ABSTRACT
Severe coronary calcium increases the complexity of percutaneous coronary interventions. It may affect the adequate preparation of the lesion, proper stent expansion and apposition and increase the risk of stent thrombosis and restenosis. The techniques available for the management of severe calcified lesions can be divided into 2 groups: non-balloon and balloon-based technologies. Rotational atherectomy has been the predominant technique to treat severe calcified lesions. As a matter of fact, there are new devices available that facilitate the modification of the plaque such as the new lithoplasty balloon that involves the use of high-energy mechanical pulses to crack coronary calcium. Coronary lithoplasty is an easy technique with a short learning curve that seems to be more effective on deep calcium by increasing luminal compliance. This may revolutionize the standard approach for the management of severe calcified coronary lesions. Also, the role of intravascular imaging is essential to select the most appropriate plaque-modification device and assess the optimal stent result. This review provides an overview of the techniques available and evidence on the currently approved devices to treat calcified lesions.
Keywords: Rotational atherectomy. Orbital atherectomy. Excimer laser. Coronary lithoplasty.
RESUMEN
El calcio coronario aumenta la complejidad del intervencionismo coronario percutáneo. La calcificación grave dificulta la preparación de la lesión, impide la adecuada expansión y la aposición del stent, y aumenta el riesgo de trombosis y de reestenosis. Las técnicas de modificación de placa se pueden dividir en 2 tipos según el tipo de dispositivo: sin balón y con balón. La aterectomía rotacional ha sido la técnica por excelencia para el tratamiento de lesiones gravemente calcificadas. Actualmente existen nuevos dispositivos que facilitan la preparación de la lesión, como el novedoso balón de litoplastia, que utiliza pulsos de alta energía mecánica para fragmentar el calcio coronario. La litoplastia coronaria es una técnica sencilla, con una curva de aprendizaje corta, que parece tener efecto sobre el calcio profundo y aumentar la distensibilidad luminal, lo que podría suponer un gran cambio en el enfoque del tratamiento de las lesiones calcificadas. Cabe destacar la relevancia de la imagen intravascular al seleccionar el dispositivo de modificación de placa más adecuado, así como para evaluar el resultado final del stent. Esta revisión proporciona una visión general sobre las técnicas disponibles y la evidencia de los dispositivos aprobados para el tratamiento de las lesiones calcificadas.
Palabras clave: Aterectomía rotacional. Aterectomía orbital. Láser de excímeros. Litoplastia coronaria.
Abbreviations CL: coronary lithoplasty. ELCA: excimer laser coronary atherectomy. IVUS: intravascular ultrasound. OA: orbital atherectomy. OCT: optical coherence tomography. PCI: percutaneous coronary intervention. RA: rotational atherectomy. SCCL: severely calcified coronary lesion.
INTRODUCTION
Severely calcified coronary lesions (SCCL) pose a tremendous challenge to perform successful percutaneous coronary interventions (PCI).1 Old age, diabetes mellitus, chronic kidney disease, and smoking are associated with increased coronary calcification.2 Coronary calcium can be underestimated on the fluoroscopy and coronary angiography, and it is necessary to use intravascular imaging modalities such as the intravascular ultrasound (IVUS) and optical coherence tomography (OCT) for an accurate assessment of the severity and characterization of the plaque.3
Severe coronary calcification increases the complexity of the PCI.4 It can affect the crossing of the lesion, the proper stent expansion and apposition, damage the drug-eluting polymer, increase the risk of stent thrombosis and restenosis, and have a negative impact on short and long-term results.5 The optimal approach for the management of SCCL requires being knowledgable of a number of factors: the characteristics of the lesion, calcium distribution, intravascular imaging modalities, and the mechanism of action of every plaque-modification device.6
To this day, plaque-modification techniques can be divided into 2 groups based on the type of device used: with and without balloon.6,7 Among the procedures with devices based on technologies without balloon we should mention rotational atherectomy ([RA], Rotablator and ROTAPro; Boston Scientific, United States), orbital atherectomy ([OA], Diamondback 360; Cardiovascular Systems, Inc., United States), and RA with excimer laser (CVX-300 Excimer Laser System, Philips, United States).8,9 Among the procedures with devices based on technologies with balloon we find the cutting balloon (WOLVERINE, Boston Scientific, United States) and the scoring balloon. The most important ones are AngioSculpt (Biotronik, Germany), Scoreflex (OrbusNeich, China), and NSE Alpha (B. Braun, Germany); the ultra-high pressure non-compliant (NC) balloon, OPN (SIS Medical AG, Switzerland); and the coronary lithoplasty device ([CL], Shockwave Medical, Inc., United States).8,9
The widespread use of these techniques and devices has been limited due to the risk of complications, the degree of technical difficulty, the operator’s experience, and the corresponding use of health resources. This review focuses on the techniques and evidence available today for the devices approved for the management of SCCLs.
ROTATIONAL ATHERECTOMY
Definition
RA is an endovascular procedure to modify atherosclerotic plaque by advancing a diamond-coated rotating metal olive-shaped burr.10,11
Operating principles
The RA device (Boston Scientific, United States) consists of an elliptical diamond crystal-coated olive-shaped burr rotating at high speed and performing differential cutting as it moves forward (figure 1 of the supplementary data). The RA pulverizes the plaque fibrocalcific components while preserving the adjacent elastic tissue by releasing microparticles into distal coronary circulation.7,10
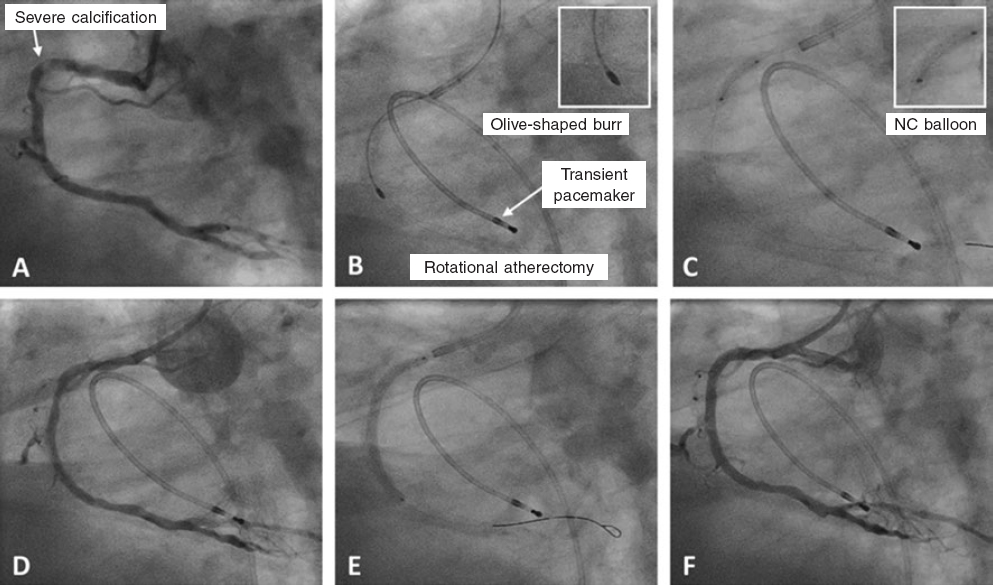
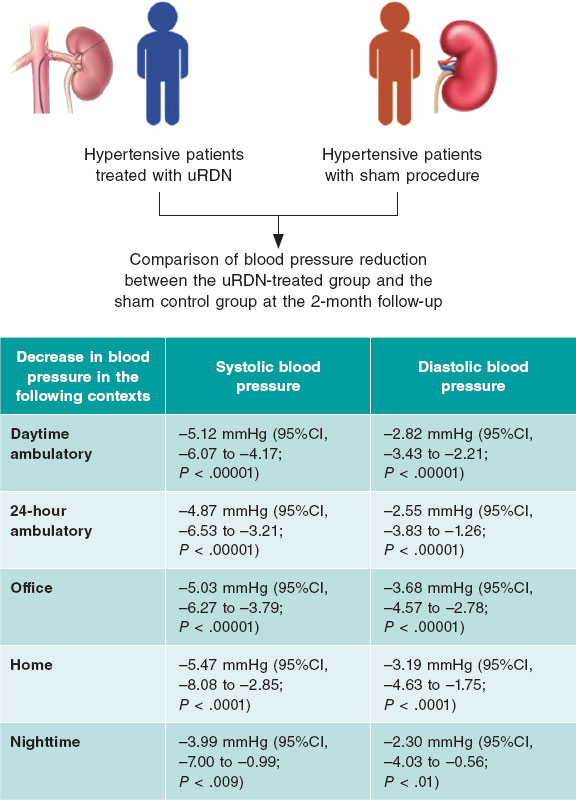
Figure 1. Case of rotational atherectomy on calcified lesion in right coronary artery. A: baseline angiography. B: rotablation of calcified lesion using a 1.5 mm olive-shaped burr. C: predilation with a 3 × 12 mm non-compliant balloon. D: angiographic result after rotablation. E: implantation of a 3 × 38 mm drug-eluting stent. F: final angiographic result after postdilation. NC, non-compliant balloon.
