Abstract
Introduction and objectives: The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes an infectious disease that can present as adult respiratory distress syndrome (ARDS). Without an effective drug therapy, extracorporeal membrane oxygenation (ECMO) is essential when invasive mechanical ventilation fails in severe cases. Our study carried out a systematic review of the studies published in 2020 to analyze the mortality of patients with ARDS due to SARS-CoV-2 who required ECMO.
Methods: A systematic review was conducted on Medline combining keywords on SARS-CoV-2 and ECMO. All studies published during 2020 with positive cases of SARS-CoV-2 treated with ECMO were included, whether observational studies or case series. However, due to the heterogeneity in the methodology of the studies, a proper statistical analysis could not be carried out, which ended up limiting our findings.
Results: Our research identified 41 publications during this period including 2007 cases of patients with severe SARS-CoV-2 infection who required invasive support with ECMO. Among these, 985 (49%) improved clinically and were decannulated or discharged from the hospital, while 660 (32.8%) died despite invasive mechanical support. Only 357 patients (17.7%) still needed ventilation support with ECMO at the time of publication of these studies without describing the final clinical outcome.
Conclusions: ECMO therapy could be useful in patients with ARDS due to SARS-CoV-2 according to the recommendations established in the clinical guidelines and based on the availability of financial resources during the pandemic. Conducting a randomized clinical trial comparing the use of ECMO with conventional invasive ventilatory therapy would provide more evidence on this regard and, consequently, more data on the management of severe SARS-CoV-2 infection.
Keywords: COVID-19. ECMO. SARS-CoV-2. Extracorporeal membrane oxygenation. Mortality. ARDS.
RESUMEN
Introducción y objetivos: El coronavirus del síndrome respiratorio agudo grave de tipo 2 (SARS-CoV-2) genera una enfermedad infecciosa que puede presentarse como síndrome de distrés respiratorio del adulto (SDRA). Sin un tratamiento farmacológico eficaz, el oxigenador extracorpóreo de membrana (ECMO) es fundamental cuando en los casos graves fracasa la ventilación mecánica invasiva. Presentamos una revisión sistemática de los trabajos publicados en el año 2020 para analizar la mortalidad de pacientes con SDRA por SARS-CoV-2 que precisaban ECMO.
Métodos: Se realizó una revisión sistemática en Medline combinando palabras clave sobre SARS-CoV-2 y ECMO. Se incluyeron todos los estudios publicados durante el año 2020 que registraran casos positivos de SARS-CoV-2 tratados con ECMO, ya fueran estudios observacionales o series de casos. Sin embargo, debido a la heterogeneidad en la metodología de los trabajos, no se pudo llevar a cabo un análisis estadístico adecuado, lo cual limita los hallazgos.
Resultados: La búsqueda identificó 41 publicaciones y se recogieron 2.007 casos de pacientes con infección grave por SARS-CoV 2 que precisaron soporte invasivo con ECMO. De estos, 985 (49%) mejoraron clínicamente y fueron descanulados o dados de alta del hospital, y 660 (32,8%) fallecieron a pesar del soporte invasivo. Solo 357 (17,7%) pacientes aún persistían con necesidad de asistencia ventilatoria con ECMO en el momento de la publicación de los estudios, sin que se describa la evolución clínica final.
Conclusiones: El tratamiento con ECMO podría ser útil en pacientes con SDRA por SARS-CoV-2, según las directrices de las guías clínicas y en función de la disponibilidad de los recursos económicos durante la pandemia. La realización de un ensayo clínico aleatorizado que compare el uso de ECMO con el tratamiento convencional ventilatorio invasivo arrojaría mayor evidencia, con el fin de aportar más datos sobre el tratamiento de la infección grave por SARS-CoV-2.
Palabras clave: COVID-19. ECMO. SARS-CoV-2. Oxigenador extracorpóreo de membrana. Mortalidad. SDRA.
Abbreviations
SARS-CoV-2: severe acute respiratory syndrome coronavirus type 2. COVID-19: coronavirus disease-2019. ARDS: acute respiratory distress syndrome. ECMO: extracorporeal membrane oxygenation.
