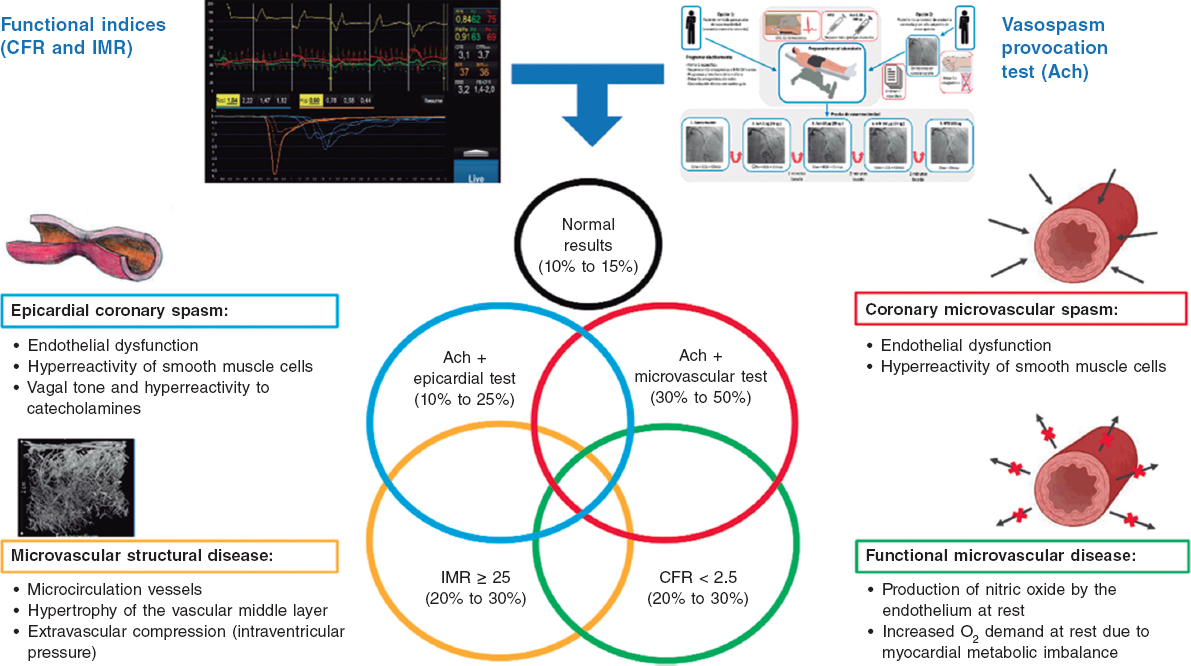
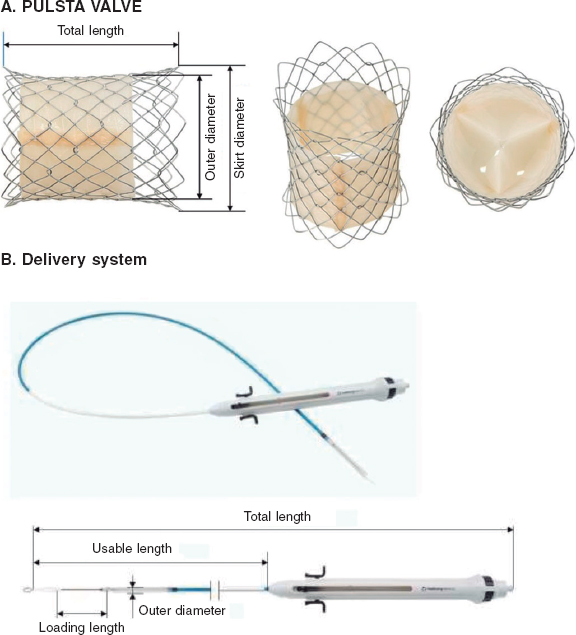
QUESTION: What types of catheter-based invasive approaches are available today to treat acute pulmonary thromboembolism? Please give us a brief description of the techniques used.
ANSWER: Catheter-directed therapy (CDT) to treat pulmonary thromboembolism falls into 3 different categories: mechanical thrombectomy, local thrombolysis, and a combination of both.