The burr has different sizes (from 1.25 mm to 2.5 mm) and is mounted on a drive shaft connected to a console that supplies rotational energy. It has 3 connections (inside a single cable with 3 outputs connected to the console): a tachometer cable, a power connector, and a compressed air/nitrogen connection. From the console, the compressed air/nitrogen provides pressure for the rotation of the engine at selected revolutions. Similarly, there is a physiological saline solution connection to which heparin and vasodilators can be added to lubricate the sheath and cool the engine down (figure 1 of the supplementary data). A 0.5:0.6 ratio between the burr and the vessel is advisable. The olive-shaped burr is advanced on a 0.009 in specific guidewire (RotaWire, Boston Scientific, United States).10,11 It should be mentioned that the RotaWire guidewire has different length diameters: the cable measures 0.009 inches and the radiopaque segment, 0.014 inches. The burr is compatible with the 0.009 in guidewire proximal segment. There are 2 different versions of RotaWire available (RotaWire Extra Support and RotaWire Floppy) used depending on the characteristics of the plaque and the support required.10,11 (figure 1 of the supplementary data).
The rotational speed recommended is between 135 000 rpm and 180 000 rpm. Decelerations > 5000 rpm should be avoided. The burr should be advanced gradually with easy back-and-forth moves and rotablation time should be < 20 seconds with pauses in between each cycle. Once rotablation has been performed, the olive-shaped burr is removed and the Dynaglide mode is activated. Unless there is a technical issue, deceleration is usually indicative of a significant resistance to the advancement of the burr due to lesion severity and calcification and makes a distinctive sound. It is advisable to carefully listen while the RA is being performed because deceleration can be indicative of the risk of burr entrapment.7,10,11 (table 1).
Table 1 General characteristics of plaque-modification devices based on technologies without balloon
| Rotational atherectomy | Orbital atherectomy | Excimer laser coronary atherectomy | |
|---|---|---|---|
| Operating principles | |||
| Type of device | High-speed rotating olive-shaped burr | Crown at high-speed elliptical rotation | High energy light catheter |
| Mechanism of action | Antegrade differential cutting | Antegrade and retrograde differential sanding | Photoablation |
| Learning curve | Long | Long | Long |
| Device size | 1.25-2.5 mm olive-shaped burr | 1.25 mm crown | 0.9-2 mm catheter |
| Compatible catheter | 6-8-Fr | 6-Fr | 5-8-Fr |
| Type of guidewire | 0.009/0.0014 in RotaWire | 0.012/0.014 in ViperWire | 0.014 in guidewire |
| Console | Small without pedal (ROTAPro) | Small without pedal | Large with pedal |
| Indications | |||
| Main indication | Plaque-modification | Plaque-modification | Lesions hard to cross, like chronic total coronary occlusion |
| Optimal calcium location | Luminal | Luminal | Luminal |
| Stent restenosis | Yes | Yes | Yes |
| Complications | |||
| Dissection | Moderate risk | Moderate risk | Moderate risk |
| Perforation | Moderate risk | Moderate risk | Moderate risk |
| Slow-flow/no-reflow | Moderate risk | Moderate risk | Moderate risk |
| Burr/crown entrapment | Moderate risk | Low risk | N/A |
| Practical advices | |||
| Speed | 135 000-180 000 | 80 000-120 000 | N/A |
| Device-vessel ratio | 0.5:0.6 | N/A | 0.5:0.6 |
| Recommendations | Pecking motion Short cycles Pauses in between cycles Avoid significant tortuosity | Continuous and slow back-and-forth moves Short cycles Pauses in between cycles | Requires the continuous infusion of fluid |
|
N/A, non-applicable. |
|||
There is an update of this device, the ROTAPro, that facilitates manipulation by a single operator and provides an improved user interface with integrated controls in the advancement device. The pedal has been replaced by a button located on top of the burr advancement control. There is another button at the back of the device to activate the Dynaglide mode. The console is smaller, has a digital screen and requires less configuration time (figure 2 of the supplementary data).
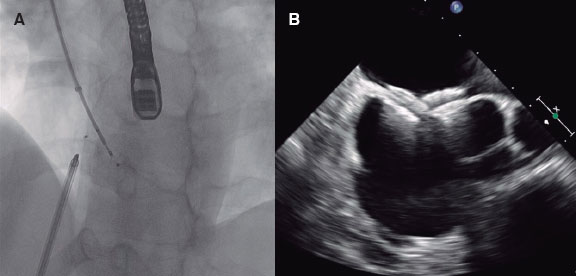
Figure 2 Case of coronary lithoplasty in a patient with inferior ST-segment elevation acute coronary syndrome. A: baseline angiography. B: result after predilation with a 2 × 15 mm semi-compliant balloon. C: failed predilation with a 2.5 × 8 mm non-compliant balloon and a 2.5 × 6 mm cutting balloon. D: coronary lithotripsy with a 3 × 12 mm lithoplasty balloon. E: angiographic result after coronary lithoplasty. F: final result after the implantation of 2 overlapping drug-eluting stents (2.75 × 33 mm and 3 × 38 mm) and postdilation with a 3 × 12 mm non-compliant balloon.
Indications
The main indication for RA is for the treatment of SCCLs that are non-dilatable through conventional methods by modifying the plaque, which facilitates the proper stent expansion and apposition.10,11 (figure 1).
