ABSTRACT
Percutaneous coronary intervention (PCI) plays a key role in the management of patients with obstructive coronary artery disease. Besides, depending on the patients’ clinical presentation, characteristics, comorbidities, and coronary anatomy, an increasing number of patients will undergo a high-risk PCI. Left ventricular assist devices, as the intra-aortic balloon pump, TandemHeart, Impella, HeartMate PHP, and extracorporeal membrane oxygenation are useful tools to provide circulatory support for high-risk PCIs. Some studies and trials have assessed its impact on this clinical scenario with controversial results. This review provides an overview on the scientific evidence available on the use of left ventricular assist devices and their potential role in high-risk PCI.
Keywords: Intra-aortic balloon pump. Left ventricular assist device. High-risk percutaneous coronary intervention. Cardiogenic shock. Right ventricle.
RESUMEN
La intervención coronaria percutánea (ICP) desempeña un papel fundamental en el tratamiento de los pacientes con enfermedad coronaria obstructiva. De ellos, un porcentaje significativo se someterán a un procedimiento de alto riesgo, en función de la presentación clínica, las características del paciente y su anatomía coronaria. Los dispositivos de asistencia ventricular izquierda, como el balón intraaórtico de contrapulsación, el dispositivo TandemHeart, el Impella, los dispositivos HeartMate PHP y las técnicas de oxigenación veno-arterial con oxigenador extracorpóreo de membrana (ECMO), son herramientas empleadas para proporcionar soporte circulatorio en la ICP de alto riesgo, con un impacto creciente en la práctica clínica. Existen numerosos trabajos en la literatura científica sobre su empleo en este escenario, con resultados controvertidos. Esta revisión proporciona una visión general de la evidencia disponible sobre el empleo de los distintos tipos de dispositivos, así como de su potencial papel en la ICP de alto riesgo.
Palabras clave: Balón intraaórtico de contrapulsación. Dispositivo de asistencia ventricular izquierda. Intervencionismo coronario percutáneo de alto riesgo. Shock cardiogénico. Ventrículo derecho.
Abbreviations: AMI: acute myocardial infarction. CHD: coronary heart disease. ECMO: extracorporeal membrane oxigenator. IABP: Intra-aortic balloon pump. LMCA: left main coronary artery. LVAD: left ventricular assist device. PCI: percutaneous coronary intervention.
INTRODUCTION
In the Western world, coronary heart disease (CHD) is a problem of public health. It is estimated that in the US population over 20, 15.5 million people suffer from CHD and nearly 635 000 will suffer from a new acute coronary event each year.1 The percutaneous coronary intervention (PCI) as the way to treat this condition is still growing exponentially and it is currently the treatment of choice for revascularization purposes,1-3 except for certain patients with multivessel or highly complex disease.4,5 The has a class I recommendation for the management of patients with acute coronary events and it is the first-line therapy in 3 clinical settings: refractory angina to medical therapy, cardiogenic shock as a complication of the acute myocardial infarction (AMI), and ST-segment elevation acute coronary syndrome.3,4
HIGH-RISK PERCUTANEOUS CORONARY INTERVENTION
The criterion to define a PCI as a high-risk PCI is not well-established, but there is a series of characteristics that give it a high periprocedural risk profile that can be divided into 3 groups: patient-specific, lesion-specific, and clinical presentation- specific.6-9
Patient-inherent factors are old age, diabetes mellitus, chronic kidney disease, previous myocardial infarction, severe peripheral vascular disease, and the presence of left ventricular systolic dysfunction defined as a value < 30%-35%.9,10
The factors dependent on the characteristics of the coronary lesion are left main coronary artery disease (LMCA)—unprotected—, ostial disease or in bifurcations, lesion to the saphenous vein bypass graft, presence of abundant calcification, and chronic occlusions.11,12 Finally, clinical presentation plays a role in the prognostic of these patients in such a way that those with a cardiogenic shock or hospitalized with an acute coronary syndrome have a higher risk of adverse events during the PCI.13
We should mention that cardiogenic shock is the leading cause of death associated with the AMI with a prevalence between 5% and 15%.13,14 There is growing evidence that the prognosis of patients with AMI complicated with cardiogenic shock could substantially improve with early PCIs and primary angioplasty.15,16
PERCUTANEOUS CIRCULATORY ASSIST DEVICES
Left ventricular assist devices (LVAD) are used to provide hemodynamic support during high-risk PCIs. These devices include the intra-aortic balloon pump (IABP), the TandemHeart device (CardiacAssist, United States), the Impella device (Abiomed, United States), the HeartMate PHP devices (St. Jude Medical, United States), and veno-arterial oxygenation techniques with extracorporeal membrane oxygenation (ECMO).17 Their main characteristic are comparatively described and shown on table 1.
Table 1. Comparison of the different type of left circulatory assist devices based on their baseline characteristics
| Device | Pump action mechanism | Cardiac chamber of action | Vascular access | Flow |
|---|---|---|---|---|
| IABP | Counterpulsation | LV | 8-9 Fr | 1 L/min |
| ECMO | Centrifugal | Biventricular | Venous (15-22 Fr) Arterial (15-21 Fr) | > 4.5 L/min |
| TandemHeart | Centrifugal | LV, RV or biventricular | Venous (15-17 Fr) Arterial (21-Fr) | 4.5 L/min |
| Impella 2.5 | Axial | LV | 12-Fr | 2.5 L/min |
| Impella CP | Axial | LV | 14-Fr | 3.33 L/min |
| Impella 5.0 | Axial | LV | 21-Fr | 5 L/min |
|
ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; LV, left ventricle; RV, right ventricle. |
||||
The intra-aortic balloon pump
Since it was first introduced back in the 1970s, the IABP has become a circulatory assist device for several indications
Their capacity to improve coronary flow,18,19 improve systemic flow by an additional increase of cardiac output of 0.5 L/min,14,20,21 and reduce the myocardial oxygen consumption22 recommends it use in all those patients in whom coronary and systemic flow needs to be increased.