INTRODUCTION
In 2020, the World Health Organization (WHO) declared a public health emergency of international concern on a new strain of coronavirus different from the severe acute respiratory syndrome (SARS-CoV) and the Middle East respiratory syndrome (MERS-CoV) with which it shares some similar characteristics.1 This new strain known as severe acute respiratory syndrome type 2 (SARS-CoV-2) causes an infectious disease called COVID-19 (coronavirus disease-2019) by the WHO.1 The first case ever reported occurred in Wuhan, China, in December 2019.1 Since then, the number of contagions and deaths attributed to COVID-19 has been growing with unprecedented numbers. Until January 2021, a total of 91,492,398 and 2,252,164 cases of COVID-19 had been diagnosed worldwide and Spain, respectively.2 A total of 1,979,507 deaths due to this virus have been confirmed across the world. In Spain 19 516 cases have required ICU admission, and 53 314 deaths have been reported.2
Clinical signs are varied and go from upper respiratory tract infections to severe respiratory distress. It is possible that the intensity of the clinical response is associated with the level of expression of proinflammatory cytokines.3 As a matter of fact, the cases that end up in an intensive care unit show overexpression of cytokines, mainly IL-2, IL-7, IL-10, granulocyte-colony stimulating factor (G-CSF), interferon gamma-induced protein 10 (IP-10), macrophage inflammatory protein-1 alpha (MIP-1α), and tumor necrosis factor alpha TNFα.3 This mechanism contributes to the development of acute respiratory distress syndrome (ARDS). Patients who develop ARDS and survive have high chances of dying due to pulmonary fibrosis in the future.4 An autopsy study of patients dead due to ARDS conducted in 2013 found that the prevalence of pulmonary fibrosis with a 1 to 3 weeks clinical course was 24%. However, when the duration of ARDS was > 3 weeks, prevalence went up to 63%.5 As a matter of fact, ARDS survivors showed reticular patterns in the computed tomography scan in up to 85% of the cases.4 This reticular pattern is often found on a CT scan in the acute phase of patients with COVD-19.1
Although the lung is the organ most commonly affected in severe cases, SARS-CoV-2 infections can damage other organs and progress to multiorgan failure. Several drugs have been used during this pandemic, but none has improved survival to this date.6 The management of ARDS in severe cases of COVID-19 includes invasive mechanical ventilation, muscle relaxation, and prone positioning.1 When these measures fail, and for the lack of an effective drug therapy, the Extracorporeal Life Support Organization guidelines suggest the use of extracorporeal membrane oxygenation (ECMO).7
The use of ECMO has proven beneficial to treat ARDS due to other viral infections. During the 2009 pandemic caused by the H1N1 influenza virus, mortality went down 21% in Australia and New Zealand in patients treated with ECMO after developing ARDS.8 These data were similar to those obtained in the United Kingdom during this same pandemic (mortality rate dropped 23% in patients on ECMO vs 52% in patients without it).9 Also, refractory respiratory distress due to MERS-CoV studied in 2014-2015 in Saudi confirmed a lower in-hospital mortality rate in the group of patients treated with ECMO.10
Therefore, the main objective of our study was to conduct a systematic review of mortality in patients with severe SARS-CoV-2 infection who required invasive support with ECMO after developing ARDS refractory to conventional therapy.
METHODS
A systematic review was conducted following the criteria established by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.11 A combination of the following keywords was used in Medline: «COVID-19», «ECMO», «SARS-CoV-2», «extracorporeal membrane oxygenator/ extracorporeal membrane oxygenation», «mortality», and «ARDS». Inclusion criteria were studies from 2020, whether observational studies or case series, that analyzed the mortality of patients with ARDS and SARS-CoV-2 infection treated with ECMO. Exclusion criteria were publications on ECMO and COVID-19 that would not include additional patients eligible for this research, with the objective of focusing on ECMO related complications, that proved its benefits compared to other therapies, with authors reporting on isolated case reports including children, pregnant and postpartum women with COVID-19 who required ECMO. However, due to the heterogeneous methodology of the studies included a proper statistical analysis could not be conducted. The study was conducted in observance of the Declaration of Helsinki regarding ethical principles on medical research with human beings. This study was approved by the Complejo Hospitalario Universitario ethics committee of the Canary Islands, Spain.
RESULTS
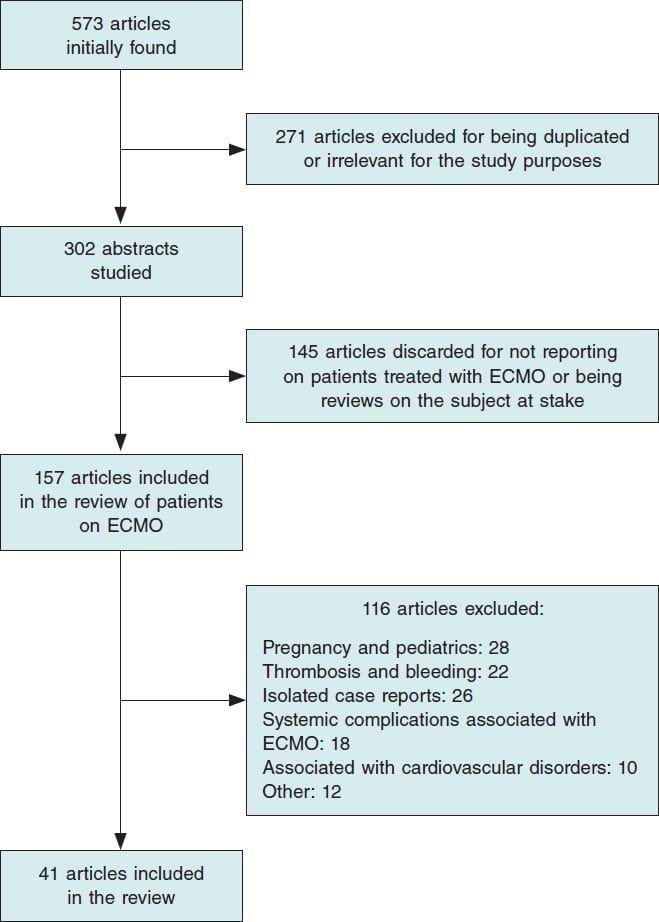
After combining the keywords, the search identified 573 publications. A total of 271 were ruled out for being duplicated or irrelevant. After reviewing the abstracts of the remaining 302 articles, 145 were excluded for not including any additional cases of patients who needed mechanical support or ECMO or for being reviews on ECMO and COVID-19. Out of the 157 studies that described cases with ECMO, 116 were discarded based on the exclusion criteria. Finally, a total of 41 publications were analyzed (figure 1) with a total of 2007 cases of patients with severe SARS-CoV-2 infections who required ECMO support with a mean age of 54 years (72% of whom were predominantly men) (table 1). Venovenous or veno-venovenous ECMO (VV-ECMO or VVV-ECMO) was administered to 1545 patients due to refractory hypoxemia yet despite prone positioning or ARDS. Venoarterial or veno-arteriovenous ECMO (VA-ECMO or VAV-ECMO) was administered to 84 patients due to cardiogenic shock. Of these, 985 (49%) patients improved clinically and were ECMO decannulated or released from the hospital. On the contrary, 660 patients (32.8%) died despite invasive mechanical support with ECMO. Finally, since 357 patients (17.7%) still needed ECMO support by the time these studies were being published, the final clinical outcome remains unknown.