The following factors significantly impact the outcomes of RA: calcium eccentricity, luminal area, and burr size. The optimal scenario to achieve proper luminal gain is a concentric lesion with a circumferential distribution of calcium and a minimal lumen area smaller than the burr size.7 Another more controversial indication is for the management of stent restenosis due to stent underexpansion. Eccentric lesions with significant tortuosity are less eligible for RA treatment since there is a higher risk of complications.9
The RA can be used as the primary strategy for the modification of calcified lesions or as a bailout strategy after a failed balloon predilation attempt of the lesion.9 It is a safe technique in both cases. However, the primary strategy is associated with shorter procedural and fluoroscopy times, fewer contrast volume, and less predilation balloons being used.10 (table 1).
Clinical data
Currently the systematic use of RA is controversial because its clinical benefit has not been clearly demonstrated yet.12-15 The ROTAXUS clinical trial16 included 240 patients with moderate-severe calcification who were randomized to RA plus drug-eluting stent or balloon predilation plus drug-eluting stent. The RA had a higher success rate and initial luminal gain (1.56 ± 0.43 vs 1.44 ± 0.49 mm; P = .01), and there was a greater late stent luminal loss at the 9-month follow-up (0.44 ± 0.58 vs 0.31 ± 0.52 mm; P = .04). No significant differences were found regarding the rate of stent restenosis or thrombosis, need for new target lesion revascularization or rate of major adverse cardiovascular events (MACE) at the 9-month follow-up.16
The PREPARE-CALC clinical trial17 included 200 patients with SCCLs randomized on a 1:1 ratio to receive treatment with cutting/scoring balloon or RA. No significant differences were seen regarding complications between the 2 groups. However, there was a higher procedural success rate with the RA and a lower percentage of residual stenosis (98% vs 81%; P = .0001). No significant differences were seen between the groups regarding the stent luminal loss or clinical outcomes at the 9-month follow-up.17
The ROTATE multicenter registry18 included 1176 patients with SCCLs treated with RA plus drug-eluting stent. The rate of MACE at 1-year follow-up was 16%. The European multicenter RA registry included data from 963 patients. Clinical success rate was 92%, mortality rate was 12.5% and the rate of MACE at 1-year follow-up was 17% (results presented at the EuroPCR 2019 congress).19
Complications
The most dreaded complications of RA are burr entrapment, perforation, and coronary dissection (table 2).10,20
Table 2. Complications of rotational atherectomy, strategy of prevention, and treatment
| Strategy of prevention | Treatment | |
|---|---|---|
| Slow-flow/no-reflow | Use smaller burrs Avoid high rotation speeds Do short cycles with pauses in between | Intracoronary administration of nitrates, nitroprusside, adenosine Keep the right perfusion in the presence of hypotension |
| Dissection | Regarding lesions at segments with significant toruosity | In the presence of significant dissection, it is advisable to stop performing the atherectomy The standard management of dissection is advised |
| Perforation | Regarding the selection of large burrs, significant vessel tortuosity, and the selection of inadequate rotation speeds | Standard treatment advised (including the use of drug-eluting stents and urgent pericardiocentesis) |
| Burr entrapment | Rare complication; it can usually be avoided with an adequate selection of cases and performing the technique the right way | Perform controlled back-and-forth moves Position a second guidewire to advance a balloon to release the burr Increase support through active intubation or the use of a catheter extension to increase traction Cardiac surgery may be necessary |
Two different types of burr entrapment can be distinguished: a) lesion entrapment (the burr cannot be moved forward or backwards) and b) distal entrapment (the burr cannot be removed but it can be moved forward). Several factors like significant lesions and very small burrs can predispose to this complication. In cases of burr entrapment, it is not advisable to activate rotablation or the Dynaglide mode.10,11 To solve this complication, these maneuvers can be performed: a) controlled push and traction with catheter active intubation; b) cut the device catheter and advance the guide catheter extension as much as possible to pull with maximum strength; or c) place a second guide catheter through which a second guidewire and a balloon are advanced to release the burr.11 This is a very serious complication that sometimes requires emergent surgery.21
Significant tortuosity and the lack of proper guide catheter coaxiality in the management of ostial lesion can lead to coronary dissections and increase the risk of perforation.10,11
The slow-flow/no-reflow phenomenon is a relatively common complication, although its incidence has dropped to 2.6% after improving the technique and with the operator’s growing experience.7,21 This phenomenon is more likely to happen in long and significant lesions where multiple ablations are performed and in the presence of a poor distal vessel. It is due to the embolization of residues towards microvasculature.11 It can be prevented through short rotablation cycles by using small burrs at first, pausing between cycles, and controlling the flow angiographically. Once diagnosis has been established, it is treated with fluid therapy, local vasodilators at distal levels, vasoactive amines in the presence of hypotension, and atropine in the presence of bradycardia.10,11
ORBITAL ATHERECTOMY
Definition
OA is an endovascular procedure to modify atherosclerotic plaque by using a diamond-coated crown whose mechanism of action consists of the antegrade and retrograde modification of the plaque.7
Operating principles
The standard OA device is the Diamondback 360 (Cardiovascular Systems, Inc., United States). It consists of a one size only diamond-coated crown (1.25 mm) connected to a drive shaft and controller and powered by a pneumatic console (figure 3 of the supplementary data). The crown is advanced on a specific 0.012/0.014 in guidewire (ViperWire; Cardiovascular Systems, Inc., St. Paul, MN, United States). The centrifugal force generated during rotation compresses the crown against the plaque eventually cracking it and increasing distensibility.22,23
The OA mechanism of action is the elliptical rotation of the crown that gradually increases orbital diameter as rotation speed increases from 80 000 rpm to 120 000 rpm.22 Increasing the orbit with higher rotation speeds allows the differential sanding of calcified lesions in vessels of up to 3.5 mm using the 1.25 mm crown.7 For optimal results, the crown needs to be moved slowly and gradually through the lesion at a speed of 1-3 mm/s, which facilitates greater luminal gain and a lower rate of complications compared to higher moving speeds.7,22
The OA effect is time-dependent; 30 second-cycles are advisable with 30 second-pauses in between them.22 The continuous infusion of a lubricant solution (ViperSlide) is required to minimize thermal lesions during OA; also, 18 mL/min of fluid are administered to cool the device down and eliminate residue, thus reducing ischemia and distal embolization.22,24
The Micro Crown system (Cardiovascular Systems, Inc., United States) is available for use. It is a technological advancement to improve the effectiveness of OA. It consists of a newly designed drive shaft to facilitate easier advances of the crown towards the lesion. It facilitates plaque modification at slower speeds (50 000 rpm-70 000 rpm)9,22 (table 1).
Indications
The main indication of OA is for the management of calcified lesions non-dilatable using conventional methods to modify the plaque, increase vessel distensibility, and facilitate the proper stent expansion.22,23 With the new OA Micro Crown system ostial and subocclusive lesions can be treated.9 (table 1).
Clinical data
The ORBIT I clinical trial25 included 50 patients and confirmed the safety and efficacy of OA for the management of calcified lesions. Procedural success was achieved in 94% of the patients and the rate of MACE was 8% at 6-month follow-up.