Recently, in the US clinical practice guidelines,3 the use of IABPs has gone from a class I a to a class II b recommendation in the cardiogenic shock setting as a complication of AMI. The European guidelines23 give IABPs a class III recommendation. This has to do with the studies that question the value of IABP as a factor worth of prognostic impact.24 The IABP SHOCK study compared the use of the IABP after PCI and the standard approach with inotropes and vasoactive amines without confirmation of short-term benefits in mortality rate.25 These findings were backed after the publication of the IABP-SHOCK II study that found no differences in 30-day mortality rate24 or all-cause mortality rate at the 12-month follow-up26 in patients with AMI complicated with cardiogenic shock.
A meta-analysis suggests that the preoperative use of IABP reduces preoperative mortality and the 30-day mortality rate in high-risk patients scheduled to undergo elective surgery of myocardial revascularization.27-32 Other authors think that the use of IABPs does not impact mortality in patients with AMI regardless of whether they show cardiogenic shock or not.14,33,34
These contradictory results set the foundations of new research studies.
Current situation in PCI procedures
The IABP has been used over decades in high-risk PCIs thanks to its circulatory support capabilities.9,35-38 A series of studies compared its elective implantation in this context with its use as a bail-out strategy in stable patients eligible for a high-risk PCI. These studies suggest that the elective implantation of an IABP prior to the procedure is associated with fewer adverse events during the PCI39,40 with a tendency towards fewer major adverse cardiovascular events. Mishra et al.38 reported that the prophylactic implantation of an IABP prior to a high-risk PCI was associated with a higher survival rate during the hospital stay and at the 6-month follow-up compared to its implantation as a bail-out strategy due to the development of hemodynamic compromise during the procedure. All of it happened at the expense of a high risk from this group of complications associated with bleeding complications.41 Although these data are relevant they all come from retrospective studies.
Back in 2010, Perera et al.39 conducted a prospective, multicenter, randomized, and controlled clinical trial on coronary interventions assisted with intra-aortic balloon pumps (BCIS-1). This study randomized 301 patients with CHD and a left ventricular systolic dysfunction < 30% to receive, or not, an elective IABP. The primary endpoint was the presence of cardiovascular adverse events at the 28-day follow-up, which occurred in 15.2% of the patients where the IABP was implanted electively compared to 16% of the patients where the IABP was not scheduled. The elective use of the IABP was associated with fewer bleeding and local complications compared to its bail-out use in the group of patients without scheduled implantations. These results are consistent with those of a meta-analysis recently published.34
Based on the results from clinical trials, the use of IABPs in high-risk PCIs has been going down.13,41 At the same time, the development and use of other LVADs in this context has been going up.42
TandemHeart
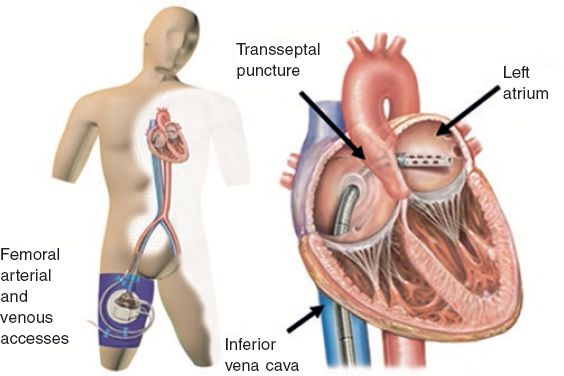
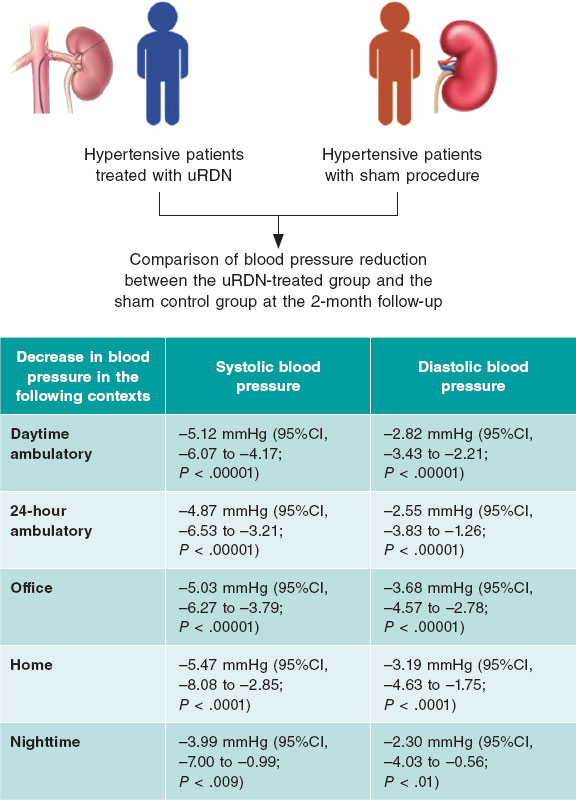
The TandemHeart (figure 1) is an external temporary mechanical circulatory support device capable of supplying a continuous flow 4 L/min.43 It includes 3 subsystems and it is the only device designed to enter the interatrial septum through a 21-Fr cannula that is allocated in the left atrium. The oxygenated blood is pumped out of the left atrium and then returned through a centrifugal pump that provides continuous flow into the femoral artery (through a 12-Fr cannula) or the iliac artery (through a 5-17-Fr cannula).
Figure 1. Scheme of the functioning of the TandemHeart device with femoral peripheral access. Oxygenated blood drainage by transseptal puncture of the left atrium that comes back though the femoral artery.
A cohort study conducted by Thiele et al.43 among 18 patients with cardiogenic shock post-AMI confirmed significantly better hemodynamic parameters after IABP implantation at the expense of a series of complications associated with the insertion and maintenance of the catheter with a 44% 30-day overall mortality rate. This study also showed that LVADs can be implanted quickly in less than 30 minutes.
Other studies have compared the efficacy of TandemHeart vs IABP for the management of cardiogenic shock such as the ones conducted by Thiele et al.42 and Burkhoff et al.44. These studies showed the capacity of the TandemHeart device to improve the patients’ hemodynamic situation assuming that there is still a risk of complications associated with the device.
