To the Editor,
Transcatheter aortic valve implantation (TAVI) can trigger significant conduction disorders due to the mechanical compression caused by the transcatheter heart valve. This is because of the proximity between the aortic annulus, the atrioventricular node, and the membranous septum (MS) of the left ventricular outflow tract. The rate of pacemaker implantation after TAVI ranges from 4% to 33%.1
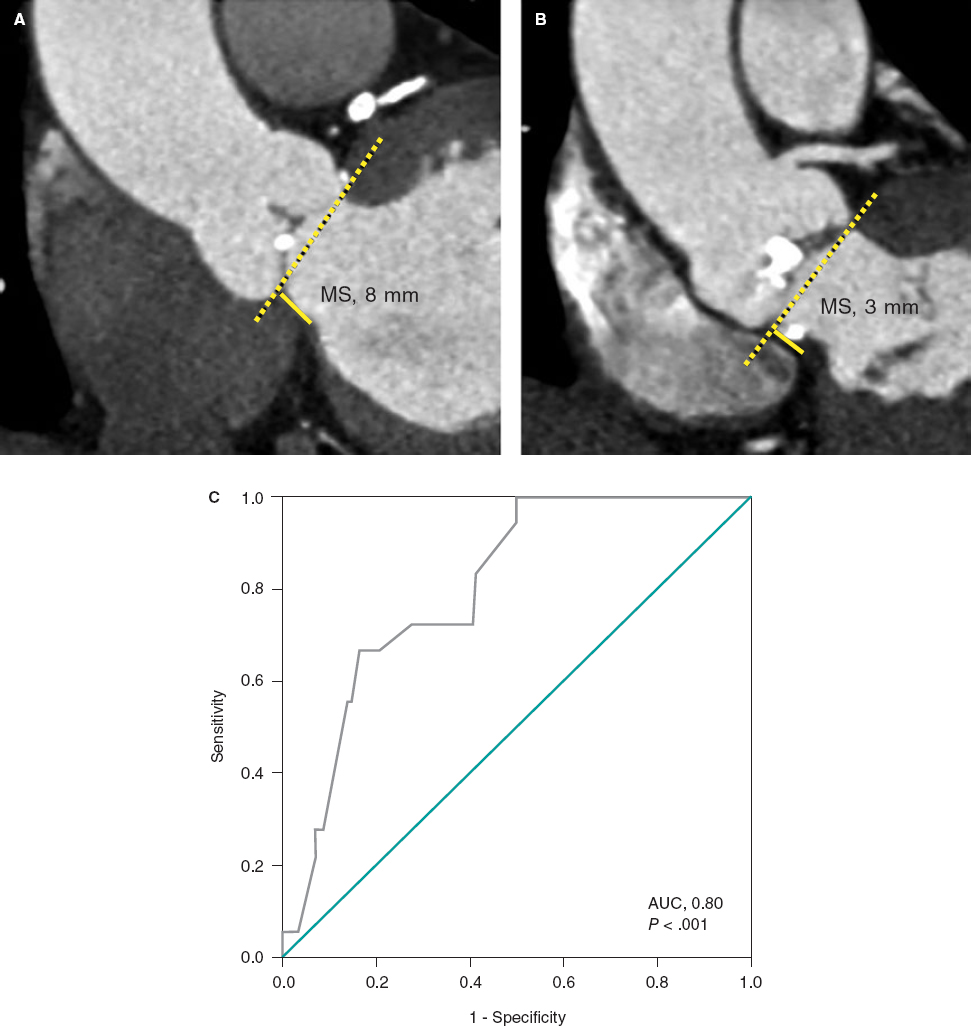
This retrospective analytical study included symptomatic patients with severe aortic stenosis referred for multidetector computed tomography as part of the TAVI protocol from December 2012 through October 2022. Written informed consent was obtained from all patients prior to the tomography scan. We excluded patients with bicuspid aortic valve anatomy, pacemaker carriers, and those with biological bioprosthetic valves. The aim of this study was to determine whether MS length is associated with the need for pacemaker implantation after TAVI. MS length was measured as the maximum distance from the plane of the aortic annulus to the top of the muscular portion of the ventricular septum in the coronal plane during systole (figure 1A,B).2 Qualitative variables were analyzed using the chi-square test or Fisher exact test, while quantitative variables were analyzed using the Mann-Whitney U test. P values < .005 were considered statistically significant. A receiver operating characteristic (ROC) curve was constructed to assess the predictive accuracy of MS length for pacemaker implantation. Data were analyzed using the IBM SPSS statistical software package, version 26 (United States).