The ORBIT II clinical trial26 included 443 patients. Procedural success was achieved in 98.6% of the patients, the rate of significant dissections was 2.3%, and the rate of MACE was 10.4% at the 30-day follow-up. The 3-year follow-up results showed a rate of MACE of 23.5%.27
The COAST clinical trial28 that used the new Micro Crown system included 100 patients. Procedural success was achieved in 85% of the patients, and the rate of MACE was 22.2% at the 1-year follow-up. The ECLIPSE trial (NCT03108456) is being conducted now and will include 2000 patients with SCCLs randomized to OA plus drug-eluting stent or balloon predilation plus drug-eluting stent.
Complications
Complications are similar to those reported for RA. However, compared to RA, since the OA performs antegrade and retrograde sanding, it reduces the chances of crown entrapment in the lesion. The residues produced are smaller and they don’t alter coronary flow during its application, thus reducing the risk of slow-flow/no-reflow phenomenon and thermal lesion of coronary endothelium.7,24 Coronary perforation is one of the most serious complications of OA (between 0.7% and 2%).26-28 OA is not advisavke when coronary anatomy shows significant tortuosity (> 90° angulations).
EXCIMER LASER CORONARY ATHERECTOMY
Definition
Excimer laser coronary atherectomy (ELCA) is an endovascular procedure for the management of significant and calcified lesions non-dilatable with the usual techniques. It uses a photochemical, photothermal, and photomechanical mechanism of action derived from applying high-energy light.29,30
Operating principles
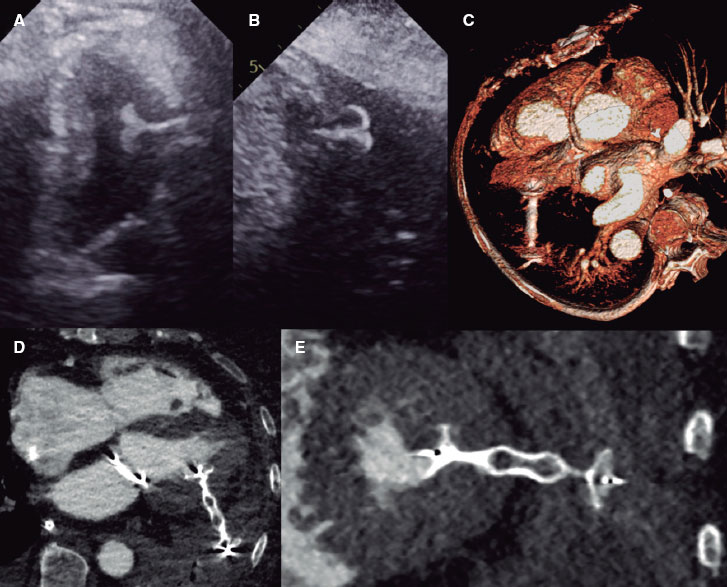
The Philips CVX-300 ELCA system uses xenon chloride and emits pulses of ultraviolet (UV) light at a 308-nm wavelength. The UV pulses generated only penetrate 50 µm deep, which disintegrates the calcified plaque through a mechanism of ablation without damage to the middle or adventitia layers (figure 4 of the supplementary data).31 There are 4 different sizes of ELCA monorail catheter available (0.9, 0.14, 1.7 and 2.0 mm) that can be advanced on a 0.014 in guidewire. The right size is selected on a 0.5:0.67 ratio between catheter and vessel.
Photomechanical effect occurs when the laser acts on a liquid environment (saline solution, contrast, or blood) with the corresponding release of expansion bubbles that act on the atherosclerotic plaque.32 Slowly moving the device forward promotes an increased luminal gain at lesion level. The number of pulses, length and total time of ELCA treatment should be individualized depending on the characteristics of the lesion. The particles generated have a diameter < 10 µm so they are reabsorbed by the reticuloendothelial system, thus avoiding microvascular obstruction31 (table 1).
Indications
The clinical use of ELCA es limited. Its main indication is for the management of lesions that are non-dilatable through conventional methods. It is rarely used as a first-line strategy for the management of SCCLs, but it is the only option when the lesion cannot be crossed with a microcatheter or with the RotaWire/ViperWire guidewires, as it occurs with chronic occlusions.33,34
Other more controversial indications are for the management of non-dilatable stent restenosis using the routine methods due to stent underexpansion,35 ostial lesions, saphenous vein graft occlusions,36 and lesions with thrombotic content.37-39 This technique should be avoided in the presence of unprotected left main coronary artery disease, significant tortuosity, and in bifurcated lesions (table 1).
Clinical data
The data available on the medical literature come from randomized studies (balloon predilation vs ELCA) are old and did not show any significant differences regarding results.29,30,40
In acute myocardial infarction, the results of the multicenter CARMEL clinical trial39 that included 151 patients with thrombotic lesions showed a device success rate in 95% of the cases. The multicenter CORAL registry36 included 98 patients with significant stenosis of the saphenous vein graft and the rate of MACE was 18.4% at the 30-day follow-up.
A study that included 81 lesions of stent restenosis due to stent underexpansion confirmed the superiority of ELCA over predilation with high-pressure balloon; the OCT confirmed the ELCA-induced crack of calcium behind the struts.35
Complications
The potential complications of ELCA are similar to the ones reported for RA and OA. The main ones are coronary dissections and coronary perforations with incidence rates of 7% and 0.5%-8%, respectively.29,30,33 However, improvements in its design, the use of a technique with continuous infusion of a saline solution, and the use of smaller caliber catheters has reduced the rate of complications.33,34
CORONARY LITHOPLASTY
Definition
CL is an innovative technique that uses high-energy mechanical pulses administered through a semi-compliant balloon that modiy the plaque by cracking coronary calcium.41,42
Operating principles
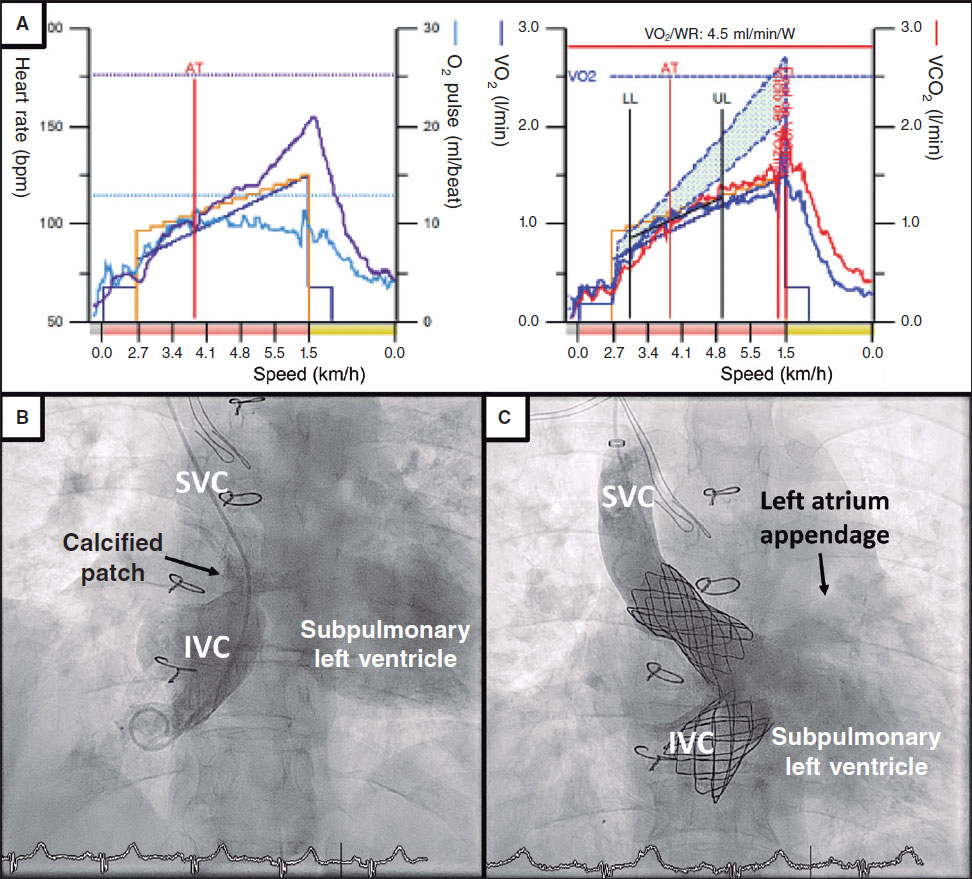
The device available is the Coronary Rx Lithoplasty System (Shockwave Medical, Inc., United States). The lithoplasty balloon (LB) is a single use 12 mm-long angioplasty balloon with diameters that go from 2.5 mm to 4 mm that is advanced on a 0.014 in
guidewire.42,43 It emits pulses of circumferential acoustic pressure to treat concentric calcified lesions (figure 5 of the supplementary data).