Current situation in PCI procedures
The first case ever reported of a TandemHeart device used in a high-risk PCI was documented by Vranckx et al.45. Since then, several retrospective studies have been conducted in an attempt to analyze its use. One of them included 9 patients with an LMCA lesion who were not eligible for surgery. This study reached a 100% success rate in the PCI.46 Four out of these 9 patients developed vascular access complications, 2 of which required vascular surgery due to the presence of lower-limb ischemia. The 6-month survival rate was 88.5% compared to 89.5% in the overall population with LMCA disease in the same hospital.47,48
Then, Aragon et al.49 analyzed the use of the TandemHeart device in 8 patients who underwent a high-risk PCI and found that hemodynamic improvement can be achieved early with angioplasty success rates close to 100% and no immediate complications after the PCI.
Back in 2012, Alli et al.50 conducted a retrospective study that analyzed 54 patients who underwent a PCI under TandemHeart support between 2004 and 2009 with a PCI success rate of 97% (62% of the patients had multivessel or LMCA disease). The overall 30-day survival rate was 90%, and it was kept for 6 months. However, the rate of vascular complications is significant (13%).50,51
Impella
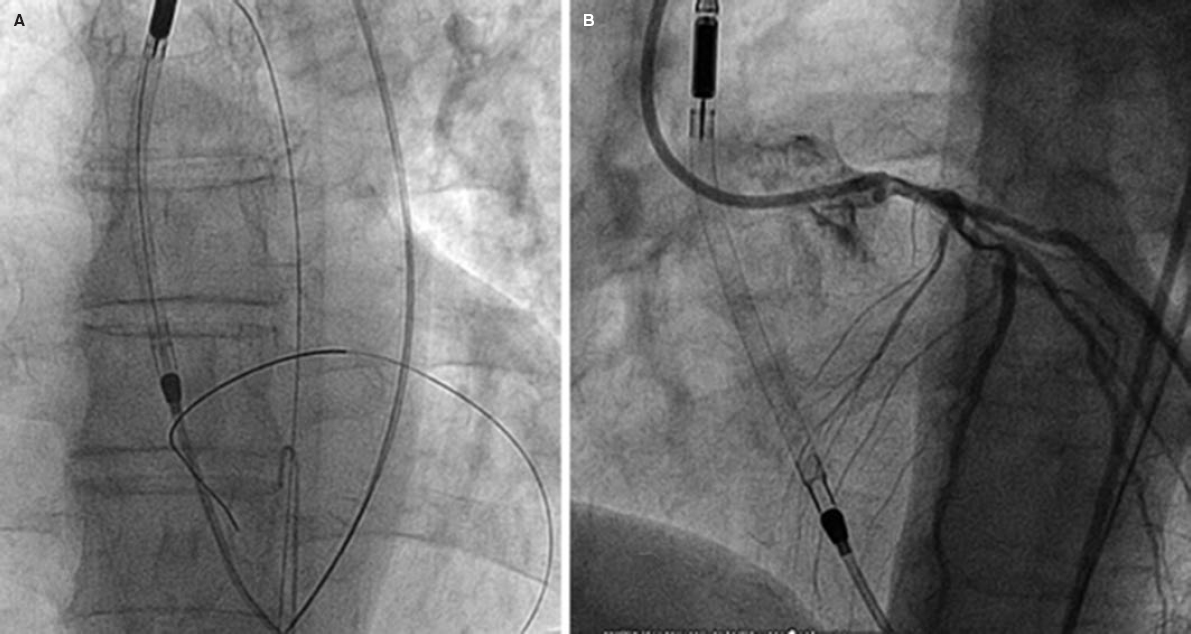
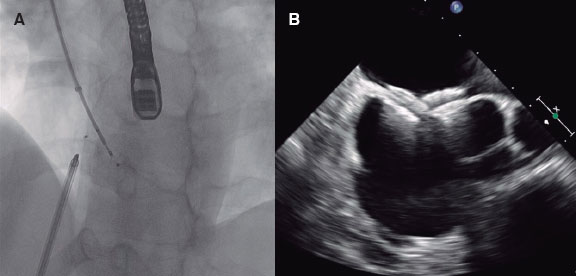
Impella devices (figure 2) use a catheter via femoral access that crosses the aortic valve that is allocated in the left ventricle where it pumps out oxygenated blood that is then returned to the ascending aorta. There are different models available: Impella 2.5, Impella CP, and Impella 5.0 supplying 2.5, 4, and 5 L/min of flow, respectively.
Figure 2. High-risk percutaneous coronary intervention with circulatory support with the Impella 2.5 device. A and B show the correct location of the device while crossing the aortic valve.
An early study conducted by Seyfarth et al.52 that compared the Impella 2.5 device and the IABP in 25 patients with cardiogenic shock post-AMI confirmed that the Impella 2.5 device provided better hemodynamic support compared to the IABP. However, it also had a higher rate of transfusions and hemolysis compared to the IABP, but no differences were seen in the 30-day mortality rate (around 46%). The EUROSHOCK registry53 included 120 patients with cardiogenic shock post-AMI who received circulatory support with an Impella 2.5 device. This registry confirmed that it is a real option resulting in better plasma lactate concentrations at the expense of a red blood cell concentrate transfusion rate of 24% and a 4.2% need for hemostatic surgery.
In this cohort study, the 30-day mortality rate was 64% and it was attributed to the high percentage of patients with clinical presentations of cardiorespiratory arrest.
Current situation in PCI procedures
Back in March 2015, the US Food and Drug Administration approved the use of the Impella 2.5 device as a LVAD in high-risk PCIs, whether elective or urgent. This followed the results of several studies that back the safety of the device in this context.54
The Europella registry55 included 144 patients who underwent high-risk PCIs under Impella 2.5 support. The primary endpoint was the development of events at the 30-day follow-up: death, major bleeding (requiring transfusion or surgery), AMI, need for urgent revascularization surgery or stroke; it also included safety events associated with the device.
In the American USpella registry56 of 175 patients with similar endpoints (except for bleeding, that was considered a secondary endpoint), the primary endpoint of death occurred in 7 patients (4%), while in the Europella registry it occurred in 8 (5.5%). Mortality results were better than the ones estimated by the STS score—predictor of surgical mortality—suggestive that support with Impella devices in this type of PCIs is a reasonable option. Complications associated with the removal of the device were reported in 8 (5.5%) and 17 (9.7%) patients, respectively. Transfusions were needed in 1 (0.7%) and 3 (1.7%) cases due to major vascular complications.
