INTRODUCTION
Right ventricular outflow tract (RVOT) disease is a common finding in children and adults with congenital heart disease and often occurs as a sequel of previous surgery. Over the past 2 decades, percutaneous pulmonary valve implantation has become more widely used and is recommended by current clinical practice guidelines1 as the preferred option for patients with previous conduits or bioprostheses.
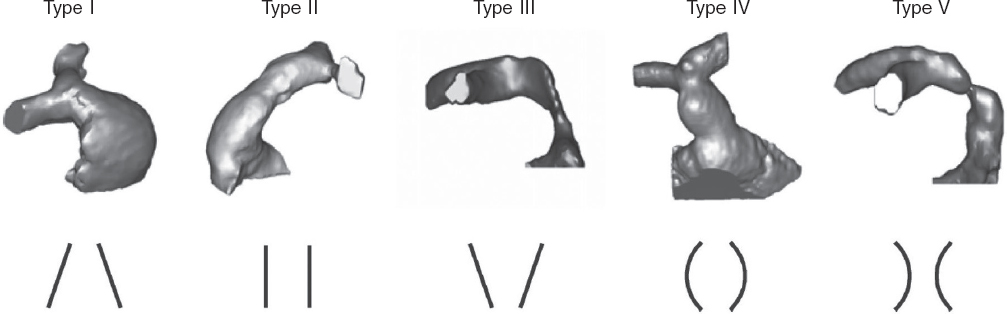
However, many patients have native or patched tracts (hereafter referred to as native RVOTs) with pulmonary regurgitation as the predominant lesion. In these patients, percutaneous valve placement is more complex due to the RVOT anatomy, its dynamic behavior, larger pulmonary annulus size, and lack of a proper landing zone for the valve. Because of the differences in underlying heart diseases, previous surgical repairs, and various pulmonary artery configurations, RVOT morphology varies widely but can be categorized into 5 subtypes2 (figure 1).
Figure 1. Five types of native RVOT anatomy: I - pyramidal; II - cylindrical or tubular; III - inverted pyramidal; IV - central enlargement; V - central narrowing. (Reproduced from Schievano et al.2 with permission from the author.)
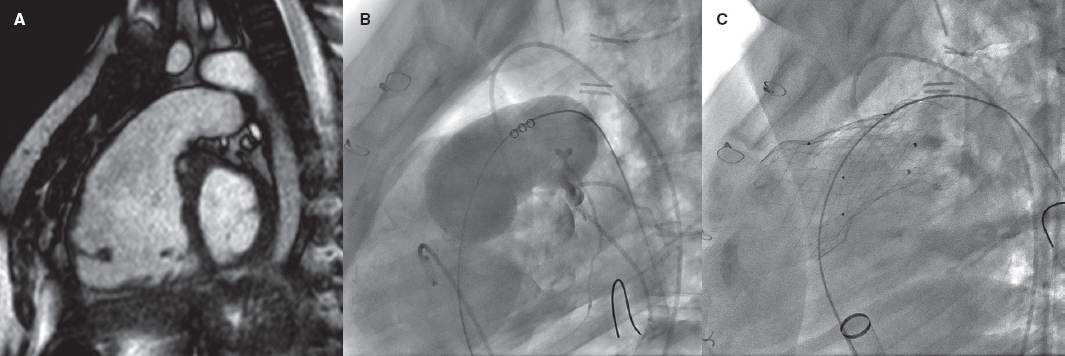
Repaired tetralogy of Fallot serves as the paradigm, and in these cases, surgery remains the standard of care. However, the development of percutaneous procedures has enabled a larger number of patients with these substrates to be eligible for percutaneous treatment (figure 2).
Figure 2. Self-expanding pulmonary valve implantation in an aneurysmal right ventricular outflow tract (RVOT). A: severely dilated RVOT on magnetic resonance imaging, with interventricular septal flattening. B: balloon sizing test in the RVOT with simultaneous injection into the left main coronary artery. C: successful venus-P valve (MedTech, China) implantation in the RVOT.
Two different models of balloon-expandable valves have been authorized to treat dysfunctional bioprostheses and conduits: the Melody (Medtronic, United States) and the SAPIEN valves (XT model, Edwards Lifesciences, United States). Although they have not yet been authorized for implantation in native RVOTs, both (along with the SAPIEN S3) have been used off-label in this setting.