The LB is inflated at calcified lesion level at a pressure of 4 atm and 1 Hz shockwaves are administered.43,44 Mechanical energy is transmitted to the lesion when the LB contacts the artery intima layer cracking the calcium in the superficial and deep layers of the vessel wall.7 This facilitates the proper stent expansion and apposition.7,42,43
Once the LB is on the lesion, it is connected to an external unit that generates pulsatile mechanical waves (figure 5 of the supplementary data). It is advisable that the LB size and vessel keep a 1:1 ratio between them.41,42 The LB is initially inflated at a pressure of 4 atm and 10 pulses are administered (around 10 seconds are required). Then, the LB is inflated at a 6 atm pressure and then it is deflated to restore the flow. New cycles are then applied; a total of 8 therapies (80 pulses) per balloon and lesion can be administered.42,43 Due to its size, if the length of the lesion is > 12 mm, the LB can be repositioned to treat the lesion entirely. The use of the LB is easy, learning curve is short, and makes PCI easier7,42 (table 3).
Table 3. General characteristics of plaque-modification devices based on technologies with balloon
| Coronary lithoplasty | Cutting/scoring balloon | Ultra-high-pressure balloon | |
|---|---|---|---|
| Operating principles | |||
| Technology | Semi-compliant balloon that emits high-energy mechanical pulses | NC balloon with microblades/semi-compliant balloon with spiral struts | Double-layer NC balloon |
| Mechanism of action | Lithotripsy/Calcium cracking | Cutting of the plaque luminal surface | It allows inflation at 35-40 atm |
| Learning curve | Short | Short | Short |
| Device size | 2.5-4 mm | 2.75-3.5 mm/2.0-3.5 mm | 1.5-4.5 mm |
| Compatible catheter | 6-Fr | 6-Fr | 6-Fr |
| Indications | |||
| Main indication | Preparation of calcified lesions | Stent restenosis | Stent optimization |
| Optimal calcium location | Luminal with circumferential distribution | Luminal | Luminal |
| Stent restenosis | Yes | Yes | Yes |
| Complications | |||
| Difficulty crossing | Yes. Improved with the new generation LB | Yes/no | Yes |
| Dissection | Low risk | Moderate risk | Moderate risk |
| Perforation | Low risk | Low risk | Low risk |
| Slow-flow/no-reflow | Low risk | Low risk | Low risk |
| Practical advices | |||
| Pulses administered | Up to 80 pulses (8 cycles) | N/A | N/A |
| Device-vessel ratio | 1:1 | 0.8:1 | 1:1 |
| Recommendations | Inflation at 4 atm, 10 pulses; then up to 6 atm and deflation Useful with tortuosity Useful in bifurcations | Slow and gradual inflation | Slow and gradual inflation It allows stent postdilation at high atm |
|
LB, lithoplasty balloon; N/A, non-applicable; NC, non-compliant balloon. |
|||
Indications
The main indication of CL is for the management of concentric, calcified lesions with a circumferential distribution of calcium.41,42 It seems to be more effective on the deepest calcium compared to other plaque-modification techniques.45-47 LC is effective in large caliber vessels since there are lithoplasty balloons of up to 4mm in diameter. This device can be used in bifurcated lesions since 2 guidewires can be released during the procedure for lateral branch protection. Similarly, the LB seems safe and effective in the presence of significant tortuosity, stent restenosis due to underexpansion,48,49 and calcification involving the left main coronary artery with severe left ventricular dysfunction50,51 (table 3). Another more controversial indication can be for the management of SCCLs in the context of an ST-segment elevation acute myocardial infarction52 (figure 2).
Clinical data
The DISRUPT CAD I was a premarket clinical trial that confirmed the safety and efficacy of CL for the management of SCCLs before stent implantation; the rate of MACE at the 6-month follow-up was 8%.45 The DISRUPT CAD II clinical trial that included 120 patients confirmed the safety profile of CL before stent implantation with a rate of MACE of 7.6% at the 30-day follow-up.46 However, larger studies with longer follow-up periods are needed to confirm these results. The DISRUPT CAD III (NCT03595176) is a multicenter clinical trial with an estimate recruitment of 392 patients that will be analyzing the safety and efficacy of LB to obtain the Food and Drug Administration approval to use this device in the United States (table 4).