The Impella CP device also has a retrospective analysis that was consistent with previous outcomes and found better survival rates in patients with cardiogenic shock due to AMI compared to the Impella 2.5 device.57
HeartMate PHP
Same as it happens with the Impella device, the HeartMate PHP uses an axial flow circulatory support system to pump blood out the left ventricle and into the ascending aorta; the main difference here is the existence of a self-expanding cannula that expands itself when it crosses the aortic valve and is implanted via femoral access with a 13-Fr/14-Fr sheath. The cannula is then expanded up to 24-Fr when it reaches the right position through the aortic valve supplying flow of up to 4 L/min.58 However, since February 2017 its use and the clinical trials that were being conducted like the SHIELD II trial (NCT02468778) have been suspended temporarily due to minor errors in its design.
Extracorporeal membrane oxygenation
ECMO can provide cardiopulmonary support similar to the extracorporeal circulation system used during cardiac surgery. Its use is well documented in the pediatric population in the severe heart or respiratory failure setting.59,60
Veno-arterial ECMO includes a circuit with cannulas of venous and arterial blood, a centrifugal pump, and a membrane oxygenator. It can be implanted via peripheral (often femoral) or central access and requires a median sternotomy.
Deoxygenated blood is drained through the venous cannula (20-Fr) from the right atrium towards the membrane oxygenator where gas exchange takes place. Oxygenated blood returns to the patient through the arterial cannula (17-Fr).
Although it is the only device capable of providing full circulatory and respiratory support, it can increase left ventricular afterload and parietal stress (due to several filling pressures), which may have negative consequences for the myocardial oxygen demand.61-64
Current situation in PCI procedures
The use of ECMO in the severe heart or respiratory failure setting has gone up 433% during the 2006-2011 period.65 Still, the experience in its use as a mechanical circulatory support system for high-risk PCIs is limited and only small retrospective studies and series of cases have been published to this day.66-68
Back in 1989, Taub et al.65 documented 7 cases of successful use of ECMO in high-risk angioplasties. The rate of complicated hematomas was high (6 patients of whom 4 required a blood transfusion); we should mention the retroperitoneal hematoma as a complication that caused the patient’s death.
In order to study the use of ECMO in high-risk PCIs, Toma-sello et al.69 published their own experience in a prospective study that included 12 patients with complex of high risk to be surgically revascularized without cardiogenic shock or cardiac arrest with veno-arterial ECMO implantation prior to the PCI. All patients tolerated the procedure and there was only 1 complication in the vascular access (1 hematoma did not require blood transfusion). No deaths or AMIs were reported at the 6-month follow-up, suggestive that ECMO can be a safe alternative in this context.
IABP vs other LVADs in PCI procedures
Several clinical trials have conducted direct comparisons between the IABP and other LVADs. The PROTECT II trial11 compared the Impella 2.5 device to the IABP in high-risk PCIs. This was a multicenter, prospective study of 452 patients eligible for a high-risk PCI (defined as LMCA disease and left ventricular ejection fraction < 35% or multivessel disease with left ventricular ejection fraction < 30%). They were randomized to receive circulatory support with the Impella 2.5 device or IABP during the procedure. Patients with recent AMI were excluded from the study. The 30-day primary endpoint was a composite of major cardiovascular events and mortality. The Impella 2.5 device provided better hemodynamic support compared to the IABP without statistically significant differences in the primary endpoint: 35.1% in the Impella 2.5 group and 40.1% in the IABP group (P = .227).
Patel et al.70 conducted a cross-sectional study during the 2008-2012 period. The study analyzed patients who underwent PCI and received circulatory support with an IABP or other LVADs (Impella, TandemHeart or a combination of IABP plus LVAD) and recorded 18 094 procedures (93% with the IABP, 6% with the Impella o TandemHeart device, and 1% with IABP plus LVAD). In the first place, the patients assisted with a LVAD were older and had more comorbidities (arterial hypertension, diabetes mellitus, renal failure, pulmonary disease) compared to those assisted with the IABP. The overall mortality rate was 19.8% (20.1% with the IABP, 12% with the LVAD, and 41% with the combination of IABP plus LVAD) and the overall rate of complications was 35.5% (36% with the IABP, 26% with the LVAD, and 52% with the combination of IABP plus LVAD). The use of the IABP was associated with a higher rate of cardiovascular (9% vs 4%) and respiratory complications (19 % vs 11%), while the use of other LVADs was associated with a higher rate of vascular complications (8.6% vs 5.5%). A subgroup analysis was conducted based on the presence, or not, of cardiogenic shock or AMI. The main conclusion was that compared to the IABP, the use of the LVAD was a predictor of a lower rate of complications and mortality only in the group of patients without AMI or cardiogenic shock.
Khera et al.71 conducted a study similar to the previous one in the 2004-2012 period but without patients who received support with both devices (combination of IABP plus other LVADs). A total of 26 556 patients underwent high-risk PCIs under IABP (96%) or LVAD (4%) support. Seven per cent of those who received LVAD support had cardiogenic shock and 2.2%, AMI. Also similar to the previous study, the authors found that patients who received LVAD support were older and had more comorbidities, but a lower rate of AMI, cardiogenic shock, and cardiorespiratory arrest compared to the group of patients who received the IABP; no significant differences were seen in the in-hospital mortality rate.
The IMPRESS trial,72 published back in October 2016, randomized 48 patients hospitalized due to ST-segment elevation and secondary acute coronary syndrome and secondary cardiogenic shock to receive support with the Impella CP device or the IABP in high-risk primary PCIs; this was the first study ever conducted with characteristics like these ones. No differences were found in the primary endpoint of death and 30-day cardiovascular events (46% mortality rate in the Impella CP group vs 50% in the IABP group; P = .92) or in the all-cause mortality at 6 months (50% in both groups), but there was a higher rate of vascular complications in the group that received support with the Impella CP device (major bleeding: 33% vs 8%) due to the larger caliber of the cannula used by this device (14-Fr vs 7.5-Fr).