To address the specific characteristics of native RVOTs, several models of self-expanding valves have been developed, such as the Venus-P (Venus MedTech, China, with CE marking for use in Europe since 2022), PULSTA (TaeWoong Medical, South Korea), and Harmony valves (Medtronic, United States, with prior FDA approval). The Alterra valve (Edwards Lifesciences) has also been used. This valve serves as a self-expanding prestent onto which a SAPIEN valve is later implanted.
The characteristics of each of these devices have already been described in detail in a previous issue of REC: Interventional Cardiology.3
RESULTS OF PERCUTANEOUS VALVES IN THE NATIVE RIGHT VENTRICULAR OUTFLOW TRACT
More information has gradually become available on the favorable results and durability of percutaneous valves. The largest multicenter registry to date,4 with 2476 patients (82% implanted with the Melody valve and 18% with the SAPIEN device, including 16% with native RVOTs), reported an 8-year survival rate of 91.1% after implantation, and a reintervention rate of 25.1%, which is similar to the rates reported in some surgical series.5 Nonrandomized comparative studies6 and a recent meta-analysis7 also found similar reintervention rates. Some series report higher rates in patients implanted with the Melody compared with the SAPIEN valve,8,9 although the 2 groups were not directly comparable, with reintervention-free survival rates in patients with SAPIEN being similar to those reported in patients with surgical valves.8
The SAPIEN device can be implanted with or without prestenting, depending on the patient’s characteristics, with good outcomes. The largest trial published to date included 774 patients implanted with the XT and S3 models10, 51% of whom had native RVOTs (table 1).
Table 1. Summary of some of the main trials of patients with native right ventricular outflow tract
| Author and year | Patients with native RVOT/Total patients | Valve type | Follow-up | Implant success | Other results | Complications |
|---|---|---|---|---|---|---|
| Malekzadeh-Milani et al.23, 2014 | 34/34 | Melody | 2.6 years | 100% | Paravalvular leak in 2 patients during follow-up | 3 acute complications (9%): 1 hemoptysis, 1 RVOT obstruction, 1 stent embolization |
| Meadows et al.18, 2014 | 31/31 | Melody | 15 months (1 month- 3.8 years) | 100% | No mortality or valve regurgitation | Stent fractures (32%) associated with a higher rate of stenosis. 3 cases of endocarditis. Reintervention in 3 patients |
| Garay et al.13, 2017 | 10/10 | Venus P | 12 months (4 to 21) | 100% | Normally functioning valve, no stent fractures, right ventricular remodeling, and NYHA functional class improvement | None |
| Martin et al.11, 2018 | 132/132 | Melody | No follow-up | Complete cohort of 229 patients, but only 58% implanted. Good immediate hemodynamic outcomes | Complication rate of 4% (mostly due to stent migration) | |
| Morgan et al.24, 2019 | 41/57 | SAPIEN (S3, XT) | 5.3 months (1 to 26) | 100% | No prestenting. Normally functioning valve at follow-up. No mortality | 1 aortic compression, 2 tricuspid valve injury, 1 valve regurgitation |
| Shahanavaz et al.10, 2020 | 397/774 | SAPIEN S3 (78%) XT (22%) | 12 months (n = 349) | 97.4% | Normally functioning valve: 91.5% | Adverse events: 10%. Emergency surgery: 14 patients (1.8%). Tricuspid injury: 3% |
| Goldstein et al.19, 2020 | 143/530 | Melody (88%) SAPIEN (22%) | 1 year | 98% | Normally functioning valve: 98% | 1 death. Reintervention rate of 13.3% (mostly unrelated to the valve) |
| Lee et al.12, 2021 | 25/25 | PULSTA | 33 (± 14) months | 100% | Zero cases of valve dysfunction | No significant adverse events |
| Gillespie et al.16, 2021 | 21/21 | Harmony | 5 years | 100% | Implantation in all but 1 patient due to pulmonary hypertension. Normally functioning valve in nonreoperated patients | Valve explantation in 2 patients, 1 death 3 years after implantation, 2 reinterventions (valve-in-valve) |
| Morgan et al.25, 2021 | 38/38 | Venus | 27 months | 97.4% | Normally functioning valve at follow-up | Migration: 2 cases (surgery in 1) |
| Houeijeh et al.9, 2023 | 99/214 | SAPIEN XT/S3 (85%) Melody (15%) | 2.8 years (3 months-11.4 years) | Only cases with successful implantation included | Reintervention-free survival at 5 to 10 years: 78.1% to 50.4% (Melody) and 94.3% to 82.2% (SAPIEN) | Severe complications: 2.3%, 1 valve-related death. Endocarditis 5.5/100 patient-years (Melody) and 0.2/100 patient-years (SAPIEN) |