Mechanical thrombectomy (MT) consists of thrombus fragmentation, aspiration or removal. Fragmentation consists of using CDT nonspecific devices such as pig-tail catheters (forced rotation inside the thrombus) or dilatation balloons to break down and fenestrate the thrombotic occlusion to improve flow towards completely occluded regions. This technique is not very precise or reproducible so as new specific devices appear it will probably fall into oblivion. It is often used as an early facilitator of aspiration or penetration of the thrombolytic drug. Thrombus aspiration or removal consists of using hollow catheters of different calibers to aspirate the thrombus. This technique is highly dependent on the age the thrombus (more effective the more acute the case is) and the caliber of the aspiration catheter. Nonspecific material for coronary interventions (guide catheters of up to 8-Fr) or structural or peripheral heart procedures can be used (long introducers or sheaths > 8 Fr), with the advantage of its wide availability and low prices; the setback, however, is that the catheter usually becomes occluded, which is why it is often used in double ‘mother-and-child’ systems.
Also, there are 2 aspiration devices that have been specifically approved to treat PTE: the Indigo system (Penumbra, United States), a 115 cm 8-Fr angled tipped catheter with an olive-shaped burr to facilitate the entry and advancement of the thrombus, which will soon will be available in 12-Fr,1 and the FlowTriever system (Inari Medical, United States), specifically designed for PTE aspiration, and including a 24-Fr catheter, a 16-Fr guide catheter extension system, and a nitinol disc-shaped thrombus retriever. Its main advantage is that its outside connections are oversized at larger diameters compared to the Luer medical device standard.2 The Nautilus—a 10-Fr catheter system from iVascular, Spain—is currently in the pipeline. Other peripheral thrombectomy non-specific PTE devices like AngioVac (Angiodynamics, United States) or AngioJet (Boston Scientific, United States) are less common since more complications have been reported.
Compared to local thrombolysis, the advantages of MT by strong aspiration are that it can facilitate the patients’ rapid hemodynamic improvement, also ending in a single procedure that can prevent the use of fibrinolytic agents when used as monotherapy.
Local thrombolysis (LT) consists of introducing 1 or 2 usually multiperforated catheters into the pulmonary artery and in the intra-thrombus position through which 1 variable dose (around 25% of the systemic dose) of a fibrinolytic agent (often rt-PA) is introduced for a certain time (6 h to 24 h) with or without an initial bolus. Its main advantages are that it is easy to use, the possibility of using peripheral vascular access (antecubital vein or veins), and its low cost (a pig-tail catheter can be used). There is an ultrasound-facilitated LT (UFLT) device manufactured by EKOS (Boston Scientific, United States) that comes with a catheter with multiple ultrasound pulse generators to facilitate the fragmentation the fibrin threads while improving drug penetration into the thrombus.
Finally, the combination of MT1 plus LT is based on the principle that MT can act upon the most proximal segments of pulmonary and lobar branches while LT can later act upon lower caliber branches in the entire pulmonary tree.
Q.: What is the clinical evidence available on intravascular thrombolysis and thrombus aspiration therapies?
A.: Most historic evidence on CDT comes from registries and case series. However, over the last few years, the development of specific devices has produced new and high-quality pieces of evidence. There are still gaps of knowledge on the use of CDT to treat high-risk PTE (scarce and heterogeneous data), and comparative data on the different CDT techniques available.