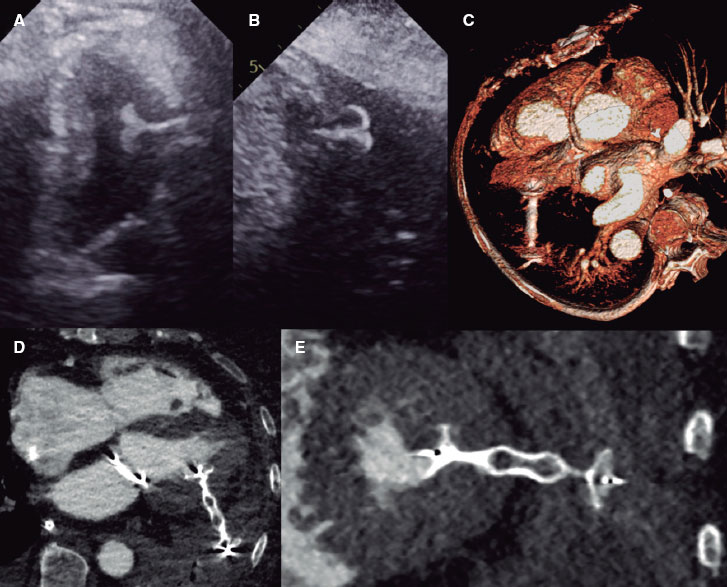
Figure 1. Measurement of membranous septum (MS) length by multidetector computed tomography in the coronal view. A: patient with an 8-mm MS not requiring no pacemaker implantation. B: patient with a 3-mm MS and left ventricular outflow tract calcification who underwent pacemaker implantation due to third-degree atrioventricular block after transcatheter aortic valve implantation. C: receiver operating characteristic (ROC) curve. Membranous septum length as a predictor of pacemaker implantation. The area under the curve (AUC) was 0.80 with cut-off, sensitivity, and specificity values of 5.5 mm, 0.67%, and 0.84%, respectively (P < .001).
A total of 134 consecutive patients were assessed: 71 (53%) were men and the mean age was 75.5 ± 7.6 years.
In the pre-TAVI electrocardiogram, 117 patients (87.3%) were in sinus rhythm, 14 (10.4%) had atrial fibrillation, and 34 (25.4%) had conduction disorders (table 1).