| Álvarez et al.14, 2023 | 8/8 | Venus | No follow-up | 100% | Normally functioning valve in all | No significant adverse events |
| Lin et al.15, 2023 | 53/53 | Venus (28%), PULSTA (72%) | 27.5 months | 98.1% | No valve regurgitation at 12 months | 1 embolization, 1 endocarditis |
|
NYHA, New York Heart Association; RVOT, right ventricular outflow tract. In studies that are not specific to native RVOT, the results and complications refer to the overall cohort, as the results of native, or non-native RVOTs are often not detailed independently. Some centers participated in > 1 study, which allowed the same patient to be included in multiple publications. The most recent series with larger numbers of patients have been prioritized. |
||||||
In a study of patients with native RVOTs that included 229 candidates for the Melody valve, the device was finally implanted in 132 patients (58%).11 The most common reason for avoiding implantation was a prohibitively large RVOT, followed by coronary or aortic root compression. The immediate outcomes of patients with successful implantation were good. However, the low implantation rate demonstrates the limitation of treating native RVOTs with these valves.
Self-expanding valves fill this gap by allowing treatment of larger RVOTs, as they adapt to the anatomy of the RVOT and provide more stable attachment. The series published to date indicate a very high implantation success rate—close to 100%—with good short- and mid-term outcomes and few complications.12-16 (table 1 illustrates a selection of series representative of patients with native RVOTs).
Drawing comparisons between the results of surgical and percutaneous pulmonary valves is challenging because the types of patients and the anatomies treated are very different. Overall, patients undergoing percutaneous valve implantation are at higher risk and often have bioprostheses or small conduits that require smaller percutaneous valves, which is associated with a higher residual gradient, leading to a greater need for reintervention,4,8,9 and a higher rate of endocarditis. This aspect has traditionally led to less favorable results with the Melody series (with a maximum diameter of 22 mm), with durability being one of the issues initially identified, although more recent series have shown positive results. Only indirect comparisons can be drawn with valve procedures in the native RVOT.
PROCEDURAL PLANNING
Multimodal imaging modalities, especially magnetic resonance imaging and computed tomography, are crucial for patient selection and procedural planning. Key aspects include measurement of the RVOT and the pulmonary annulus, and assessment of both the RVOT and the coronary anatomy. Recently, computed tomography guidelines for RVOT assessment17 have been published with detailed information on the measurements that should be taken, and the cardiac cycle phase.
POTENTIAL PROBLEMS
Valve or stent migration or embolization is a potential complication in patients with large RVOTs due to the lack of an adequate landing zone, with incidence rates between 0% and 4.5%,11,10,18 and consequently proper valve sizing is of paramount importance.
Stent fractures can lead to loss of integrity and contribute to prosthetic valve dysfunction. Nonetheless, the implications of the occasional finding of an isolated strut fracture remain unclear.
Tricuspid valve injury has been reported in 3% to 6% of patients.10 However, this rate has dropped significantly with the use of DrySeal introducer sheaths (W.L. Gore & Associates, United States).10
Coronary compression is a rare complication nowadays, because coronary angiography is simultaneous and systematically performed during RVOT balloon inflation testing. However, it can be a reason for not performing percutaneous implantation in nearly 3% of patients.11
A higher incidence of infective endocarditis has been reported, especially after Melody valve implantation,6,8,9 with a higher risk when smaller valves are implanted and there is a greater residual gradient. The risk involved with other types of device such as the SAPIEN—whether because of its different composition (bovine jugular vein graft in the Melody compared with bovine pericardium in the SAPIEN) or because of its larger size—is much lower9 and seems comparable to that of surgical series.
