscript and final content approval: A. Pérez de Prado, R. Ocaranza-Sánchez, F. Lozano Ruiz-Poveda, J. Moreu Burgos, R. Álvarez Ramos, A. Rodrigues, L. Fernández González, P. Aguar, B. García del Blanco, E. Pinar, V. Peral, F. Sainz Laso, J.R. Rumoroso, A. Torres, M. Sabaté, and R. Trillo Nouche.
CONFLICTS OF INTEREST
A. Pérez de Prado, and M. Sabaté received consulting honoraria and research grants from iVascular, and Cardiva S.L. F. Lozano Ruiz-Poveda received honoraria for his lectures from Abbott and Medtronic. All authors have declared payments to their centers from Cardiva S.L.
WHAT IS KNOWN ABOUT THE TOPIC?
- Current DES offer superior efficacy in terms of reducing restenosis with very low rates of stent thrombosis. The Angiolite stent (iVascular, Barcelona, Spain) is a thin-strut cobalt-chromium sirolimus-eluting stent with biostable coating of thrombus-resistant fluoroacrylate loaded with sirolimus. This stent has been comprehensively tested in preclinical studies, in a first-in-human study (ANCHOR study), and in a randomized clinical trial (compared to a cobalt-chromium everolimus- eluting stent) with consistent positive results. We designed an observational, prospective, and registry to endorse the previous results in our daily routine practice.
WHAT DOES THIS STUDY ADD?
- The results of this observational registry on the use of the Angiolite stent in a real-world, high-risk population confirm the excellent results seen in previous studies at the 2-year follow-up. Both the rate of device-related outcomes (target lesion and vessel failure) and patient-related outcomes (MACE) were lower compared to former data.
SUPPLEMENTARY DATA
REFERENCES
1. Neumann F-J, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. European Heart Journal. 2018;40:87-165.
2. Lüscher TF, Steffel J, Eberli FR, Joner M, Nakazawa G, Tanner FC, et al. Drug-eluting stent and coronary thrombosis:biological mechanisms and clinical implications. Circulation. 2007;115:1051-8.
3. Gori T, Polimeni A, Indolfi C, Räber L, Adriaenssens T, Münzel T. Predictors of stent thrombosis and their implications for clinical practice. Nature Reviews Cardiology. 2019;16:243-256.
4. Estevez-Loureiro R, Perez de Prado A, Perez-Martinez C, Cuellas-Ramon C, Regueiro-Purrinos M, Gonzalo-Orden JM, et al. Safety and Efficacy of New Sirolimus-eluting stent Models in a Preclinical Study. Rev Esp Cardiol (Engl Ed). 2015;68:1118-24.
5. Puri R, Otaegui I, Sabate M, Serra-Penaranda A, Puigfel M, Perez de Prado A, et al. Three- and 6-month optical coherence tomographic surveillance following percutaneous coronary intervention with the Angiolite(R) drug-eluting stent :The ANCHOR study. Catheter Cardiovasc Interv. 2018;91:435-443.
6. Moreu J, Moreno-Gomez R, Perez de Prado A, Garcia Del Blanco B, Trillo R, Pinar E, et al. First-in-man randomised comparison of the Angiolite durable fluoroacrylate polymer-based sirolimus-eluting stent versus a durable fluoropolymer-based everolimus-eluting stent in patients with coronary artery disease:the ANGIOLITE trial. EuroIntervention. 2019;15:e1081-e1089.
7. Valgimigli M, Bueno H, Byrne RA, Collet J-P, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS:The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). European Heart Journal. 2017;39:213-260.
8. Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, et al. Standardized End Point Definitions for Coronary Intervention Trials:The Academic Research Consortium-2 Consensus Document. Circulation. 2018;137:2635-2650.
9. Stone GW, Rizvi A, Sudhir K, Newman W, Applegate RJ, Cannon LA, et al. Randomized comparison of everolimus- and paclitaxel-eluting stents. 2-year follow-up from the SPIRIT (Clinical Evaluation of the XIENCE V Everolimus Eluting CoronarystentSystem) IV trial. J Am Coll Cardiol. 2011;58:19-25.
10. Waltenberger J, Hoffmann S, Brachmann J, Van Der Heijden J, Richardt G, Froebert O, et al. Bioflow-III:one year target lesion failure data of an all-comers registry with a drug eluting stent. European Heart Journal. 2013;34:P3036-P3036.
11. Pilgrim T, Heg D, Roffi M, Tüller D, Muller O, Vuilliomenet A, et al. Ultrathin strut biodegradable polymer sirolimus-eluting stent versus durable polymer everolimus-eluting stent for percutaneous coronary revascularisation (BIOSCIENCE):a randomised, single-blind, non-inferiority trial. Lancet. 2014;384:2111-22.
12. Kereiakes DJ, Meredith IT, Windecker S, Lee Jobe R, Mehta SR, Sarembock IJ, et al. Efficacy and safety of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent :the EVOLVE II Randomized Trial. Circ Cardiovasc Interv. 2015;8.
13. Kereiakes DJ, Meredith IT, Masotti M, Carrie D, Moreno R, Erglis A, et al. Safety and efficacy of a bioabsorbable polymer-coated, everolimus-eluting coronary stent in patients with diabetes:the EVOLVE II diabetes substudy. EuroIntervention. 2017;12:1987-1994.
14. Kim CH, Lee E, Kang J, Han J-K, Yang H-M, Park KW, et al. TCT-754 One-year clinical outcome of patients treated with Resolute Onyx versus Resolute Integrity:A Comparison of the HOST-ONYX and HOST-RESOLINTE Registries. Journal of the American College of Cardiology. 2017;70:B319-B319.
15. Tam CC, Chan K, Lam S, Yung A, Lam YM, Chan C, et al. One-year clinical outcomes of patients implanted with a Resolute Onyx zotarolimus-eluting stent. J Int Med Res. 2018;46:457-463.
16. von Birgelen C, Zocca P, Buiten RA, Jessurun GAJ, Schotborgh CE, Roguin A, et al. Thin composite wire strut, durable polymer-coated (Resolute Onyx) versus ultrathin cobalt–chromium strut, bioresorbable polymer-coated (Orsiro) drug-eluting stents in allcomers with coronary artery disease (BIONYX):an international, single-blind, randomised non-inferiority trial. The Lancet. 2018;392:1235-1245.
17. Wijns W, Valdes-Chavarri M, Richardt G, Moreno R, Iniguez-Romo A, Barbato E, et al. Long-term clinical outcomes after bioresorbable and permanent polymer drug-eluting stent implantation:final five-year results of the CENTURY II randomised clinical trial. EuroIntervention. 2018;14:e343-e351.
18. Jeger RV, Farah A, Ohlow M-A, Mangner N, Möbius-Winkler S, Leibundgut G, et al. Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2):an open-label randomised non-inferiority trial. The Lancet. 2018;392:849-856.