HOW WOULD I APPROACH IT?
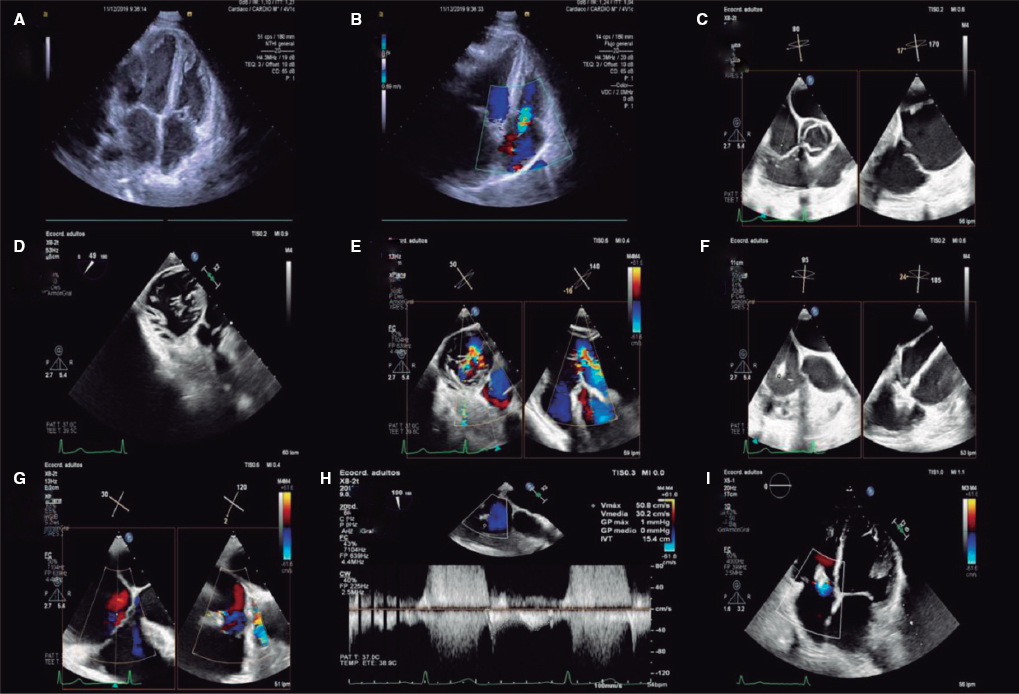
This is a a challenging case that combines severe coronary artery disease of trifurcated left main coronary artery and severe aortic stenosis in an elderly female patient with chronic kidney disease.
According to the current clinical guidelines, this patient whose score in the Society of Thoracic Surgeons score is > 10% and with good femoral accesses has an I-B indication for a transcatheter aortic valve implantation (TAVI). No other factor supports performing surgery except for the existence of coronary artery disease, which could be considered eligible for coronary artery bypass graft given the complexity of the left main lesion and the presence of good distal beds. However, what makes this patient’s surgical risk nearly unacceptable is the combination of a valve replacement procedure plus coronary bypasses, so if a better percutaneous option is available, and we believe there is, such an option should be pursued. After establishing the indication for TAVI, there is a II-A indication for percutaneous revascularization since the percent diameter stenosis is > 70% in proximal segments, the Syntax I score estimated using the data available is 27 points (29 if intense calcification is considered) and after adding clinical data tinto consideration, the Syntax II score shows a 4-year mortality rate after percutaneous coronary intervention (PCI) of 44.2% vs 33.6% after surgery. All this leaves the decision making process open since the risk involved in both strategies is high. In this case we might choose PCI plus TAVI.
Another controversial aspect is whether PCI and TAVI should be performed simultaneously or as a 2-staged procedure.
The arguments in favor of performing both procedures separately are:
One of the most important factors to consider when choosing which procedure should come first is the amount of contrast that will be used since the glomerular filtration rate (52 mL/min) and the patient’s age elevate the risk of contrast-induced nephropathy. If we follow to the current recommendations that establish a ratio < 3.7 between the amount of contrast administered and the glomerular filtration rate to reduce the risk of contrast-induced nephropathy, the maximum amount of contrast for this patient should be 192.4 cc, which may seem somehow shorthanded for the concomitant management of a trifurcated left main coronary artery and a TAVI procedure. Also, it is necessary to perform a computed tomography scan to plan the TAVI which, in turn, increases the amount of contrast that will eventually be needed. This is why a two-staged procedure seems to be the safest approach.
The time elapsed between the PCI and the TAVI (we would suggest 4-6 weeks) would allow to assess the response to the PCI after stent endothelization.
Possibility to perform an aortic valvuloplasty as bridge therapy to TAVI as long as the baseline aortic regurgitation is not significant (in this case it was described as mild in the aortography).
The arguments in favor of performing both procedures simultaneously are:
Using the secondary femoral access for the PCI with a 8-Fr catheter.
Using the guiding catheter system and the coronary guidewire for the TAVI with protected left main stem (LMS) during valve implantation.
Shorter hospital stay.