Figure 1. Flowchart depicting the search for articles on extracorporeal membrane oxygenation (ECMO) and COVID-19.
Table 1. Registry of the studies available, number of patients on extracorporeal membrane oxygenation (ECMO), and number of patients released from the hospital, deceased, and still on ECMO by the time this study was being published
| Study | Journal | Patients with COVID-19 | Mean age, years (range) | Sex masculine/feminine | Total Patients on ECMO | Patients on VV- or VVV/VA- or VAV-ECMO | Patients decannulated or released from the hospital (%) | Dead patients (%) | Patients still on ECMO (%) |
|---|---|---|---|---|---|---|---|---|---|
| Total | 6636 | 54 (44-71) | 1448/457 | 2007 | 1545/84 | 985 (49%) | 660 (32.8%) | 357 (17.7%) | |
| Ahmadi ZH et al.34 | J Card Surg | 7 | 46 | 6/1 | 7 | 7/0 | 2 | 5 | 0 |
| Akhtar W et al.35 | Indian J Thorac Cardiovasc Surg | 18 | 47 | 16/2 | 18 | 15/3 | 14 | 4 | 0 |
| Alnababteh M et al.36 | Perfusion | 59 | 44 | 8/5 | 13 | 13/0 | 7 | 6 | 0 |
| Barbaro RP et al.24 | Lancet | 1035 | 49 | 764/269 | 1035 | 978/57 | 599 | 380 | 56 |
| Charlton M et al.37 | J Infect | 34 | 46 | 27/7 | 34 | NA | 18 | 16 | 0 |
| Cousin N et al.38 | ASAIO J | 30 | 57 | 24/6 | 30 | 30/0 | 14 | 16 | 0 |
| Falcoz PE et al.39 | Am J Respir Crit Care Med | 377 | 56 | 16/1 | 17 | 16/1 | 11 | 6 | 0 |
| Guo Z et al.40 | J Cardiothorac Vasc Anesth | 667 | 63 | 7/1 | 8 | 8/0 | 4 | 4 | 0 |
| Hu H et al.41 | Curr Med Sci | 55 | 50 | 4/5 | 9 | 9/0 | 5 | 4 | 0 |
| Huang S et al.42 | J Clin Anesth | 3 | 62 | 1/2 | 3 | 3/0 | 0 | 2 | 1 |
| Huette P et al.43 | Can J Anaesth | 12 | NA | NA | 12 | NA | 8 | 4 | 0 |
| Jacobs JP et al.44 | ASAIO J | 32 | 52 | 22/10 | 32 | 26/5b | 5 | 10 | 17 |
| Kon ZN et al.45 | Ann Thorac Surg | 1900 | 40 | 23/4 | 27 | 27/0 | 11 | 1 | 15 |
| Le Breton C et al.46 | J Crit Care | 13 | 58 | 10/3 | 13 | 13/0 | 11 | 2 | 0 |
| Li J et al.47 | Am J Med Sci | 74 | 71 | NA | 2 | NA | 0 | 2 | 0 |
| Loforte A et al.48 | ASAIO J | 4 | 49 | 4/0 | 4 | 4/0 | 1 | 2 | 1 |
| Marullo AG et al.31 | Minerva Cardioangiol | 333 | 52 | 285/48 | 333 | 150/9b,c | 54 | 57 | 222c |
| Miike S et al.49 | J Infect Chemother | 14 | 58 | 2/1 | 3 | NA | 2 | 1 | 0 |
| Mustafa AK et al.50 | JAMA Surg | 40 | 48 | 30/10 | 40 | NA | 29 | 6 | 5 |
| Osho AA et al.51 | Ann Surg | 6 | 47 | 5/1 | 6 | 6/0 | 5 | 1 | 0 |
| Riera J et al.52 | Crit Care Explor | 19 | 50 | 16/3 | 19 | 19/0 | 13 | 4 | 2 |
| Rieg S et al.53 | PLoS One | 213 | 65 | NA | 23 | NA | 9 | 14 | 0 |
| Ruan Q et al.19 | Intensive Care Med | 150 | 67 | NA | 7 | NA | 0 | 7 | 0 |
| Santos-Martínez S et al.54 | REC Interv Cardiol | 14 | 48 | 11/3 | 14 | 12/2 | 8 | 4 | 0 |
| Schmidt M et al.30 | Lancet Respir Med | 492 | 49 | 61/22 | 83 | 81/2 | 52 | 30 | 1 |
| Schroeder I et al.55 | Anaesthesist | 70 | 66 | 5/2 | 7 | NA | 1 | 6 | 0 |
| Shen C et al.56 | JAMA | 5 | 36-65 | 1/0 | 1 | NA | 1 | 0 | 0 |
| Sromicki et al.57 | Circ J | 9 | 59 | 6/3 | 9 | 7/2 | 7 | 2 | 0 |
| Sultan I et al.32 | J Card Surg | 10 | 31-62a | 7/3 | 10 | 10/0 | 2 | 1 | 7 |
| Wu C et al.58 | JAMA Intern Med | 201 | 51 | NA | 1 | NA | 0 | 1 | 0 |
| Xu Y et al.59 | Front Med (Lausanne) | 45 | 56 | NA | 10 | NA | 6 | 2 | 2 |
| Xuan W et al.60 | J Clin Anesth | 5 | 61 | NA | 5 | 4/1b | 2 | 3 | 0 |
| Yang X et al.61 | Crit Care Med | 21 | 58 | 12/9 | 21 | NA | 9 | 12 | 0 |
| Yang X et al.62 | Lancet Respir Med | 52 | 59 | NA | 6 | NA | 0 | 5 | 1 |