Table 4. Characteristics and results of the main studies on different plaque-modification techniques
| Study and year | Number of patients and treatment groups | Characteristics of the lesion | Study results | |
|---|---|---|---|---|
| Rotational atherectomy | ||||
| ROTAXUS16 (2014) | 240 patients RA + stent vs standard PCI | Lesion with moderate- severe calcification | Luminal loss in the stent at the 9-month follow-up Rate of MACE at the 9-month follow-up | 0.44 vs 0.31 mm; P = .04 24.2% vs 28.3%; P = .46 |
| PREPARE-CALC17 (2018) | 200 patients Cutting/scoring balloon vs RA | Severely calcified lesion | Luminal loss at the 9-month follow-up Target lesion revascularization | 0.16 ± 0.39 vs 0.22 ± 0.40 mm; P = .21 7% vs 2%; P = .17 |
| ROTATE18 (2016) | 1176 patients RA + drug-eluting stent | Severely calcified lesion | Rate of MACE at the 1-year follow-up Rate of MACE at the 2-year follow-up | 16% 24.9% |
| European RA Registry19 (2019) | 963 patients | Severely calcified lesion | Clinical success Rate of MACE at the 1-year follow-up | 92% 17% |
| Orbital atherectomy | ||||
| ORBIT I25 (2013) | 50 patients | Calcified lesion | Procedural success Rate of MACE at the 6-month follow-up | 94% 8% |
| ORBIT II26,27 (2014) | 443 patients | Severely calcified lesion | Procedural success Rate of MACE at the 1 and 3-year follow-up | 98.6% 16.4% and 23.5% |
| COAST28 (2017) | 100 patients Micro Crown OA | Severely calcified lesion | Procedural success Rate of MACE at the 1-year follow-up | 85% 22.2% |
| Coronary atherectomy with excimer laser | ||||
| AMRO29 (1996) | 308 patients (157/151) Standard PCI/laser | Severely calcified lesion/stent restenosis | Luminal gain at the 6-month follow-up Rate of MACE at the 6-month follow-up | 0.48 vs 0.44 mm; P = .34 29.9 vs 33.1%; P = .55 |
| LAVA30 (1997) | 215 patients (98/117) Standard PCI/laser | Severely calcified lesion/stent restenosis | Procedural success at the 1-year follow-up | 96.9% vs 96.6%; P = .88 No significant differences |
| ERBAC40 (1997) | 222/232/231 patients Standard PCI vs ELCA vs RA | Lesion with moderate- severe calcification | Procedural success Target lesion revascularization | 80% vs 77% vs 89%; P = .0019 32% vs 46% vs 42.4%; P = .013 |
| Coronary lithoplasty | ||||
| DISRUPT CAD I44 (2019) | 60 patients | Severely calcified lesion | Angiographic success Rate of MACE at the 6-month follow-up | 100% 8.3% |
| DISRUPT CAD II45 (2019) | 120 patients | Severely calcified lesion | Angiographic success Rate of MACE at the 30-day follow-up | 100% 7.6% |
|
AMRO, Amsterdam-Rotterdam trial; COAST; Coronary Orbital Atherectomy System Study; ELCA, excimer laser coronary atherectomy; ERBAC: Excimer Laser, Rotational Atherectomy, and Balloon Angioplasty Comparison; LAVA, Laser Angioplasty Versus Angioplasty; MACE, major cardiovascular adverse events; OA, orbital atherectomy; PCI, percutaneous coronary intervention; RA, rotational atherectomy; ROTAXUS, Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease. |
||||
Complications
Perioperative complications (dissection and perforation) are uncertain. Although the device has proven successful in the short-term, there are few real-world data published on the use of LBs. It seems that the calcium crack caused by the LB remains in situ without causing distal embolization, which reduces the rate of the slow-flow/no-reflow phenomenon.7,42,43
OTHER TECHNIQUES AND DEVICES
Non-compliant balloon
The NC balloon sustains very few size changes event at high pressures. This facilitates focusing its power on a given point to dilate calcified lesions without causing excessive dilatation in other vessel segments. It is often used for stent postdilation and to guarantee proper stent expansion and apposition.53 However, caution is required because the use of NC balloons for the management of SCCLs can cause coronary perforations and dissections of stent borders when used with postdilation purposes.53
Ultra-high-pressure balloon
The ultra-high-pressure balloon is a double-layer system that facilitates the balloon uniform expansion and reduces the risk of fracture and coronary perforation.54 The OPN device (SIS Medical AG, Switzerland) provides pressures of up to 35-40 atm without tearing the balloon (table 3). These characteristics facilitate the management of stent underexpansion when other options have failed. The main limitation of this type of balloon is its crossing profile due to its greater rigidity and double-layer technology.7,54
Cutting balloon and scoring balloon
The WOLVERINE cutting balloon (Boston Scientific, Marlborough, MA, United States) consists of a NC balloon with 3 micro-blades longitudinally arranged on its surface. These blades create incisions inside the calcified lesion during balloon inflation; sequential inflation up to 6 atm is advisable.55 The main limitations are its crossing profile and the risk of dissection and coronary perforation (table 3). The balloon crossing profile and navigability have improved with the new generation of devices: the AngioSculpt, Scoreflex, and NSE Alpha scoring balloons (table 3). It consists of a low-profile semi-compliant balloon surrounded by 3 nitinol spiral struts for better anchoring to the plaque, fewer chances of balloon gliding, and lower risk of dissection and perforation.56
COMBINING THE TECHNIQUES
The combination of RA plus cutting balloon for the management of SCCLs facilitates calcium cracking and better stent expansion and apposition.57
The RASER technique consists of combining ELCA plus RA or OA.58 This combination can be used in lesions that don’t allow the advancement of a microcatheter. The laser can do enough plaque modification to eventually advance the microcatheter and change it for a RotaWire/ViperWire guidewire to perform the RA or OA and achieve the proper stent expansion.58
The combination RA plus CL (RotaTripsy technique) has been described recently.59 It can be useful in very serious and calcified lesions with circumferential distribution of calcium. In this type of lesions where the LB is hard to release on the target lesion, the RA can initially modify the plaque to advance the LB, thus increasing luminal distensibility for a proper stent expansion.59
INTRAVASCULAR IMAGING IN CALCIFIED LESIONS
Intravascular imaging modalities (IVUS and OCT) improve the identification of SCCLs and provide thorough assessments of the calcium load, distribution, and eccentricity.7,42
Thanks to the ultrasound deeper penetration, IVUS can detect calcified deposits at the deepest layers of the vessel wall. However, due to the acoustic shadowing, only the calcic arch can be seen and no information on its thickness.60,61 The OCT has greater spatial resolution and higher definition. Calcium is seen as an attenuation region with a well-established luminal border. Compared to the IVUS it is a more precise technique to define calcium load because it provides information on the different degrees of calcic arch and the area, thickness, length, and volume of calcium distribution.42,61-63
Intravascular imaging is essential before selecting the plaque-modification device and to assess the stent final outcome. The OCT has greater sensitivity to detect stent underexpansion and malapposition and to assess the outcomes after postdilation.7,61
CONCLUSIONS
Coronary calcification is associated with complex lesions and patients with significant comorbidities, which is a predictor of poor prognosis in the short and long-term. More complex patients with more calcified lesions are being treated these days. This means plaque preparation is key in these cases to promote a proper stent expansion and apposition and avoid stent restenosis and thrombosis.
The ideal plaque-modification device is easy to use and implement,safe and effective during the procedure, and with good short and long-term results. With the appearance of CL and the upgrade of Rotablator and the cutting balloon, this field has a bright future ahead. There are many devices available today that can be classified into plaque-modification techniques with and without balloon.
We still lack much evidence from randomized studies and real-world registries to know what the exact role of each of these techniques is, and what benefit can be derived from combining them because they may be complementary, not exclusive. Regardless of the plaque-modification technique used, it is advisable to perform intravascular imaging to guarantee the proper stent expansion and apposition.
CONFLICTS OF INTEREST
None reported.
References
1. Bourantas CV, Zhang YJ, Garg S, et al. Prognostic implications of coronary calcification in patients with obstructive coronary artery disease treated by percutaneous coronary intervention:a patient-level pooled analysis of 7 contemporary stent trials. Heart. 2014;100:1158-1164.
2. Otsuka F, Sakakura K, Yahagi K, et al. Has our understanding of calcification in human coronary atherosclerosis progressed?Arterioscler Thromb Vasc Biol. 2014;34:724-736.
3. Wang X, Matsumura M, Mintz GS, et al. In Vivo Calcium Detection by Comparing Optical Coherence Tomography, Intravascular Ultrasound, and Angiography. JACC Cardiovasc Imaging. 2017;10:869-879.
4. Généreux P, Madhavan MV, Mintz GS, et al. Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes. Pooled analysis from the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) and ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) TRIALS.J Am Coll Cardiol. 2014;63:1845-1854.