The results published by Koen et al. back in 2019 are interesting too.73 This retrospective, single-center study analyzed the progression and prognosis of patients treated with high-risk PCI during the 2011-2018 period based on whether they received mechanical circulatory support or not. The primary endpoint was a composite of periprocedural mortality (< 24 hours), cardiac arrest, need for vasoactive drugs, need for circulatory support as a bail-out strategy, endotracheal intubation, and peripheral ischemia. One-hundred and ninety-eight patients treated with high-risk PCIs were recruited. Sixty-nine (35%) of these benefited from LVAD support: 18 with the Impella CP device, 25 with the HeartMate PHP device, and 26 with the Pulsecath iVAC 2L device (PulseCath BV, The Netherlands; it is a transfemoral pulsatile ventricular assist device that enables a cardiac output of up to 2 L/min). In this study the rate of the rate for the primary endpoint was 20% in the group of patients without circulatory support compared to 9% in the group that received periprocedural circulatory support.
Amin et al.74 published a retrospective study including 48 306 patients treated with high-risk PCIs circulatory suppport (43 524 with the IABP and 4782 with the Impella device). This study was conducted throughout a 13-year period (2004- 2016) in 432 hospitals from the United States. A pre-Impella era until 2007 was identified (the Impella 2.5 device was approved by the US Food and Drug Administration to be used in high-risk PCIs in 2008). The use of the Impella device grew exponentially until 2016. In the group of patients received support with the Impella device, the authors saw more adverse events in the form of death, bleeding complications, and strokes. Still, these patients were not in a more critical situation compared to the group of patients that received IABP support.
These findings prompted an interesting discussion. Yet despite the sample size, there are different factors that may explain such results, but they seem insufficient to stop recommending the use of this device in patients treated with high-risk PCIs. In the first place, this was a retrospective study with substantial differences in the experience and volume of cases managed in each center. Similarly, the use of the new antiplatelet therapies—that grew significantly from 2009—may partially justify the higher rate of bleeding complications reported.
On the other hand, the authors did not provide a detailed description of the characteristics of the patients’ coronary anatomy (only a higher prevalence of multivessel disease, bifurcation lesions, and chronic occlusions was reported in the Impella group). They did not report either on the rates of PCI success, the patient’s clinical and hemodynamic tolerance to the procedure, the main reason for using a LVAD in this context or the causes for the mortality seen. The authors clarify that patients with the Impella device were not more critical since the rate of cardiogenic shock and need for invasive mechanical ventilation was lower compared to patients with the IABP. However, after a thorough review of the results, it stands out that in the Impella group there was a higher prevalence of previous heart failure, chronic obstructive pulmonary disease and chronic kidney disease, comorbidities that may be behind the results seen. Also, we should mention that the average hospital and ICU stays combined were lower in the group that used the Impella device as the LVAD.
For these reasons, taking the above-mentioned limitations into consideration, and yet despite the study sample size and its surprising results, some associated confounding factors were seen, which is why it may be risky to stop recommending the use Impella devices in the high-risk PCI setting.
Right ventricular failure in patients implanted with a LVAD. Circulatory support devices
Generally speaking, right ventricular (RV) failure occurs in nearly 20% to 50% of the patients after a LVAD implantation procedure.75 However, no uniform requirements to define RV failure are to be found in the medical literature (table 2). Its pathogenesis is multifactorial. Left ventricular unloading by LVADs induces a loss of septal contribution to the right function (septal contraction represents 60% of the power of RV contractility).76
Table 2. Definitive criteria for right ventricular failure after left ventricular assist device implantation
| Postoperative support with inotropes for over 14 days |
| Use of inhaled nitric oxide for over 48 hours |
| Need for inotropic treatment at the hospital discharge |
| Right circulatory support |
| 2 or more of the following hemodynamic parameters: |
| Mean arterial pressure < 55 mmHg |
| Central venous pressure > 16 mmHg |
| Mixed venous saturation < 55% |
| Cardiac index (flow supplied by LVAD) < 20 L/min/m2 |
| Inotropic support score > 20 U |
|
LVAD, left ventricular assist device. |
Due to the significant morbimortality associated, the right selection of patients who are eligible for LVAD implantation is key. These are some predictors of RV failure:77
-
– Right atrial pressure prior to implantation > 20 mmHg.
-
– Transpulmonary gradient prior to implantation > 16 mmHg.
-
– Sudden drop (> 10 mmHg) of the pulmonary arterial pressure after implantation.
-
– Central venous pressure/pulmonary capillary wedge pressure ratio > 0.63.
-
– Tricuspid regurgitation grade > III prior to implantation.
-
– RV short axis/long axis ratio > 0.6.
-
– Need for circulatory support prior to LVAD implantation.
-
– Hypertransaminasemia, hyperbilirubinemia or renal im- pairment.
-
– Need for invasive mechanical ventilation prior to im- plantation.
-
– Right ventricular free wall global longitudinal strain < –9.6%.
The management of RV failure is basically preventive. The proper selection of patients eligible for LVADs is key as well as optimizing their RV preload and afterload situation in order to reduce central venous pressure. As general measures, it is essential to perform anti-infective prophylaxis, avoid cardiac arrhythmias, and schedule protective mechanical ventilation towards the RV (with low positive end-expiratory pressure). Dobutamine, adrenaline, and milrinone are the main inotropic agents used to treat RV failure after LVAD implantation and they can be associated with drugs used to reduce pulmonary arterial pressure.
Circulatory assist devices have a role in the clinical setting too. Veno-arterial ECMO—already described in this manuscript—mimics the RV function. Another member of the Impella family is the Impella RP model that has a single 22-Fr cannula that pumps blood out of the inferior vena cava and into the pulmonary artery and supplies flow at a rate of 4 L/min with promising results in the RECOVER RIGHT trial.78 This study recruited 30 patients with acute RV failure (after LVAD implantation and due to an AMI with right ventricular involvement).
Economic impact of the use of LVADs
The economic impact left by the technical advances made in the percutaneous management of cardiovascular heart disease is growing. An analysis of the costs involved in the healthcare provided in the PROTECT II trial shows that hospitalization related costs were higher in the Impella group compared to the IABP group ($47 667 vs $33 684). A difference that would not only be explained by the cost of the device.79 In contrast, the costs derived from the hospital stay and rehospitalizations were lower in the Impella 2.5 device group ($11 007 vs $21 834).