Self-expanding valves are larger, and the proximal end often remains inside the RVOT, which could increase the risk of ventricular arrhythmias. The incidence of nonsustained ventricular tachycardia varies widely (between 0.6% and 40%11,19,20), although it is usually a transient phenomenon during the early postimplantation phase, and its long-term implications remain unclear. Of note, when comparing surgical with percutaneous pulmonary valve replacement, the early incidence of arrhythmias was lower in the latter.21 A potential caveat is that catheter access to the arrhythmic substrate can be limited after valve implantation.
The presence of the valve metal mesh with or without previous stents inside the RVOT can pose additional challenges for the surgeon if surgical valve replacement is subsequently required. This is a relative problem, because surgical pulmonary valve replacement also increases the risk of future reinterventions related to resternotomy.
BENEFITS OF PERCUTANEOUS VALVE IMPLANTATION
The possibility of performing percutaneous pulmonary valve implantation offers clear advantages: the procedure is much less invasive, length of stay is shorter,8 recovery is faster, the mortality rate is very low (from 0.2% to 0.8%),19 and the cost-effectiveness ratio is more favorable.22 In patients at high surgical risk, it might be the only available treatment option. The path followed by its “left-sided relatives”—transcatheter heart valves in the aortic position—illustrates that the threshold for the use of percutaneous techniques is decreasing as more experience is gained and technology becomes further refined. In cases of intermediate or low risk, percutaneous valve implantation may delay or avoid the need for surgery in patients who often require multiple interventions during their lifetime. This concept of avoiding sternotomies is relevant due to the added risk of further surgeries and for patients who are candidates for heart transplants. The decision on the best approach to valve implantation in each patient should be made by a multidisciplinary heart team, including health professionals experienced in these types of heart disease.
CONCLUSIONS
Percutaneous pulmonary valve implantation is particularly challenging in patients with native RVOTs. Nonetheless, it is a feasible option that is being used with a high success rate and few complications. However, appropriate candidate selection is essential. Several models of self-expanding valves have been specifically developed for this purpose, with good short- and mid-term results, allowing the treatment of patients with large RVOTs that were previously not amenable to balloon-expandable devices. The latest information suggests that the durability of percutaneous valves may be comparable to that of surgical bioprostheses, although long-term data are lacking, especially with the latest models. Although more studies and follow-up are necessary, percutaneous techniques are already an option for many patients and will likely become an alternative to surgical treatment in the near future.
FUNDING
None declared.
CONFLICTS OF INTEREST
None declared.
REFERENCES
1. Baumgartner H, De Baker J, Babu-Narayan S, et al. 2020 ESC Guidelines for the management of adult congenital heart disease. The Task Force for the management of adult congenital heart disease of the European Society of Cardiology (ESC). Eur Heart J. 2021;42:563-645.
2. Schievano S, Coats L, Migliavacca F, et al. Variations in Right Ventricular Outflow Tract Morphology Following Repair of Congenital Heart Disease:Implications for Percutaneous Pulmonary Valve Implantation. J Cardiovasc Magn Reson. 2007;9:687-695.
3. Gutiérrez-Larraya Aguado F, Pardeiro CA, Domingo EJB. Percutaneous treatment of pulmonary valve and arteries for the management of congenital heart disease. REC Interv Cardiol. 2021;3:119-128.
4. McElhinney DB, Zhang Y, Levi DS, et al. Reintervention and Survival After Transcatheter Pulmonary Valve Replacement. J Am Coll Cardiol. 2022;79:18-32.
5. Buber J, Egidy G, Huang A, et al. Durability of large diameter right ventricular out fl ow tract conduits in adults with congenital heart disease. Int J Cardiol. 2023;175:455-463.
6. Georgiev S, Ewert P, Eicken A, et al. Munich Comparative Study:Prospective Long-Term Outcome of the Transcatheter Melody Valve Versus Surgical Pulmonary Bioprosthesis with up to 12 Years of Follow-Up. Circ Cardiovasc Interv. 2020;13:1-7.
7. Ribeiro JM, Gonc L, Costa M. Transcatheter Versus Surgical Pulmonary Valve Replacement:A Systemic Review. Ann Thorac Surg. 2020;110:1751-1761.
8. Hribernik I, Thomson J, Ho A, et al. Comparative analysis of surgical and percutaneous pulmonary valve implants over a 20-year period. Eur J Cardiothoracic Surg. 2022;61:572-579.