Table 1. Clinical and electrocardiographic characteristics before and after TAVI, tomographic parameters, types of transcatheter heart valve, and complications after TAVI
| Variables | Total n = 134 | With pacemaker implantation n = 18 | Without pacemaker implantation n = 116 | P |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, years | 75.5 ± 7.6 | 76.2 ± 7.8 | 75.5 ± 7.5 | .63 |
| Masculine sex, n (%) | 71 (53%) | 12 (66.7%) | 59 (51%) | .21 |
| BMI, kg/m2 | 26 ± 4.3 | 28 ± 6.8 | 25.7 ± 3.7 | .2 |
| Hypertension, n (%) | 91 (67.9%) | 10 (55.6%) | 81 (69.8%) | .23 |
| Ischemic heart disease, n (%) | 74 (55.2%) | 9 (50%) | 65 (56%) | .63 |
| Diabetes mellitus, n (%) | 43 (32.1%) | 8 (44.4%) | 35 (30.2%) | .23 |
| Dyslipidemia, n (%) | 36 (26.9%) | 5 (27.8%) | 31 (26.7%) | 1 |
| Smoking, n (%) | 33 (24.6%) | 3 (16.7%) | 30 (25.9%) | .56 |
| Kidney disease, n (%) | 18 (13.4%) | 3 (16.7%) | 15 (12.9%) | .71 |
| Electrographic characteristics prior to TAVI | ||||
| Rhythm | ||||
| Sinus, n (%) | 117 (87.3%) | 17 (94.4%) | 100 (86.2%) | .47 |
| Atrial fibrillation, n (%) | 14 (10.4%) | 0 | 14 (12.1%) | .21 |
| Flutter, n (%) | 3 (2.2%) | 1 (5.6%) | 2 (1.7%) | .35 |
| Conduction disorder | ||||
| RBBB, n (%) | 13 (9.7%) | 4 (22.2%) | 9 (7.8%) | .07 |
| LBBB, n (%) | 10 (7.5%) | 2 (11.1%) | 8 (6.9%) | .66 |
| First-degree AVB, n (%) | 8 (5.9%) | 3 (16.7%) | 5 (4.3%) | .13 |
| LBBB + First-degree AVB, n (%) | 2 (1.5%) | 0 | 2 (1.7%) | 1 |
| Incomplete left bundle branch block, n (%) | 1 (0.7%) | 0 | 1 (0.9%) | 1 |
| Tomographic parameters | ||||
| MS length, mm | 6.86 ± 1.72 | 5.3 ± 1.2 | 7.1 ± 1.7 | < .001 |
| Presence of LVOT calcification, n (%) | 39 (29.1%) | 9 (50%) | 30 (25.9%) | .036 |
| Type of transcatheter heart valve | ||||
| Balloon-expandable | ||||
| Edward SAPIEN 3, n (%) | 60 (44.8%) | 8 (44.4%) | 52 (44.8%) | .9 |
| Edward SAPIEN, n (%) | 14 (10.4%) | 3 (16.7%) | 11 (9.5%) | .4 |
| Edward SAPIEN XT, n (%) | 8 (6%) | 1 (5.6%) | 7 (6%) | 1 |
| Self-expandable | ||||
| Evolute R, n (%) | 17 (12.7%) | 1 (5.6%) | 16 (13.8%) | .47 |
| ACCURATE Neo, n (%) | 14 (10.4%) | 0 | 14 (12.1%) | .21 |
| Portico, n (%) | 11 (8.2%) | 1 (5.6%) | 10 (8.6%) | 1 |
| CoreValve, n (%) | 10 (7.5%) | 4 (22.2%) | 6 (5.2%) | .029 |
| Electrocardiographic characteristics after TAVI | ||||
| Third-degree AVB, n (%) | 16 (11.9%) | 16 (88.9%) | 0 | < .001 |
| Isolated persistent new-onset LBBB, n (%) | 10 (7.4%) | 0 | 10 (8.6%) | .5 |
| Persistent LBBB + AF + NSVT, n (%) | 1 (0.8%) | 1 (5.6%) | 0 | .13 |
| Persistent LBBB + nodal rhythm, n (%) | 1 (0.8%) | 1 (5.6%) | 0 | .13 |
| Transient third-degree AVB, n (%) | 8 (6%) | 0 | 8 (6.9%) | .65 |
| First-degree AVB, n (%) | 6 (4.5%) | 0 | 6 (5.2%) | .41 |
| Transient LBBB, n (%) | 4 (3%) | 0 | 4 (3.4%) | .56 |
| Isolated AF, n (%) | 2 (1.5%) | 0 | 2 (1.7%) | .75 |
| Transient nodal rhythm, n (%) | 1 (0.8%) | 0 | 1 (0.9%) | .87 |
| Flutter, n (%) | 1 (0.8%) | 0 | 1 (0.9%) | .87 |
| Complications after TAVI | ||||
| Local: Iliac or femoral artery dissection, hematoma | 8 (6%) | 0 | 8 (6.9%) | .30 |