CONCLUSIONS
The future will shed light on the true role of LVADs in the cath lab. All of these devices are used to improve the cardiac output, mean arterial pressure, coronary perfusion by reducing the pulmonary capillary wedge pressure in patients with a reduced cardiac reserve.
Yet despite the controversial results offered by different studies, registries, and clinical trials, the use of LVADs is on the rise in high-risk PCIs allowing us to preserve hemodynamic stability during the procedure.
FUNDING
The authors did not declare any sources of funding while this study was being conducted.
CONFLICTS OF INTEREST
None declared.
REFERENCES
1. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statis-tics--2015 update:a report from the American Heart Association. Circulation. 2015;131:e29-322.
2. Pulido JN, Park SJ, Rihal CS. Percutaneous left ventricular assist devices:clinical uses, future applications, and anesthetic considerations. J Cardiothorac Vasc Anesth. 2010;24:478-486.
3. O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction:a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362-425.
4. Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients With ST-Elevation Myocardial Infarction:An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction. J Am Coll Cardiol. 2016;67:1235-1250.
5. Epstein AJ, Polsky D, Yang F, Yang L, Groeneveld PW. Coronary revascularization trends in the United States, 2001-2008. JAMA. 2011;305:1769-1776.
6. Rihal CS, Naidu SS, Givertz MM, et al. 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care:Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion;Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d'Intervention. J Am Coll Cardiol. 2015;65:e7-e26.
7. Sakakura K, Ako J, Wada H, Kubo N, Momomura S. ACC/AHA classification of coronary lesions reflects medical resource use in current percutaneous coronary interventions. Catheter Cardiovasc Interv. 2012;80: 370-376.
8. Krone RJ, Shaw RE, Klein LW, et al. Evaluation of the American College of Cardiology/American Heart Association and the Society for Coronary Angiography and Interventions lesion classification system in the current “stent era“of coronary interventions (from the ACC-National Cardiovascular Data Registry). Am J Cardiol. 2003;92:389-394.
9. Hartzler GO, Rutherford BD, McConahay DR, Johnson WL, Giorgi LV. “High-risk“percutaneous transluminal coronary angioplasty. Am J Cardiol. 1988;61:33G-37G.
10. Bass TA. High-Risk Percutaneous Coronary Interventions in Modern Day Clinical Practice:Current Concepts and Challenges. Circ Cardiovasc Interv. 2015;8:e003405.
11. O'Neill WW, Kleiman NS, Moses J, et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention:the PROTECT II study. Circulation. 2012;126:1717-1727.
12. Teirstein PS, Vogel RA, Dorros G, et al. Prophylactic versus standby cardiopulmonary support for high risk percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1993;21:590-596.
13. Carnendran L, Abboud R, Sleeper LA, et al. Trends in cardiogenic shock:report from the SHOCK Study. The SHould we emergently revascularize Occluded Coronaries for cardiogenic shocK?Eur Heart J. 2001;22:472-478.
14. Goldberg RJ, Gore JM, Alpert JS, et al. Cardiogenic shock after acute myocardial infarction. Incidence and mortality from a community-wide perspective, 1975 to 1988. N Engl J Med. 1991;325:1117-1122.
15. Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999;341:625-634.
16. Yip HK, Wu CJ, Chang HW, et al. Comparison of impact of primary percutaneous transluminal coronary angioplasty and primary stenting on short-term mortality in patients with cardiogenic shock and evaluation of prognostic determinants. Am J Cardiol. 2001;87:1184-1188.
17. Stretch R, Sauer CM, Yuh DD, Bonde P. National trends in the utilization of short-term mechanical circulatory support:incidence, outcomes, and cost analysis. J Am Coll Cardiol. 2014;64:1407-1415.
18. Kern MJ, Aguirre F, Bach R, Donohue T, Siegel R, Segal J. Augmentation of coronary blood flow by intra-aortic balloon pumping in patients after coronary angioplasty. Circulation. 1993;87:500-511.
19. Fuchs RM, Brin KP, Brinker JA, Guzman PA, Heuser RR, Yin FC. Augmentation of regional coronary blood flow by intra-aortic balloon counterpulsation in patients with unstable angina. Circulation. 1983;68:117-123.
20. Bregman D, Kripke DC, Goetz RH. The effect of synchronous unidirectional intra-aortic balloon pumping on hemodynamics and coronary blood flow in cardiogenic shock. Trans Am Soc Artif Intern Organs. 1970;16:439-446.
21. Scheidt S, Wilner G, Mueller H, et al. Intra-aortic balloon counterpulsation in cardiogenic shock. Report of a co-operative clinical trial. N Engl J Med. 1973;288:979-984.
22. Williams DO, Korr KS, Gewirtz H, Most AS. The effect of intraaortic balloon counterpulsation on regional myocardial blood flow and oxygen consumption in the presence of coronary artery stenosis in patients with unstable angina. Circulation. 1982;66:593-597.
23. Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization:The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541-2619.
24. Thiele H, Schuler G, Neumann FJ, et al. Intraaortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock:design and rationale of the Intraaortic Balloon Pump in Cardiogenic Shock II (IABP-SHOCK II) trial. Am Heart J. 2012;163:938-945.
25. Prondzinsky R, Lemm H, Swyter M, et al. Intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock:the prospective, randomized IABP SHOCK Trial for attenuation of multiorgan dysfunction syndrome. Crit Care Med. 2010;38:152-160.
26. Thiele H, Zeymer U, Neumann FJ, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II):final 12 month results of a randomised, open-label trial. Lancet. 2013;382:1638-1645.
27. Zangrillo A, Pappalardo F, Dossi R, et al. Preoperative intra-aortic balloon pump to reduce mortality in coronary artery bypass graft:a meta-analysis of randomized controlled trials. Crit Care. 2015;19:10.
28. Ranucci M, Castelvecchio S, Biondi A, et al.;Surgical and Clinical Outcome Research (SCORE) Group. A randomized controlled trial of preoperative intra-aortic balloon pump in coronary patients with poor left ventricular function undergoing coronary artery bypass surgery. Crit Care Med. 2013;41:2476-2483.