5. Maehara A, Mintz GS, Witzenbichler B, et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents:the assessment of dual antiplatelet therapy with drug-eluting stents (ADAPT-DES) study. Circulation. 2014;129:463-470.
6. Barbato E, Shlofmitz E, Milkas A, Shlofmitz R, Azzalini L, Colombo A. State of the art:evolving concepts in the treatment of heavily calcified and undilatable coronary stenoses —from debulking to plaque modification, a 40-year-long journey. EuroIntervention. 2017;13:696-705.
7. De Maria GL, Scarsini R, Banning AP. Management of Calcific Coronary Artery Lesions:Is it Time to Change Our Interventional Therapeutic Approach?JACC Cardiovasc Interv. 2019;12:1465-1478.
8. Kassimis G, Raina T, Kontogiannis N. How should we treat heavily calcified coronary artery disease in contemporary practice?From atherectomy to intravascular lithotripsy. Cardiovasc Revasc Med. 2019. https://doi.org/10.1016/j.carrev.2019.01.010.
9. Shavadia JS, Vo MN, Bainey KR. Challenges With Severe Coronary Artery Calcification in Percutaneous Coronary Intervention:A Narrative Review of Therapeutic Options. Can J Cardiol. 2018;34:1564-1572.
10. Sharma SK, Tomey MI, Teirstein PS, et al. North American Expert Review of Rotational Atherectomy. Circ Cardiovasc Interv. 2019;12:e007448.
11. Barbato E, CarriéD, Dardas P, et al. European expert consensus on rotational atherectomy. EuroIntervention. 2015;11:30-36.
12. Bittl JA, Chew DP, Topol EJ, et al. Meta-analysis of randomized trials of percutaneous transluminal coronary angioplasty versus atherectomy, cutting balloon atherectomy, or laser angioplasty. J Am Coll Cardiol. 2004;43:936-942.
13. Whitlow PL, Bass TA, Kipperman RM, et al. Results of the study to determine Rotablator and transluminal angioplasty strategy (STRATAS). Am J Cardiol. 2001;87:699-705.
14. Safian RD, Feldman T, Muller DW, et al. Coronary angioplasty and Rotablator atherectomy trial (CARAT):immediate and late results of a prospective multicenter randomized trial. Catheter Cardiovasc Interv. 2001;53:213-220.
15. Sakakura K, Inohara T, Kohsaka S, et al. Incidence and Determinants of Complications in Rotational Atherectomy:Insights From the National Clinical Data (J-PCI Registry). Circ Cardiovasc Interv. 2016;9:e004278.
16. Abdel-Wahab M, Richardt G, Joachim Buttner H, et al. High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions:the randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial. JACC Cardiovasc Interv. 2013;6:10-19.
17. Abdel-Wahab M, Toelg R, Byrne RA, et al. High-Speed Rotational Atherectomy Versus Modified Balloons Prior to Drug-Eluting Stent Implantation in Severely Calcified Coronary Lesions. The Randomized PREPARE-CALC. Circ Cardiovasc Interv. 2018;11:e007415.
18. Kawamoto H, Latib A, Ruparelia N, et al. In-hospital and midterm clinical outcomes of rotational atherectomy followed by stent implantation:The ROTATE multicentre registry. EuroIntervention. 2016;12:1448-1456.
19. CarriéD, Reczuch K, Dobrzycki K, et al.;on behalf of the EURO4C Registry investigators. Results of the prospective European Registry on Rotational Atherectomy. EuroPCR Congress 2019. Available online:https://media.pcronline.com/diapos/EuroPCR2019/3355-20190523_1630_Theatre_Bordeaux_Carrie_Didier_1111_(12324)/Carrie_Didier_20190523_1630_Theatre_Bordeaux.pdf. Accessed 12 Nov 2019.
20. Sulimov DS, Abdel-Wahab M, Toelg R. Stuck Rotablator:the nightmare of rotational atherectomy. EuroIntervention. 2013;9:251-258.
21. Tomey MI, Kini AS, Sharma SK. Current status of rotational atherectomy. JACC Cardiovasc Interv. 2014;7:345-353.
22. Shlofmitz E, Shlofmitz R, Lee MS. Orbital Atherectomy:A Comprehensive Review. Interv Cardiol Clin. 2019;8:161-171.
23. Sotomi Y, Cavalcante R, Shlofmitz RA, et al. Quantification by optical coherence tomography imaging of the ablation volume obtained with the orbital atherectomy system in calcified coronary lesions. EuroIntervention. 2016;12:1126-1134.
24. Yamamoto MH, Maehara A, Karimi Galougahi K, et al. Mechanisms of orbital versus rotational atherectomy plaque modification in severely calcified lesions assessed by optical coherence tomography. JACC Cardiovasc Interv. 2017;10:2584-2586.
25. Parikh K, Chandra P, Choksi N, et al. Safety and feasibility of orbital atherectomy for the treatment of calcified coronary lesions:the ORBIT I trial. Catheter Cardiovasc Interv. 2013;81:1134-1139.
26. Chambers JW, Feldman RL, Himmelstein SI, et al. Pivotal trial to evaluate the safety and efficacy of the orbital atherectomy system in treating de novo, severely calcified coronary lesions (ORBIT II). JACC Cardiovasc Interv. 2014;7:510-518.
27. Lee M, Genereux P, Shlofmitz R, et al. Orbital atherectomy for treating de novo, severely calcified coronary lesions:3-year results of the pivotal ORBIT II trial. Cardiovasc Revasc Med. 2017;18:261-264.
28. Sharma S, Saito S, Shlofmitz R, et al. LBT-3 treatment of severely calcified coronary lesions with the coronary orbital atherectomy system Micro Crown:1-year results from the COAST trial. JACC Cardiovasc Interv. 2017;3:S1-2.
29. Appelman YE, Piek JJ, Strikwerda S, et al. Randomized trial of excimer laser angioplasty versus balloon angioplasty for treatment of obstructive coronary artery disease. Lancet. 1996;347:79-84.
30. Stone GW, de Marchena E, Dageforde D, et al. Prospective, randomized, multicenter comparison of laser-facilitated balloon angioplasty versus stand-alone balloon angioplasty in patients with obstructive coronary artery disease. The Laser Angioplasty Versus Angioplasty (LAVA) Trial Investigators. J Am Coll Cardiol. 1997;30:1714-1721.
31. Ambrosini V, Sorropago G, Laurenzano E, et al. Early outcome of high energy laser (Excimer) facilitated coronary angioplasty on hard and complex calcified and balloon-resistant coronary lesions:LEONARDO Study. Cardiovasc Revasc Med. 2015;16:141-146.
32. Deckelbaum LI, Natarajan MK, Bittl JA, et al. Effect of intracoronary saline infusion on dissection during excimer laser coronary angioplasty:a randomized trial. The Percutaneous Excimer Laser Coronary Angioplasty (PELCA) Investigators. J Am Coll Cardiol. 1995;26:1264-1269.
33. Fernandez JP, Hobson AR, McKenzie D, et al. Beyond the balloon:excimer coronary laser atherectomy used alone or in combination with rotational atherectomy in the treatment of chronic total occlusions, non-crossable and nonexpansible coronary lesions. EuroIntervention. 2013;9:243-250.