| Pericardial effusion/Tamponade | 3 (2.2%) | 1 (5.6%) | 2 (1.7%) | .35 |
| Ischemic stroke | 3 (2.2%) | 0 | 3 (2.6%) | .35 |
| Kidney disease | 3 (2.2%) | 1 (5.6%) | 2 (1.7%) | .35 |
| Intraoperative mortality | 5 (3.73%) | |||
| Massive ischemic stroke | 1 (0.8%) | 0 | 1 (0.9%) | .86 |
| Hypovolemic shock due to iliac artery perforation | 1 (0.8%) | 0 | 1 (0.9%) | .86 |
| Cardiogenic shock due to acute myocardial infarction | 3 (2.2%) | 0 | 3 (2.6%) | .65 |
| Out-of-hospital mortality > 30 days after TAVI | ||||
| Non-cardiac causes | 4 (2.98%) | 0 | 4 (3.4%) | .55 |
|
AF, atrial fibrillation; AVB, atrioventricular block; BMI, body mass index; LBBB, left bundle branch block; LVOT, left ventricular outflow tract; MS, membranous septum; NSVT, non-sustained ventricular tachycardia; RBBB, right bundle branch block; TAVI, transcatheter aortic valve implantation. |
||||
The most commonly used balloon-expandable valve was Edwards SAPIEN 3 (Edwards Lifesciences, United States), which was used in 60 patients (44.8%), while the most widely used self-expanding valve was Evolut R, which was implanted in 17 patients (12.7%) (table 1).
After TAVI, 16 patients (11.9%) developed third-degree atrioventricular block, and 12 (9%) developed persistent new left bundle branch block (table 1).
Pacemaker implantation was performed in 18/134 patients (13.4%). Of these, balloon-expandable valves were implanted in 12/82 (14.6%), while self-expanding valves were implanted in 6/52 (11.5%). There was a significant correlation between CoreValve (Medtronic, United States) and pacemaker implantation (odds ratio [OR], 5.24; 95% confidence interval [CI], 1.32-20.86; P = .029).
In our Mexican population, the mean body mass index (BMI) was 26 kg/m2, while MS length was 6.86 mm. In patients receiving a pacemaker (n = 18), MS length was significantly shorter (5.3 ± 1.2 vs 7.1 ± 1.7 mm; P < .001), with a cut-off value of 5.5 mm (P < .001) (figure 1C). On univariate analysis, the OR for the association between MS length < 5.5 mm and need for pacemaker implantation was 6.80 (95%CI, 2.36-19.58).
The MS lengths reported in the literature vary. In a Japanese population with a mean BMI of 21.7 kg/m2, the mean MS length was5.3 mm ± 1.3 mm in pacemaker carriers vs 6.6 mm in noncarriers (P = .001).3 In a North American population with a mean BMI of 28 kg/m2, MS length was 7.5 mm with measurements of 6.4 mm ± 1.7 mm in pacemaker carriers vs 7.7 mm ± 1.9 mm in patients without pacemakers (P = .001).4
Pre-existing right bundle branch block was a risk factor for high- degree atrioventricular block. Among the 13 patients with pre-existing right bundle branch block, 4 (22.2%) underwent pacemaker implantation (P = .07). The mean MS length was 7.22 mm with measurements of 5.78 mm in the 4 pacemaker carriers vs 7.86 mm in noncarriers (P = .063).
Ten patients had baseline left bundle branch block with a mean MS length of 5.85 mm. In the 2 patients who underwent pacemaker implantation, the mean length was 4.8 mm vs 6.11 mm in those without a pacemaker (P = .3).