29. Christenson JT, Badel P, Simonet F, Schmuziger M. Preoperative intraaortic balloon pump enhances cardiac performance and improves the outcome of redo CABG. Ann Thorac Surg. 1997;64:1237-1244.
30. Christenson JT, Simonet F, Schmuziger M. The effect of preoperative intra-aortic balloon pump support in high risk patients requiring myocardial revascularization. J Cardiovasc Surg (Torino). 1997;38:397-402.
31. Lomivorotov VV, Cherniavskiy AM, Boboshko VA, Kornilov IA, Lomivorotov VN, Karaskov AM. Levosimendan vs. intra-aortic balloon pump in high-risk cardiac surgery. Asian Cardiovasc Thorac Ann. 2011;19:154-159.
32. Shi M, Huang J, Pang L, Wang Y. Preoperative insertion of an intra-aortic balloon pump improved the prognosis of high-risk patients undergoing off-pump coronary artery bypass grafting. J Int Med Res. 2011;39:1163-1168.
33. Ahmad Y, Sen S, Shun-Shin MJ, et al. Intra-aortic Balloon Pump Therapy for Acute Myocardial Infarction:A Meta-analysis. JAMA Intern Med. 2015;175:931-939.
34. Kantrowitz A, Tjonneland S, Freed PS, Phillips SJ, Butner AN, Sherman JL, Jr. Initial clinical experience with intraaortic balloon pumping in cardiogenic shock. JAMA. 1968;203:113-118.
35. Szatmary LJ, Marco J. Haemodynamic and antiischaemic protective effects of intra-aortic balloon counterpulsation in high risk coronary heart patients undergoing percutaneous transluminal coronary angioplasty. Cor Vasa. 1987;29:183-191.
36. Brodie BR, Stuckey TD, Hansen C, Muncy D. Intra-aortic balloon counterpulsation before primary percutaneous transluminal coronary angioplasty reduces catheterization laboratory events in high-risk patients with acute myocardial infarction. Am J Cardiol. 1999;84:18-23.
37. Briguori C, Sarais C, Pagnotta P, et al. Elective versus provisional intra-aortic balloon pumping in high-risk percutaneous transluminal coronary angioplasty. Am Heart J. 2003;145:700-707.
38. Mishra S, Chu WW, Torguson R, et al. Role of prophylactic intra-aortic balloon pump in high-risk patients undergoing percutaneous coronary intervention. Am J Cardiol. 2006;98:608-612.
39. Perera D, Stables R, Thomas M, et al. Elective intra-aortic balloon counterpulsation during high-risk percutaneous coronary intervention:a randomized controlled trial. JAMA. 2010;304:867-74.
40. Sandhu A, McCoy LA, Negi SI, et al. Use of mechanical circulatory support in patients undergoing percutaneous coronary intervention:insights from the National Cardiovascular Data Registry. Circulation. 2015;132:1243-1251.
41. Khera R, Cram P, Lu X, et al. Trends in the use of percutaneous ventricular assist devices:analysis of national inpatient sample data, 2007 through 2012. JAMA Intern Med. 2015;175:941-950.
42. Thiele H, Sick P, Boudriot E, et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005;26:1276-1283.
43. Thiele H, Lauer B, Hambrecht R, Boudriot E, Cohen HA, Schuler G. Reversal of cardiogenic shock by percutaneous left atrial-to-femoral arterial bypass assistance. Circulation. 2001;104:2917-2922.
44. Burkhoff D, Cohen H, Brunckhorst C, O'Neill WW. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J. 2006;152:469.e1-8.
45. Vranckx P, Foley DP, de Feijter PJ, Vos J, Smits P, Serruys PW. Clinical introduction of the Tandemheart, a percutaneous left ventricular assist device, for circulatory support during high-risk percutaneous coronary intervention. Int J Cardiovasc Intervent. 2003;5:35-39.
46. Vranckx P, Schultz CJ, Valgimigli M, et al. Assisted circulation using the TandemHeart during very high-risk PCI of the unprotected left main coronary artery in patients declined for CABG. Catheter Cardiovasc Interv. 2009;74:302-310.
47. Valgimigli M, van Mieghem CA, Ong AT, et al. Short- and long-term clinical outcome after drug-eluting stent implantation for the percutaneous treatment of left main coronary artery disease:insights from the Rapamycin-Eluting and Taxus Stent Evaluated At Rotterdam Cardiology Hospital registries (RESEARCH and T-SEARCH). Circulation. 2005;111:1383-1389.
48. Kar B, Butkevich A, Civitello AB, et al. Hemodynamic support with a percutaneous left ventricular assist device during stenting of an unprotected left main coronary artery. Tex Heart Inst J. 2004;31:84-86.
49. Aragon J, Lee MS, Kar S, Makkar RR. Percutaneous left ventricular assist device:“TandemHeart“for high-risk coronary intervention. Catheter Cardiovasc Interv. 2005;65:346-352.
50. Alli OO, Singh IM, Holmes DR, Jr., Pulido JN, Park SJ, Rihal CS. Percutaneous left ventricular assist device with TandemHeart for high-risk percutaneous coronary intervention:the Mayo Clinic experience. Catheter Cardiovasc Interv. 2012;80:728-734.
51. Nascimbene A, Loyalka P, Gregoric ID, Kar B. Percutaneous coronary intervention with the TandemHeart percutaneous left ventricular assist device support:Six years of experience and outcomes. Catheter Cardiovasc Interv. 2016;87:1101-1110.
52. Seyfarth M, Sibbing D, Bauer I, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008;52:1584-1588.
53. Lauten A, Engstrom AE, Jung C, et al. Percutaneous left-ventricular support with the Impella-2.5-assist device in acute cardiogenic shock:results of the Impella-EUROSHOCK-registry. Circ Heart Fail. 2013;6:23-30.
54. Henriques JP, Remmelink M, Baan J, Jr., et al. Safety and feasibility of elective high-risk percutaneous coronary intervention procedures with left ventricular support of the Impella Recover LP 2.5. Am J Cardiol. 2006;97:990-992.