| Yang Y et al.63 | Card Fail Rev | 7 | 45 | 3/4 | 7 | 6/1b | 6 | 1 | 0 |
| Yankah CA et al.64 | Thorac Cardiovasc Surg | 42 | 51 | 30/12 | 42 | 42/0 | 17 | 7 | 18 |
| Yao K et al.65 | J Infect Chemother | 101 | 60 | NA | 11 | NA | 9 | 2 | 0 |
| Zayat R et al.66 | Artif Organs | 17 | 57 | 11/6 | 17 | 16/1 | 9 | 8 | 0 |
| Zeng Y et al.67 | Critical Care | 12 | 51 | 11/1 | 12 | NA | 3 | 5 | 4 |
| Zhang G et al.13 | J Clin Virol | 221 | 55 | NA | 10 | NA | 2 | 3 | 5 |
| Zhang J et al.68 | ERJ Open Res | 43 | 46 | 20/13 | 43 | 43/0 | 29 | 14 | 0 |
| Zhou F et al.69 | Lancet | 191 | 56 | NA | 3 | NA | 0 | 3 | 0 |
|
COVID-19, coronavirus disease-2019; ECMO, extracorporeal membrane oxygenation; NA, not available; VA, venoarterial; VAV, veno-arteriovenous; VV, venovenous; VVV, veno-venovenous. aStudy age range. bIndication for VA- or VAV-ECMO not available. cIncomplete data. |
|||||||||
DISCUSSION
We present a systematic review of publications on patients with severe SAR-CoV-2 infections treated with ECMO during 2020 since the beginning of the COVID-19 pandemic. This study includes one of the largest series of patients requiring ECMO due to severe SARS-CoV-2 infection published on the medical literature to this date.
The main clinical presentation of COVID-19 is a mild infection with dry cough and fever as the most common symptoms; the overall rate of ARDS is 3.4%.12 However, after studying series of patients who develop pneumonia and require hospitalization, the rate of ARDS can be up to 17% to 21%.13,14 The systemic inflammatory response of patients ith COVID-19 can affect, to a greater or lower extent, the pulmonary epithelium and endothelium.15 However, the endothelium seems less affected by SARS-CoV-2, which produces fewer alveolar exudates, thus contributing to the production of dry cough. On the other hand, in patients with severe COVID-19 ARDS does not show the reduction of compliance that a standard ARDS would cause, suggestive that other mechanisms are responsible for severe hypoxemia.15 This milder endothelial aggression can contribute to a small viral affectation of distal organs.15
Myocardial damage is present in 7.2% to 20% of the cases15-18 and kidney injuries in 2.9% to 15% depending on the sources.15 Myocardial damage can be associated with higher in-hospital mortality16-18 and should tip us off to discard cardiogenic shock due to fulminant myocarditis in case of hemodynamic instability after severe SARS-CoV-2 infection19. Myocardial damage is multifactorial and could be the result of the virus direct cardiotoxicity on cardiomyocytes.16 This possibility may be associated with the compatibility that exists between the virus and the angiotensin-II receptor, present in over 7.5% of cardiomyocytes. We should not forget the systemic inflammatory response following the infection that can cause the direct inflammation and suppression of myocardial contractility.16 Similarly, the fewer visits to the emergency room due to acute coronary syndrome reported and the drop in the activity of the infarction code during the pandemic have both increased the rate of cardiogenic shock of ischemic origin.20 This has reduced the healthcare activity provided during the pandemic with fewer coronary interventions being performed. This serious complication may have increased the need for ventricular assist devices, particularly ECMO, in the context of a lower availability of this device due to being used by patients with severe SARS-CoV-2 infection.