The use of contrast may be limited by using additional imaging modalities such as intravascular ultrasound (IVUS) for the management of the left main coronary artery, transesophageal ultrasound for the TAVI, fusion imaging technology such as the Heart/EchoNavigator system or ultrasound-guided femoral access. Also, pigtail catheters can be placed in every aortic sinus to find the coplanar view and guide the implant without the need for contrast.
Taking all this into consideration, and only if the patient’s clinical situation allows it, the first step would be to plan the PCI with aortic valvuloplasty and 4-6 weeks later, the TAVI procedure.
Therefore, this is how the management of the LMS should be:
•Secondary femoral access (left) of 8-Fr or 9-Fr based on the type of balloon that will be used for the valvuloplasty.
•3.5 or 4.0 8-Fr EBU guiding catheter.
•Guidewires to the anterior descending artery (DA) and to both intermediate branches.
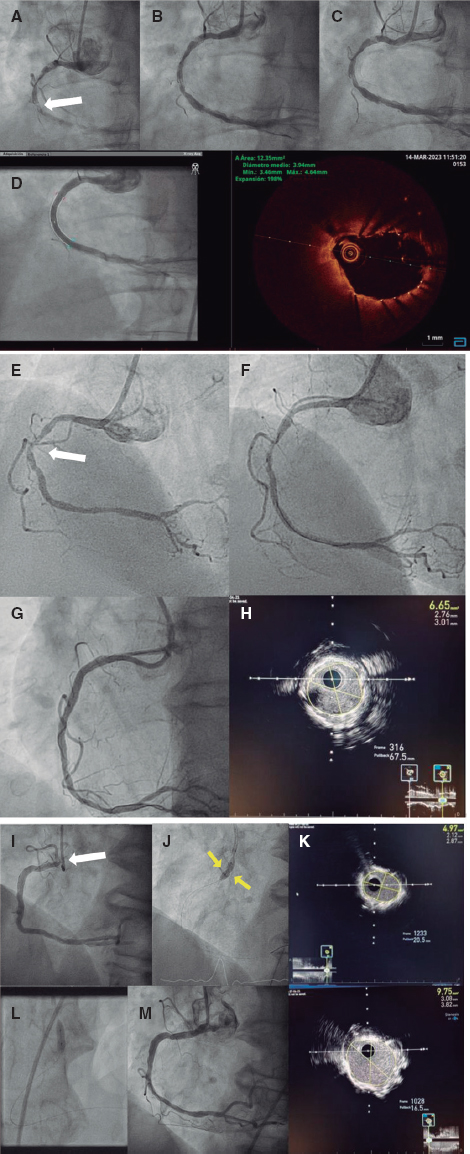
•IVUS from the LMS to the DA, intermediate and circumflex branches to assess the distal LMS and the degree of calcification in the ostium.
•In case of circumferential calcification, use rotational atherectomy: if not, predilatation with scoring or cutting balloon.
•Use the DA as the main branch and based on the IVUS findings:
–If the IVUS on the intermediate branches shows limited damage: use the provisional stenting technique, predilatation of both secondary branches, and stent implantation from the LMS to the DA using the proximal optimization technique to adapt to the disproportionate caliber. If necessary due to poor outcomes in 1 or 2 branches, we would use the T-stenting technique and protrusion or inverted T-stenting with recrossing plus final triple kissing balloon. The use of a drug-eluting balloon may be considered for accessory branches.
–If the IVUS conducted on both intermediate branches and LMS shows disease with poor predictors of good outcomes without stent implantation: use the triple-stenting technique, the double kissing crush stent technique (stent to both intermediate branches as secondary branches covering the ostium and slightly protruding into the LMS), one final stent from the LMS to the DA, recrossing towards both branches with sequential kissing-balloon plus final triple kissing balloon.
–In both options the circumflex branch is considered a secondary branch with the strategy of keeping it open but not treating it right away.
–Percutaneous closure using the Perclose ProGlide Suture Mediated Closure (Abbott Vascular, Redwood, CA, United States).
Regarding the type of valve, in this decision it is of paramount importance to take into consideration the possible need to access the LMS after the TAVI especially if we think that the rate of restenosis in trifurcations treated with 2 or more stents is usually high. The height of the LMS ostium the length and calcification of the leaflets and the width of the sinuses should all be studied by computed tomography scan prior to planning the implantation and choosing the device. The advantage of the expandable balloon device is that it is shorter preventing in many cases the jailing of the LMS; the setback is that overpacing is required for implantation purposes. Self-expandable and fully recapturable devices can be implanted without overpacing and with little contrast if using fusion imaging technology or transesophageal ultrasound; the setback is that, although these devices have wider cells, the LMS is jailed, which could make access difficult after implantation. Nevertheless, the interventional team should use the model they are most experienced with.
Corresponding author: Unidad de Hemodinámica y Cardiología Intervencionista, Hospital Regional Universitario de Málaga, Avda. de Carlos Haya 84, 29010 Málaga, Spain.
E-mail address: cristobalurbano@gmail.com (C.A. Urbano-Carrillo).