9. Houeijeh A, Batteux C, Karsenty C, et al. Long-term outcomes of transcatheter pulmonary valve implantation with melody and SAPIEN valves. Int J Cardiol. 2023;370:156-166.
10. Shahanavaz S, Zahn EM, Levi DS, et al. Transcatheter Pulmonary Valve Replacement With the SAPIEN Prosthesis. J Am Coll Cardiol. 2020;76:2847-2858.
11. Martin MH, Meadows J, McElhinney DB, et al. Safety and Feasibility of Melody Transcatheter Pulmonary Valve Replacement in the Native Right Ventricular Outflow Tract:A Multicenter Pediatric Heart Network Scholar Study. JACC Cardiovasc Interv. 2018;11:1642-1650.
12. Lee S, Kim S, Kim Y. Mid-term outcomes of the Pulsta transcatheter pulmonary valve for the native right ventricular outflow tract. Catheter Cardiovasc Interv. 2021;98:E724-E732.
13. Garay F, Pan X, Zhang YJ, Wang C, Springmuller D. Early experience with the Venus p-valve for percutaneous pulmonary valve implantation in native outflow tract. Netherlands Hear J. 2017;25:76-81.
14. Álvarez-Fuente M, Toledano M, Hernández I, et al. Initial experience with the new percutaneous pulmonary self-expandable Venus P-valve. REC Interv Cardiol. 2023;5:263-269.
15. Lin MT, Chen CA, Chen SJ, et al. Self-Expanding Pulmonary Valves in 53 Patients With Native Repaired Right Ventricular Outflow Tracts. Can J Cardiol. 2023;39:997-1006.
16. Gillespie MJ, Bergersen L, Benson LN, Weng S, Cheatham JP. 5-Year Outcomes From the Harmony Native Outflow Tract Early Feasibility Study. JACC Cardiovasc Interv. 2021;14:816-817.
17. Han BK, Garcia S, Aboulhosn J, et al. Technical recommendations for computed tomography guidance of intervention in the right ventricular outflow tract:Native RVOT conduits and bioprosthetic valves:A white paper of the Society of Cardiovascular Computed Tomography (SCCT), Congenital Heart Surgeons'Society (CHSS), and Society for Cardiovascular Angiography &Interventions (SCAI). J Cardiovasc Comput Tomogr. 2023;18(2024):75-99.
18. Meadows JJ, Moore PM, Berman DP, et al. Congenital Heart Disease Use and Performance of the Melody Transcatheter Pulmonary Valve in Native and Postsurgical, Nonconduit Right Ventricular Outflow Tracts. Circ Cardiovasc Interv. 2014;7:374-380.
19. Goldstein BH, Bergersen L, Armstrong AK, et al. Adverse Events, Radiation Exposure, and Reinterventions Following Transcatheter Pulmonary Valve Replacement. J Am Coll Cardiol. 2020;75:363-376.
20. Taylor A, Yang J, Dubin A, et al. Ventricular arrhythmias following transcatheter pulmonary valve replacement with the harmony TPV25 device. Catheter Cardiovasc Interv. 2022;100:766-773.
21. Wadia SK, Lluri G, Aboulhosn JA, et al. Ventricular arrhythmia burden after transcatheter versus surgical pulmonary valve replacement. Heart. 2018;104:1791-1796.
22. Vergales JE, Wanchek T, Novicoff W, Kron IL, Lim DS. Cost-Analysis of Percutaneous Pulmonary Valve Implantation Compared to Surgical Pulmonary Valve Replacement. Catheter Cardiovasc Interv. 2013;82:1147-1153.
23. Malekzadeh-Milani S, Ladouceur M, Cohen S, Iserin L, Boudjemline Y. Results of transcatheter pulmonary valvulation in native or patched right ventricular outflow tracts. Arch Cardiovasc Dis. 2014;107:592-598.
24. Morgan GJ, Sadeghi S, Salem MM, et al. SAPIEN valve for percutaneous transcatheter pulmonary valve replacement without “pre-stenting“:A multi-institutional experience. Catheter Cardiovasc Interv. 2019;93:324-329.
25. Morgan G, Prachasilchai P, Promphan W, et al. Medium-term results of percutaneous pulmonary valve implantation using the Venus P-valve:international experience. EuroIntervention. 2019;14:1363-1370.