Several registries prior to the development of specific devices like the PERFECT showed, for the very first time, better clinical outcomes with low rates of bleeding too. A meta-analysis of multiple trials on CDT conducted over the last decade provides aggregate data of 1168 patients showing overall rates of procedural success (95% confidence interval [95%CI], 72.5-89.1%), 30-day mortality (95%CI, 3.2-14.0%), and major bleeding (95%CI, 1.0-15.3%) of 81.3%, 8.0%, and 6.7%, respectively, in high-risk PTE. In intermediate-risk PTE, the rates of procedural success (95%CI, 95.3-99.1%), 30-day mortality (95%CI, 0%-0.5%), and major bleeding (95%CI, 0.3-2.8%) were 97.5%, 0% and > 1.4%, respectively.4
MT (aspiration) with the Indigo device was included in the single arm EXTRACT-PE clinical trial5 of 119 patients with intermediate-risk PTE. The efficacy (a 0.43 reduction in the right ventricle/left ventricle (RV/LV) ratio at 48 hours) and safety profile (rates of major bleeding, and intracranial hemorrhage of 1.7%, and 0%, respectively) was confirmed in a protocol with almost non-use of LT (98.3%). The FlowTriever device was used to conduct the FLARE trial6 of 106 intermediate-risk patients treated without thrombolytic drugs. The device proved its efficacy (a reduction of 0.38 in the RV/LV ratio at 48 hours) and safety profile (rates of major adverse events, major bleeding, and intracranial hemorrhage of 3.8%, 1%, and 0%, respectively). These results were confirmed in the first 250 patients of the FLASH registry7 with a rate of major adverse events of 1.2% (3 hemorrhages, none of them intracranial).
The EKOS device was studied in the ULTIMA randomized clinical trial8 of 59 patients with acute intermediate-risk PTE and a RV/LV ratio > 1 who were randomized to receive fixed doses of rt-PA (10 mg or 20 mg in bilaterals) in UFLT. It confirmed further reductions of the RV/LV ratio at 24 hours compared to standard therapy with heparin (mean reduction of 0.30 ± 0.20 vs 0.03 ± 0.16 (P < .001). The SEATTLE II registry9 of 150 patients most of them with submassive PTE demonstrated a similar efficacy profile with reasonable safety data (rates of 30-day major bleeding and intracranial hemorrhage of 10% and 0%, respectively).
The only comparative randomized clinical trial published to this date of 2 CDT strategies is the SUNSET sPE10 that found no differences in the radiologic thrombus load reduction with UFLT or simple LT with similar doses of thrombolytic drugs.
Q.: What are today’s indications for invasive treatment and how does the routine clinical practice at your center look like?
A.: In the clinical practice guidelines published by the European Society of Cardiology in 2019, CDT is given an indication IIa, level of evidence C, for patients with high-risk PTE in whom systemic thrombolysis (ST) is contraindicated or in cases when it has failed. Also, these guidelines rank CDT as an alternative to ST as a bailout therapy in patients initially treated with anticoagulation (often intermediate-high-risk PTE) with hemodynamic impairment (indication IIa, level of evidence C).
The alternative to CDT with a similar indication but a higher level of recommendation (indication I – level of evidence C) is surgical embolectomy. However, the availability of surgical teams ready to operate on these patients is very limited, as well as evidence compared to CDT.
In some centers with PTE response teams, CDT is used to treat intermediate-risk PTE (often intermediate-high-risk PTE) as coadjuvant therapy to anticoagulation. Still, there is not such a thing as a formal indication for ST. This strategy is based on studies that show that surrogate parameters like right ventricular function improve faster. However, there is no solid evidence regarding improved clinical parameters compared to anticoagulation therapy only.
Added to the indications approved in the European clinical guidelines, at our center, CDT is used in patients with an indication for LT and high risk of bleeding. Evidence shows that 30% to 50% of the patients with an indication for ST won’t receive this therapy afraid that complications will arise (basically severe or intracranial hemorrhages). Factors associated with high risk of bleeding in ST studies are active neoplasms, age ≥ 75 years, low weight (< 50 kg), acute kidney injury (glomerular filtration rate < 30) or active anticoagulation. These patients can benefit from CDT thanks to their lower risk of bleeding—particularly intracranial—based on data of indirect comparisons with ST.
Q.: Please tell us which trials are currently in the pipeline assessing these invasive strategies.