55. Sjauw KD, Konorza T, Erbel R, et al. Supported high-risk percutaneous coronary intervention with the Impella 2.5 device the Europella registry. J Am Coll Cardiol. 2009;54:2430-2434.
56. Maini B, Naidu SS, Mulukutla S, et al. Real-world use of the Impella 2.5 circulatory support system in complex high-risk percutaneous coronary intervention:the USpella Registry. Catheter Cardiovasc Interv. 2012;80:717-725.
57. Algin A, Tonino CM. TCT-187. 30-day survival in patients with cardiogenic shock:Impella 2.5 versus Impella 4.0. J Am Coll Cardiol. 2015;66:B70.
58. Dudek D. Temporary Cardiac Support During High-Risk PCI:HeartMate PHP and the SHIELD I Study. En:27th Annual Transcatheter Cardiovascular Therapeutics;2015 Oct 14;San Francisco, United States. Disponible en:https://www.tctmd.com/slide/temporary-cardiac-support-during-high-risk-pci-heartmate-php-and-shield-i-study. Consultado 20 Abr 2020.
59. Bartlett RH, Gazzaniga AB, Jefferies MR, Huxtable RF, Haiduc NJ, Fong SW. Extracorporeal membrane oxygenation (ECMO) cardiopulmonary support in infancy. Trans Am Soc Artif Intern Organs. 1976;22:80-93.
60. Bartlett RH, Andrews AF, Toomasian JM, Haiduc NJ, Gazzaniga AB. Extracorporeal membrane oxygenation for newborn respiratory failure:forty-five cases. Surgery. 1982;92:425-433.
61. Anderson H, 3rd, Steimle C, Shapiro M, et al. Extracorporeal life support for adult cardiorespiratory failure. Surgery. 1993;114:161-172.
62. Kawashima D, Gojo S, Nishimura T, et al. Left ventricular mechanical support with Impella provides more ventricular unloading in heart failure than extracorporeal membrane oxygenation. ASAIO J. 2011;57:169-176.
63. Koeckert MS, Jorde UP, Naka Y, Moses JW, Takayama H. Impella LP 2.5 for left ventricular unloading during venoarterial extracorporeal membrane oxygenation support. J Card Surg. 2011;26:666-668.
64. Sauer CM, Yuh DD, Bonde P. Extracorporeal membrane oxygenation use has increased by 433% in adults in the United States from 2006 to 2011. ASAIO J. 2015;61:31-36.
65. Taub JO, L'Hommedieu BD, Raithel SC, et al. Extracorporeal membrane oxygenation for percutaneous coronary angioplasty in high risk patients. ASAIO Trans. 1989;35:664-666.
66. Ricciardi MJ, Moscucci M, Knight BP, Zivin A, Bartlett RH, Bates ER. Emergency extracorporeal membrane oxygenation (ECMO)-supported percutaneous coronary interventions in the fibrillating heart. Catheter Cardiovasc Interv. 1999;48:402-405.
67. Shammas NW, Roberts S, Early G. Extracorporeal membrane oxygenation for unprotected left main stenting in a patient with totally occluded right coronary artery and severe left ventricular dysfunction. J Invasive Cardiol. 2002;14:756-759.
68. Sheu JJ, Tsai TH, Lee FY, et al. Early extracorporeal membrane oxygenator-assisted primary percutaneous coronary intervention improved 30-day clinical outcomes in patients with ST-segment elevation myocardial infarction complicated with profound cardiogenic shock. Crit Care Med. 2010;38:1810-1817.
69. Tomasello SD, Boukhris M, Ganyukov V, et al. Outcome of extracorporeal membrane oxygenation support for complex high-risk elective percutaneous coronary interventions:A single-center experience. Heart Lung. 2015;44:309-313.
70. Myat A, Patel N, Tehrani S, Banning AP, Redwood SR, Bhatt DL. Percutaneous circulatory assist devices for high-risk coronary intervention. JACC Cardiovasc Interv. 2015;8:229-244.
71. Khera R, Cram P, Vaughan-Sarrazin M, Horwitz PA, Girotra S. Use of Mechanical Circulatory Support in Percutaneous Coronary Intervention in the United States. Am J Cardiol. 2016;117:10-16.
72. Ouweneel DM, Eriksen E, Sjauw KD, et al. Impella CP versus Intra-aortic balloon pump in acute myocardial infarction complicated by cardiogenic shock. The IMPRESS trial. J Am Coll Cardiol. 2017;69:278-287.
73. Ameloot K, Bastos MB, Daemen J, et al. New-generation mechanical circulatory support during high-risk PCI:a cross-sectional analysis. EuroIntervention. 2019;15:427-433.
74. Amin AP, Spertus JA, Curtis JP, et al. The Evolving Landscape of Impella Use in the United States Among Patients Undergoing Percutaneous Coronary Intervention with Mechanical Circulatory Support. Circulation. 2020;141:273-284.
75. Gregory D, Scotti DJ, de Lissovoy G, et al. A value-based analysis of hemodynamic support strategies for high-risk heart failure patients undergoing a percutaneous coronary intervention. Am Health Drug Benefits. 2013;6:88-99.
76. Kaul TK, Fields BL. Postoperative acute refractory right ventricular failure:Incidence, pathogenesis, management and prognosis. Cardiovasc Surg. 2000;8:1-9.
77. Cordtz J, Nilsson JC, Hansen PB, et al. Right ventricular failure after implantation of a continuous-flow left ventricular assist device:Early haemodynamic predictors. Eur J Cardiothorac Surg. 2014;45:847-853.
78. Anderson MB, Goldstein J, Milano C, et al. Benefits of a novel percutaneous ventricular assist device for right heart failure:The prospective RECOVER RIGHT study of the Impella RP device. J Heart Lung Transplant. 2015;34:1549-1560.
79. Roos JB, Doshi SN, Konorza T, et al. The cost-effectiveness of a new percutaneous ventricular assist device for high-risk PCI patients:mid-stage evaluation from the European perspective. J Med Econ. 2013;16:381-390.
Corresponding author: Servicio de Cardiología, Hospital Clínico San Carlos, Profesor Martín Lagos s/n, 28040 Madrid, Spain.
E-mail address: jc.gomezpolo@gmail.com (J.C. Gómez Polo).