A significant association was found between left ventricular outflow tract calcification with pacemaker implantation (OR, 2.86; 95%CI, 1.04-7.89; P = .036) and conduction disorders (OR, 2.65; 95%CI, 1.22-5.72; P = .012).
None of the 14 patients with pre-existing atrial fibrillation underwent pacemaker implantation. Mentias et al.5 reported that the rate of pacemaker implantation was significantly lower (P = .001) in patients with pre-existing atrial fibrillation (24.9%) than in those with baseline sinus rhythm (25.3%) and new-onset atrial fibrillation (28.2%).
The rate of new-onset left bundle branch block after TAVI ranges from 8% to 30% with balloon-expandable valves and from 22% to 50% with self-expanding valves such as CoreValve.2 In the present study, the rate was lower, at 9% (12/134), with 5/82 patients (6.1%) being implanted with balloon-expandable valves and 7/52 patients (13.5%) with self-expanding valves. Sammour et al.6 demonstrated that the depth of transcatheter heart valve implantation is a predictor of new left bundle branch block. A limitation of the present study is that we did not measure the depth of valve implantation. Other limitations are the small sample size drawn from a single hospital and the lack of measurement of the degree of annular overexpansion.
In conclusion, both MS length and left ventricular outflow tract calcification, assessed by multidetector computed tomography, are important predictors of the need for pacemaker implantation.
FUNDING
None.
ETHICAL CONSIDERATIONS
The article was not approved by any ethical committee because it was a retrospective study and the decision to perform the tomography was taked at the doctor’s discretion. Informed consent was not obtained from the patients for the publication of their case because they were anonymized. According to the SAGER guidelines, sex and gender variables were taken into consideration.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence tools were used.
AUTHORS’ CONTRIBUTIONS
D.I. Katekaru-Tokeshi conceived the study, conducted data collection and analysis, and drafted the manuscript. H.A. Ale-Gonzáles collected and analyzed data and contributed to manuscript drafting. P. Custodio-Sánchez analyzed the data and reviewed the manuscript. M. Jiménez-Santos interpreted the computed tomography studies and reviewed the manuscript. E. Kimura-Hayama reviewed both the manuscript and the images. F. Castillo-Castellón interpreted the computed tomography studies and critically revised the manuscript. All authors approved the manuscript final version.
CONFLICTS OF INTEREST
None.
REFERENCES
1. Muñoz-García AJ, Muñoz-García E, Alonso-Briales JH, Hernández-García JM. Trastornos de la conducción auriculoventricular tras el implante valvular aórtico transcatéter. Rev Esp Cardiol Supl. 2015;15(C):44-48.
2. Hamdan A, Guetta V, Klempfner R, et al. Inverse relationship between membranous septal length and the risk of atrioventricular block in patients undergoing transcatheter aortic valve implantation. J Am Coll Cardiol Intv. 2015;8:1218-1228.
3. Miki T, Senoo K, Ohkura T, et al. Importance of Preoperative Computed Tomography Assessment of the Membranous Septal Anatomy in Patients Undergoing Transcatheter Aortic Valve Replacement With a Balloon-Expandable Valve. Circ J. 2020;84:269-276.
4. Maeno Y, Abramowitz Y, Kawamori H, et al. A highly predictive risk model for pacemaker implantation after TAVR. JACC Cardiovasc Imaging. 2017;10:1139-1147.
5. Mentias A, Saad M, Girotra S, et al. Impact of Pre-Existing and New-Onset Atrial Fibrillation on Outcomes After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol Intv. 2019;12:2119-2129.
6. Sammour Y, Lak H, Chahine J, et al. Clinical and echocardiographic outcomes with new-onset left bundle branch block after SAPIEN-3 transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2023;101:187-196.
* Corresponding author.
E-mail address: diakatekaru@hotmail.com (D.I. Katekaru-Tokeshi).