To fight severe COVID-19 cases due to ARDS refractory to protective invasive mechanical ventilation, muscle relaxation, and prone positioning or cardiogenic shock refractory to inotropic and vasopressor support, VV-ECMO or VA-ECMO are available options according to the guidelines recently published by the Extracorporeal Life Support Organization (ELSO).7 The problem with this therapy is that it is an expensive and limited resource. Therefore, during this health crisis, it should be used in young populations with high mortality rates and fewer comorbidities.7 Kidney disease is not an absolute contraindication and it should not be used in patients on invasive mechanical ventilation for more than 7 days because of the worse outcomes reported.7 For all these reasons, thorough assessments prior to indicating the most appropriate ECMO support is needed in patients with severe SARS-CoV-2 infection.21 The best time to implant this device is when protective invasive mechanical ventilation and prone positioning fail, and as long as the patient does not develop septic shock or multiorgan failure.22 After implantation, it is recommended to assess the blood concentrations of IL-6 and lymphocytes because if the numbers of these markers do not improve with this therapy, these patients’ prognosis is often less promising.23
The search conducted found higher mortality rates in patients who received ECMO due to ARDS after severe SARS-CoV-2 infection compared to those who developed the disease caused by the H1N1 influenza virus in the United Kingdom during the pandemic of 2009: 32.8% vs 23%,9 respectively. These findings are consistent with those from the registry conducted by Barbaro et al., one of the largest registries ever published, of 1035 patients with a 39% in-hospital mortality rate.24 On the other hand, during the MERS-CoV pandemic of 2015, the mortality of the group that received ECMO therapy was analyzed (64% compared to 100% in the group without this device).10 However, due to the lack of clinical trials in the medical literature with control groups of treatment without ECMO for the management of SARS-CoV-2-induced ARDS, we still should not say that its use is beneficial. Also, the high pressure exerted on the health centers at the beginning of the pandemic may have contributed to the worse results reported like the ones published by Ruan et al.19 compared to other series that studied mortality with ECMO in these patients when this pressure on the healthcare system had probably gone down.24,30
During the first few months of 2020, 2 meta-analyses of patients with SARS-CoV-2-induced ARDS treated with ECMO were conducted. The first one included 4 Chinese studies and proved the poor benefits of this therapy in 17 patients since only 1 managed to survive.25 The other meta-analysis includes 6 series of 17 patients in total. Fourteen of these patients died and mortality rate was close to 82.3%.26 The limitation of these studies is the small number of patients included for analysis and both recommended conducting new studies.
There are reviews already currently available on the medical literature. However, one of them only includes 274 patients who required ECMO, meaning that mortality could not be properly analyzed since 45.6% of the patients remained hospitalized by the time the studies included were being published.27 A different review of 479 patients from 25 studies showed a 19.83% mortality rate. However, the authors claim that it is just an estimate since some of the studies did not report on the mortality rate of the subjects.28 Finally, Melhuish et al.29 grouped 331 cases from 10 different studies and 4 database registries and estimated a 46% mortality rate. A common limitation of these studies is that none of them includes the registry conducted by Barbaro et al.24 the largest published to this date. Our review widens and consolidates these findings after including the 3 largest series published to this date of 83, 333, and 1035 patients.24,30,31 Although we found a higher mortality rate compared to the H1N1 pandemic of 2009,8,9 ECMO support in these patients may be acceptable for the lack of another therapeutic option. However, every case should be treated individually; patients over 60 and with associated comorbidities like cardiovascular disease and diabetes have a higher mortality risk.17,28,31
Due to the complexity of ECMO support, the need for the proper learning curve and clinical experience, the results of this therapy can be biased. From 2003 through 2019, the number of centers that used this device across the world quadrupled, and the number of devices implanted has multiplied by a factor of 6.32 This is so to such an extent that during an unexpected pandemic when resources need to be immediately restructured, the results obtained by the studies within the first few months of 2020 should be interpreted with caution. For example, during the pandemic of 2009, much more ECMO systems were used, which may have generated higher chances of recovery compared to the current limitation of resources available for the implantation of this device. This means that mortality results may be different too.1
Finally, we should mention that despite the fact that patients survive with the invasive support provided by ECMO, the chances of experiencing pulmonary fibrosis in the future are non-negligible with the corresponding higher mortality rate.5 Further studies are needed to identify patients with greater chances of developing this complication; also, antithrombotic therapy may be useful for the management of SARS-CoV-2 infections causing parenchymal pulmonary fibrosis.5
Limitations
The first limitation of our study is that unpublished multicenter registers on scientific journals were excluded.33 Also, patients treated with ECMO from studies focused on analyzing ECMO related complications and isolated case reports were excluded. The characteristics of patients from each study or the methodologies used have not been compared because they were different.