34. Mohandes M, Rojas S, Moreno C, Fernández F, Fuertes M, Guarinos J. Excimer laser in percutaneous coronary intervention of device uncrossable chronic total and functional occlusions. Cardiovasc Revasc Med. 2019. https://doi.org/10.1016/j.carrev.2019.08.022.
35. Lee T, Shlofmitz RA, Song L, et al. The effectiveness of excimer laser angioplasty to treat coronary in-stent restenosis with peri-stent calcium as assessed by optical coherence tomography. EuroIntervention 2019;15:e279-288.
36. Giugliano GR, Falcone MW, Mego D, et al. A prospective multicenter registry of laser therapy for degenerated saphenous vein graft stenosis:the COronary graft Results following Atherectomy with Laser (CORAL) trial. Cardiovasc Revasc Med. 2012;13:84-89.
37. Nishino M, Mori N, Takiuchi S, et al. Indications and outcomes of excimer laser coronary atherectomy:Efficacy and safety for thrombotic lesions —The ULTRAMAN registry. J Cardiol. 2017;69:314-319.
38. Dorr M, Vogelgesang D, Hummel A, et al. Excimer laser thrombus elimination for prevention of distal embolization and no-reflow in patients with acute ST elevation myocardial infarction:results from the randomized LaserAMI study. Int J Cardiol. 2007;116:20-26.
39. Topaz O, Ebersole D, Das T, et al. Excimer laser angioplasty in acute myocardial infarction (the CARMEL multicenter trial). Am J Cardiol. 2004;93:694-701.
40. Reifart N, Vandormael M, Krajcar M, et al. Randomized comparison of angioplasty of complex coronary lesions at a single center. Excimer Laser, Rotational Atherectomy, and Balloon Angioplasty Comparison (ERBAC) Study. Circulation. 1997;96:91-98.
41. De Silva K, Roy J, Webb I, et al. A Calcific, Undilatable Stenosis:Lithoplasty, a New Tool in the Box?JACC Cardiovasc Interv. 2017;10:304-306.
42. Ali ZA, Brinton TJ, Hill JM, et al. Optical Coherence Tomography Characterization of Coronary Lithoplasty for Treatment of Calcified Lesions:First Description. JACC Cardiovasc Imaging. 2017;10:897-906.
43. Serruys PW, Katagiri Y, Onuma Y. Shaking and Breaking Calcified Plaque:Lithoplasty, a Breakthrough in Interventional Armamentarium?JACC Cardiovasc Imaging. 2017;10:907-911.
44. Rodriguez Costoya I, Tizón Marcos H, Vaquerizo Montilla B, et al. Coronary Lithoplasty:Initial Experience in Coronary Calcified Lesions. Rev Esp Cardiol. 2019;72:788-790.
45. Brinton TJ, Ali ZA, Hill JM, et al. Feasibility of Shockwave Coronary Intravascular Lithotripsy for the Treatment of Calcified Coronary Stenoses. Circulation. 2019;139:834-836.
46. Ali ZA, Nef H, Escaned J, et al. Safety and Effectiveness of Coronary Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Stenoses:The Disrupt CAD II Study. Circ Cardiovasc Interv. 2019;12:e008434.
47. Wong B, El-Jack S, Newcombe R, Glenie T, Armstrong G, Khan A. Shockwave Intravascular Lithotripsy for Calcified Coronary Lesions:First Real-World Experience. J Invasive Cardiol. 2019;31:46-48.
48. Tovar Forero MN, Wilschut J, Van Mieghem NM, Daemen J. Coronary lithoplasty:a novel treatment for stent underexpansion. Eur Heart J. 2019;40:221.
49. Watkins S, Good R, Hill J, Brinton TJ, Oldroyd KG. Intravascular lithotripsy to treat a severely underexpanded coronary stent. EuroIntervention. 2019;15:124-125.
50. Wong B, E-Jack S, Khan A, et al. Treatment of Heavily Calcified Unprotected Left Main Disease With Lithotripsy:The First Case Series. J Invasive Cardiol. 2019;31:E143-E147.
51. Ristalli F, Maiani S, Mattesini A, et al. Intravascular lithotripsy and Impella support to assist complex LM angioplasty. Cardiovasc Revasc Med. 2019. https://doi.org/10.1016/j.carrev.2019.06.014.
52. Wong B, El-Jack S, Newcombe R, et al. Shockwave Intravascular Lithotripsy of Calcified Coronary Lesions in ST-Elevation Myocardial Infarction:First-in-Man Experience. J Invasive Cardiol. 2019;31:E73-E75.
53. Romagnoli E, Sangiorgi GM, Cosgrave J, et al. Drug-eluting stenting:the case for post-dilation. JACC Cardiovasc Interv. 2008;1:22-31.
54. Secco GG, Ghione M, Mattesini A, et. al. Very high-pressure dilatation for undilatable coronary lesions:indications and results with a new dedicated balloon. EuroIntervention. 2016;12:359-365.
55. Mauri L, Bonan R, Weiner BH, et al. Cutting balloon angioplasty for the prevention of restenosis:results of the Cutting Balloon Global Randomized Trial. Am J Cardiol. 2002;90:1079-1083.
56. de Ribamar Costa JJr, Mintz GS, Carlier SG, et al. Nonrandomized comparison of coronary stenting under intravascular ultrasound guidance of direct stenting without predilation versus conventional predilation with a semi-compliant balloon versus predilation with a new scoring balloon. Am J Cardiol. 2007;100:812-817.
57. Amemiya K, Yamamoto MH, Maehara A, et al. Effect of cutting balloon after rotational atherectomy in severely calcified coronary artery lesions as assessed by optical coherence tomography. Catheter Cardiovasc Interv. 2019. https://doi.org/10.1002/ccd.28278.
58. Fernandez JP, Hobson AR, McKenzie D, et al. Beyond the balloon:excimer coronary laser atherectomy used alone or in combination with rotational atherectomy in the treatment of chronic total occlusions, non-crossable and nonexpansible coronary lesions. EuroIntervention. 2013;9:243-250.
59. Jurado-Román A, Gonzálvez A, Galeote G, Jiménez-Valero S, Moreno R. RotaTripsy:Combination of Rotational Atherectomy and Intravascular Lithotripsy for the Treatment of Severely Calcified Lesions. JACC Cardiovasc Interv. 2019;12:e127-e129.
60. Hoffmann R, Mintz GS, Popma JJ, et al. Treatment of calcified coronary lesions with Palmaz-Schatz stents. An intravascular ultrasound study. Eur Heart J. 1998;19:1224-1231.
61. Sharma SK, Vengrenyuk Y, Kini AS. IVUS, OCT, and coronary artery calcification:is there a bone of contention?JACC Cardiovasc Imaging. 2017;10:880-882.
62. Fujino A, Mintz GS, Matsumura M, et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention. 2018;13:e2182-e2189.
63. Zhang M, Matsumura M, Usui E, et al. TCT 51:IVUS Predictors of Stent Expansion in Severely Calcified Lesions. J Am Coll Cardiol. 2019;74(13 Supplement) B51.http://www.onlinejacc.org/content/74/13_Supplement/B51.
Corresponding author: Hospital del Mar, Parc de Salut Mar, Passeig Marítim 25-29, 08003 Barcelona, Spain.
Correo electrónico: beavaquerizo@yahoo.es (B. Vaquerizo).