A.: Scientific societies have been developing European multicenter registries like EuroPE-CDT and NCT04037423 and, at national level, the TROMPA registry that is backed by the Interventional Cardiology Association of the Spanish Society of Cardiology.11
Several studies have already been started by device manufactures and are currently in the pipeline. Regarding the MT strategy, the Indigo device is being studied to collect efficacy, safety, and functional recovery data from 600 patients selected in the already started STRIKE-PE registry (NCT04798261). There is also an active registry currently recruiting patients to test the FlowTriever device that will include 1300 participants (FLASH, NCT03761173). Also, the upcoming PEERLESS clinical trial (NCT05111613) will recruit 550 patients with PTE and intermediate-high risk who will be randomized to receive the FlowTriever aspiration system or CDT based on the local routine clinical practice with other devices available in the market. The study primary endpoint will be death, intracranial hemorrhage, major bleeding, clinical impairment or length of stay at an intensive care unit.
The most relevant studies that are being conducted on the EKOS device are the KNOCOUT PE registry (NCT03426124)—in a phase of active recruitment—that will include up to 1500 patients, and the HI-PEITHO trial (NCT04790370). The latter is an international, prospective, multicenter clinical trial that will be comparing anticoagulation vs anticoagulation plus UFLT. The study primary endpoint is a composite of PTE-related mortality, cardiorespiratory decompensation or nonfatal PTE recurrence at 7 days. We should also mention the NCT03581877 trial (Peripheral systemic thrombolysis vs catheter-directed thrombolysis for submassive PE) that is comparing UFLT vs the same dose (24 mg) of rt-PA administered via peripheral vein for 12 hours.
Today’s challenges regarding research are to define procedural success, standardize the endpoints of clinical trials, and establish what intermediate-risk patients with PTE may benefit the most from CDT compared to standard therapy.
FUNDING
None whatsoever.
CONFLICTS OF INTEREST
None reported.
REFERENCES
1. De Gregorio MA, Guirola JA, Kuo WT, et al. Catheter-directed aspiration thrombectomy and low-dose thrombolysis for patients with acute unstable pulmonary embolism: Prospective outcomes from a PE registry. Int J Cardiol. 2019;287:106-110.
2. Salinas, P. Tromboaspiración con sistema FlowTriever en embolia pulmonar aguda. Med Clin (Barc). 2022. https://doi.org/10.1016/j.medcli.2022.02.022.
3. Kuo WT, Banerjee A, Kim PS, et al. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT): Initial Results From a Prospective Multicenter Registry. Chest. 2015;148:667-673.
4. Avgerinos ED, Saadeddin Z, Abou Ali AN, et al. A meta-analysis of outcomes of catheter-directed thrombolysis for high- and intermediate-risk pulmonary embolism. J Vasc Surg Venous Lymphat Disord. 2018;6:530-540.
5. Sista AK, Horowitz JM, Tapson VF, et al. Indigo Aspiration System for Treatment of Pulmonary Embolism. JACC: Cardiovascular Interventions. 2021;14:319-329.
6. Tu T, Toma C, Tapson VF, et al. A Prospective, Single-Arm, Multicenter Trial of Catheter-Directed Mechanical Thrombectomy for Intermediate-Risk Acute Pulmonary Embolism: The FLARE Study. JACC Cardiovasc Interv. 2019;12:859-869.
7. Toma C, Bunte MC, Cho KH, et al. Percutaneous mechanical thrombectomy in a real-world pulmonary embolism population: Interim results of the FLASH registry. Catheterization and Cardiovascular Interventions. 2022;99:1345-1355.
8. Kucher N, Boekstegers P, Müller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014;129:479-486.
9. A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism: The SEATTLE II Study - PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/26315743/. Accessed 20 Feb 2022.
10.Avgerinos ED, Jaber W, Lacomis J, et al. Randomized Trial Comparing Standard Versus Ultrasound-Assisted Thrombolysis for Submassive Pulmonary Embolism: The SUNSET sPE Trial. Cardiovascular Interventions. 2021;14(12):1364-1373.
11. Registro TROMPA | SHCI - Sección de Hemodinámica y Cardiología Intervencionista de la SEC (Sociedad Española de Cardiología). Available online: https://www.hemodinamica.com/cientifico/registros-y-trabajos/registros-y-trabajos-actuales/registro-trompa/. Accessed 20 Feb 2022.
* Corresponding author:
E-mail address: salinas.pablo@gmail.com (P. Salinas).