CONCLUSIONS
We believe that invasive support with ECMO may be useful for certain patients based on the recommendations established by the clinical guidelines and the availability of resources despite the dissimilar results obtained. A randomized clinical trial comparing the use of ECMO to conventional invasive mechanical ventilation would bring further evidence on this regard.
FUNDING
This study received no funding whatsoever.
AUTHORS’ CONTRIBUTIONS
N. Báez-Ferrer was involved in the reference search, data analysis, and writing of this manuscript. A. Bompart-Cairós, and D. López-Rial both participated in the reference search. P. Abreu-González, and D. Hernández-Vaquero participated in the review and writing of this manuscript. A. Domínguez-Rodríguez conducted the manuscript final review.
CONFLICTS OF INTEREST
None reported.
WHAT IS KNOWN ABOUT THE TOPIC?
- ARDS can be the clinical presentation of SARS-CoV-2 infection.
- Multiple drug therapies fail during the management of this entity. The use of ECMO is especially important in patients who are refractory to mechanical ventilation, muscle relaxation, and prone positioning.
- Since the beginning of the COVID-19 pandemic and all across 2020 several articles of patients with severe SARS-CoV-2 infection manifested as ARDS have been published. These articles have analyzed the mortality rate associated with ECMO therapy. However, to this date, no randomized clinical trial has assessed the clinical benefit of ECMO in these patients.
WHAT DOES THIS STUDY ADD?
- We presented the results of a systematic review of the studies published in 2020 during the COVID-19 pandemic to analyze the mortality rate of patients with SARS-CoV-2-induced ARDS requiring ECMO.
- A total of 41 publications were identified during 2020, and 2007 cases of patients with severe SARS-CoV-2 infection who required invasive support with ECMO were collected.
- Of all the cases collected, a mortality rate associated with ECMO in patients with severe SARS-CoV-2 was found to be 32.8%; 660 patients died despite therapy with invasive mechanical support.
- ECMO therapy may be useful in patients with SARS-CoV-2-induced ARDS. However, it would be interesting to conduct a randomized clinical trial to compare the use of ECMO to conventional invasive ventilation therapy during this pandemic.
REFERENCES
1. Tang D, Comish P, Kang R. The hallmarks of COVID-19 disease. PLoS Pathog. 2020;16:1-24.
2. Actualización nº291. Enfermedad por el coronavirus (COVID-19). 15.01.2021. Available online https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Actualizacion_291_COVID-19.pdf. Accessed 18 Jan 2021.
3. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:18-21.
4. Yan-Rong G, Qing-Dong C. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak –an update on the status. Mil Med Res. 2020;7:11.
5. George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19:the potential role for antifibrotic therapy. Lancet Respir Med. 2020;8:807-815.
6. Thille AW, Esteban A, Fernández-Segoviano P, et al. Chronology of histological lesions in acute respiratory distress syndrome with diff use alveolar damage:A prospective cohort study of clinical autopsies. Lancet Respir Med. 2013;1:395-401.
7. Bartlett RH, Ogino MT, Brodie D, et al. Initial ELSO Guidance Document:ECMO for COVID-19 Patients with Severe Cardiopulmonary Failure. ASAIO J. Published online 2020:472-474.
8. Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators, Davies A, Jones D, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. J Am Med Assoc. 2009;302:1888-1895.
9. Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). J Am Med Assoc. 2011;306:1659-1668.
10. Alshahrani MS, Sindi A, Alshamsi F, et al. Extracorporeal membrane oxygenation for severe Middle East respiratory syndrome coronavirus. Ann Intensive Care. 2018;8:3.
11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions:Explanation and Elaboration. Vol 62.;2009.
12. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720.
13. Zhang G, Hu C, Luo L, et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020;127:104364.
14. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China:a descriptive study. Lancet. 2020;395:507-513.
15. Li X, Ma X. Acute respiratory failure in COVID-19:Is it “typical“ARDS?Crit Care. 2020;24:1-5.
16. Kang Y, Chen T, Mui D, et al. Cardiovascular manifestations and treatment considerations in COVID-19. Heart. 2020;106:1132-1141.
17. Li X, Guo Z, Li B, et al. Extracorporeal Membrane Oxygenation for Coronavirus Disease 2019 in Shanghai, China. ASAIO J. 2020:475-481.
18. Shi S, Qin M, Shen B, et al. Association of Cardiac Injury with Mortality in Hospitalized Patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802-810.
19. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-848.
20. Rodríguez-Leor O, Cid-Álvarez B, Ojeda S, et al. Impact of the COVID-19 pandemic on interventional cardiology activity in Spain. REC Interv Cardiol. 2020;2:82-89.
21. Khan R, Anandamurthy B, McCurry K, Krishnan S. Utility of extracorporeal membrane oxygenation in COVID-19. Cleve Clin J Med. 2020:1-3.
22. Li L, Li R, Wu Z, et al. Therapeutic strategies for critically ill patients with COVID-19. Ann Intensive Care. 2020;10:45.
23. Henry BM. COVID-19, ECMO, and lymphopenia:a word of caution. Lancet Respir Med. 2020;8:e24.
24. Barbaro R, MacLaren G, Boonstra P, Iwashyna, TH, Slutsky A, Fan E. Extracorporeal membrane oxygenation support in COVID-19:an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396:1071-1078.
25. Henry BM, Lippi G. Poor survival with extracorporeal membrane oxygenation in acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19):Pooled analysis of early reports. J Crit Care. 2020;58:27-28.
26. Ñamendys-Silva SA. ECMO for ARDS due to COVID-19. Hear Lung. 2020;49:348-349.
27. Oliveira TF de, Rocha CA de O, Santos AGG dos, et al. Extracorporeal Membrane Oxygenation in COVID-19 Treatment:a Systematic Literature Review. Brazilian J Cardiovasc Surg. 2021;8:84-123.
28. Haiduc AA, Alom S, Melamed N, Harky A. Role of extracorporeal membrane oxygenation in COVID-19:A systematic review. J Card Surg. 2020;35:2679-2687.
29. Melhuish TM, Vlok R, Thang C, Askew J, White L. Outcomes of extracorporeal membrane oxygenation support for patients with COVID- 19:A pooled analysis of 331 cases. Am J Emerg Med. 2020;39:245-246.
30. Schmidt M, Hajage D, Lebreton G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19:a retrospective cohort study. Lancet Respir Med. 2020;8:1121-1131.
31. Marullo A, Cavaretta E, Biondi-zoccai G, et al. Extracorporeal membrane oxygenation for critically ill patients with coronavirus-associated disease 2019 :an updated perspective of the European experience. Minerva Cardioangiol. 2020;68:368-372.
32. Sultan I, Habertheuer A, Usman AA, et al. The role of extracorporeal life support for patients with COVID-19:Preliminary results from a statewide experience. J Card Surg. 2020;35:1410-1413.
33. The Extracorporeal Life Support Organization (ELSO):ECMO in COVID-19. Available online:https://www.elso.org/Registry/FullCOVID19RegistryDashboard.aspx. Accessed 18 Jan 2021.
34. Ahmadi ZH, Jahangirifard A, Farzanegan B, et al. Extracorporeal membrane oxygenation and COVID-19:The causes of failure. J Card Surg. 2020;35:2838-2843.
35. Akhtar W, Olusanya O, Baladia MM, Young H, Shah S. SARS-CoV-2 and ECMO:early results and experience. Indian J Thorac Cardiovasc Surg. 2021;37:53-60.
36. Alnababteh M, Hashmi MD, Vedantam K, et al. Extracorporeal membrane oxygenation for COVID-19 induced hypoxia:Single-center study. Perfus (United Kingdom). 2020. http://dx.doi.org/10.1177/0267659120963885.
37. Charlton M, Dashey S, Stubbs A, Lai FY, Tang JW. Comparing SARS-CoV-2 and influenza A(H1N1)pdm09-infected patients requiring ECMO. A single-centre, retrospective observational cohort experience. J Infect. 2020. http://dx.doi.org/10.1016/j.jinf.2020.11.003.
38. Cousin N, Bourel C, Carpentier D, et al. SARS-CoV-2 versus influenza associated acute respiratory distress syndrome requiring veno-venous extracorporeal membrane oxygenation support. ASAIO J. 2021 Feb 1;67(2):125-131.
39. Falcoz P-E, Puyraveau M, Perrier S, et al. Extracorporeal Membrane Oxygenation for Critically Ill Patients with COVID-19–related Acute Respiratory Distress Syndrome:Worth the Effort?. Am J Respir Crit Care Med. 2020;202:460-463.
40. Guo Z, Sun L, Li B. Anticoagulation Management in Severe Coronavirus Disease 2019 Patients on Extracorporeal Membrane Oxygenation. J Cardiothorac Vasc Anesth. 2021;35:389-397.
41. Hu H, Xu S, Wang J, Rao X. Respiratory Support in Severely or Critically Ill ICU Patients With COVID-19 in Wuhan, China. Curr Med Sci. 2020;40:636-641.
42. Huang S, Xia H, Wu Z, et al. Clinical data of early COVID-19 cases receiving extracorporeal membrane oxygenation in Wuhan, China. J Clin Anesth. 2021;68:110044.
43. Huette P, Beyls C, Guilbart M, et al. Extracorporeal membrane oxygenation for respiratory failure in COVID-19 patients:outcome and time-course of clinical and biological parameters. Can J Anesth. 2020;67:1486-1488.
44. Jacobs JP, Stammers AH, Louis J, et al. Extracorporeal Membrane Oxygenation in the Treatment of Severe Pulmonary and Cardiac Compromise in Coronavirus Disease 2019:Experience with 32 Patients. ASAIO J. 2020. http://dx.doi.org/10.1097/MAT.0000000000001185.
45. Kon ZN, Smith DE, Chang SH, et al. Extracorporeal Membrane Oxygenation Support in Severe COVID-19. Ann Thorac Surg. 2020. http://dx.doi.org/10.1016/j.athoracsur.2020.07.002.
46. Breton C Le, Besset S, Amouretti M, Billiet PA, Dao M. Extracorporeal membrane oxygenation for refractory COVID-19 acute respiratory distress syndrome. J Crit Care. 2020;60:10-12.
47. Li J, Xu G, Yu H, Peng X, Luo Y, Cao C. Clinical Characteristics and Outcomes of 74 Patients With Severe or Critical COVID-19. Am J Med Sci. 2020;360:229-235.
48. Loforte A, Dal Checco E, Gliozzi G, et al. Veno-venous extracorporeal membrane oxygenation support in covid-19 respiratory distress syndrome:Initial experience. ASAIO J. 2020;66:734-738.
49. Miike S, Sakamoto N, Washino T, et al. Critically ill patients with COVID-19 in Tokyo, Japan:A single-center case series. J Infect Chemother. 2021;27:291-295.
50. Mustafa AK, Alexander PJ, Joshi DJ, et al. Extracorporeal Membrane Oxygenation for Patients with COVID-19 in Severe Respiratory Failure. JAMA Surg. 2020;155:990-992.
51. Osho AA, Moonsamy P, Hibbert KA, et al. Veno-venous Extracorporeal Membrane Oxygenation for Respiratory Failure in COVID-19 Patients:Early Experience From a Major Academic Medical Center in North America. Ann Surg. 2020;272:e75-e78.
52. Riera J, Argudo E, Martínez-Martínez M, et al. Extracorporeal Membrane Oxygenation Retrieval in Coronavirus Disease 2019:A Case-Series of 19 Patients Supported at a High-Volume Extracorporeal Membrane Oxygenation Center. Crit Care Explor. 2020;2:e0228.
53. Rieg S, von Cube M, Kalbhenn J, et al. COVID-19 in-hospital mortality and mode of death in a dynamic and non-restricted tertiary care model in Germany. PLoS One. 2020;15:1-16.
54. Santos-Martínez S, Martín Moreiras J, Vázquez-Álvarez M, Peñasco Y, Uribarri A, Amat-Santos I. Oxigenador extracorpóreo de membrana con implante percutáneo durante la pandemia de COVID-19. Registro multicéntrico español. REC Interv Cardiol. 2020;2:312-314.
55. Schroeder I, Scharf C, Zoller M, et al. Characteristics and outcome of 70 ventilated COVID-19 patients:Summary after the first wave at a university center. Anaesthesist. 2020. http://dx.doi.org/10.1007/s00101-020-00906-3.
56. Shen C, Wang Z, Zhao F, et al. Treatment of 5 Critically Ill Patients with COVID-19 with Convalescent Plasma. J Am Med Assoc. 2020;323:1582-1589.
57. Sromicki J, Schmiady M, Maisano F, Mestres CA. ECMO therapy in COVID-19:The Zurich experience. J Card Surg. 2020. 2021;36:1707-12.
58. Wu C, Chen X, Cai Y, et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934-943.
59. Xu Y, Xu Z, Liu X, et al. Clinical Findings of COVID-19 Patients Admitted to Intensive Care Units in Guangdong Province, China:A Multicenter, Retrospective, Observational Study. Front Med. 2020;7:1-9.
60. Xuan W, Chen C, Jiang X, et al. Clinical characteristics and outcomes of five critical COVID-19 patients treated with extracorporeal membrane oxygenation in Leishenshan Hospital in Wuhan. J Clin Anesth. 2020;67:110033.
61. Yang X, Cai S, Luo Y, et al. Extracorporeal Membrane Oxygenation for Coronavirus Disease 2019-Induced Acute Respiratory Distress Syndrome:A Multicenter Descriptive Study. Crit Care Med. 2020;3:1289-1295.
62. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China:a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-481.
63. Yang Y, Rali AS, Inchaustegui C, et al. Extracorporeal Membrane Oxygenation in Coronavirus Disease 2019-associated Acute Respiratory Distress Syndrome:An initial US Experience at a High-volume Centre. Card Fail Rev. 2020;6:7-9.
64. Yankah CA, Trimlett R, Sandoval E, et al. COVID-19 Pulmonary Failure and Extracorporeal Membrane Oxygenation:First Experience from Three European Extracorporeal Membrane Oxygenation Centers. Thorac Cardiovasc Surg. 2021;69:259-62.
65. Yao K, Hasegawa S, Tagashira Y, et al. Experience of 101 patients with coronavirus infectious disease 2019 (COVID-19) at a tertiary care center in Japan. J Infect Chemother. 2021;27:413-417.
66. Zayat R, Kalverkamp S, Grottke O, et al. Role of extracorporeal membrane oxygenation in critically Ill COVID-19 patients and predictors of mortality. Artif Organs. 2020. http://dx.doi.org/10.1111/aor.13873.
67. Zeng Y, Cai Z, Xianyu Y, Yang BX, Song T, Yan Q. Prognosis when using extracorporeal membrane oxygenation (ECMO) for critically ill COVID-19 patients in China:A retrospective case series. Crit Care. 2020;24:8-10.
68. Zhang J, Merrick B, Correa GL, et al. Veno-venous extracorporeal membrane oxygenation in coronavirus disease 2019:a case series. ERJ Open Res. 2020;6:00463-02020.
69. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China:a retrospective cohort study. Lancet. 2020;395:1054-1062.