ABSTRACT
Introduction and objectives: Percutaneous closure of ventricular septal defect (VSD) can be an alternative to surgery reducing length of stay, and complications. The high risk of atrioventricular block (AVB) involved during percutaneous closure has encouraged the development of new devices such as the KONAR-MF (Lifetech, China). This device is very flexible and has a low radial force that adapts to the anatomy of the VSD without exerting any pressure to the adjacent structures. This is our early experience with this new device.
Methods: Retrospective review of patients and VSD closure procedures using the KONAR-MF device at 2 Spanish centers from February 2020—date of the first implantation in our country—through September 2021.
Results: A total of 7 closure procedures of VSD were performed being the device successfully implanted in 6 of the 7 patients. A total of 4 native perimembranous VSDs and 3 residual VSDs after tetralogy of Fallot repair were reported. The size of the VSD measured through transesophageal echocardiography and angiography was consistent in all the cases except for 1. In this patient device embolization occurred. At the follow-up [1.2 months (IQR, 0.9-15.5), (maximum 17 months)] we saw worsening atrioventricular conduction in a patient with a previous AVB who required a pacemaker. The immediate residual shunt rate was 83% (5/6) with persistent residual shunt beyond the 1-month follow-up in 1 patient (16%). All patients were discharged from the hospital within the first 48 hours following the intervention.
Conclusions: The percutaneous closure of VSD with the KONAR-MF device is a feasible alternative to surgery in selected patients. An adequate anatomical evaluation of the VSD is one of the keys of successful procedures. The implantation of this device is no stranger to complications like AVB or device embolization.
Keywords: Ventricular septal defect. Catheterizations in congenital heart disease. Ventricular septal defect. Closure devices.
RESUMEN
Introducción y objetivos: El cierre percutáneo de la comunicación interventricular (CIV) puede ser una alternativa a la cirugía y reduce el tiempo de hospitalización y las complicaciones. El alto riesgo de bloqueo auriculoventricular (BAV) en el cierre percutáneo ha incentivado el desarrollo de nuevos dispositivos, como el KONAR-MF (Lifetech, China), muy flexible y con poca fuerza radial para adaptarse a la anatomía de la CIV sin presionar las estructuras adyacentes. Se presenta la experiencia inicial con este nuevo dispositivo.
Métodos: Revisión retrospectiva de pacientes y procedimientos de implante del dispositivo KONAR-MF, en 2 centros españoles, desde febrero de 2020, fecha del primer implante en nuestro país, hasta septiembre de 2021.
Resultados: Se han realizado 7 procedimientos de cierre de CIV con KONAR-MF, implantándolo con éxito en 6 de los casos. Fueron 4 CIV perimembranosas nativas y 3 CIV residuales tras reparación de tetralogía de Fallot. El tamaño de la CIV medido por ecocardiografía transesofágica y angiografía fue concordante en todos los casos salvo en uno; en este paciente se produjo una embolización del dispositivo. En el seguimiento (1,2 meses [rango intercuartílico: 0,9-15,5], máximo 17 meses) se observó un empeoramiento de la conducción auriculoventricular en un paciente con BAV previo, que precisó marcapasos. La tasa de shunt residual inmediato fue del 83% (5/6), persistiendo el shunt residual más allá del mes de seguimiento en 1 paciente (16%). Todos los pacientes recibieron el alta hospitalaria en las primeras 48 horas tras la intervención.
Conclusiones: El cierre percutáneo de CIV con el dispositivo KONAR-MF es una alternativa factible a la cirugía en pacientes seleccionados, siendo la adecuada valoración anatómica de la CIV una de las claves para el éxito del procedimiento. El implante de este dispositivo no está exento de complicaciones, como el BAV y la embolización.
Palabras clave: Comunicación interventricular. Intervencionismo en cardiopatías congénitas. Dispositivos de cierre de comunicación interventricular.
Abbreviations
AVB: atrioventricular block. TOE: transesophageal echocardiography. VSD: ventricular septal defect.
INTRODUCTION
Ventricular septal defect (VSD) is one of the most common congenital heart diseases. Its prevalence is 5.3 cases for every 1000 live births.1 It can occur in isolation or as part of a more complex congenital heart disease. Standard therapy is surgical closure with very low morbidity and mortality rates. However, it is no stranger to complications.
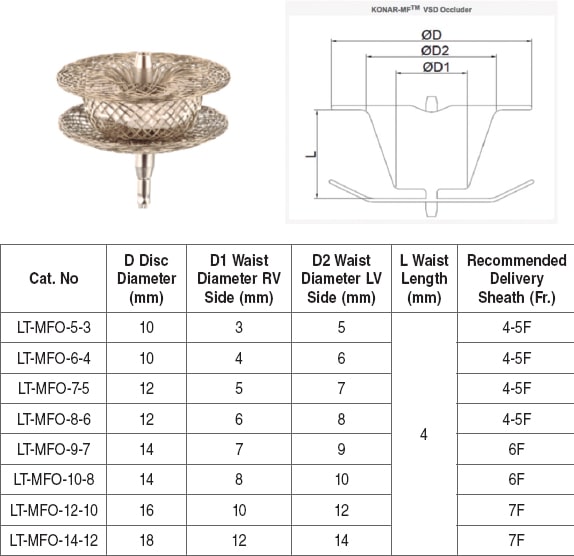
Percutaneous closure can be an alternative to surgery in selected anatomies, thus reducing the length of hospital stay, and complications. Both percutaneous and surgical closures have a potential risk of atrioventricular block (AVB)—< 2% for surgical closure, and 0.5% to 6.8% for percutaneous closure.2-5 The high risk of AVB has led to the development of new and more flexible sheaths and devices to close the VSD with less radial strength that minimize the risk of damage to the cardiac conduction system. In this context, the KONAR-MF VSD device occluder (Lifetech, China) was developed. It obtained the CE marking in Europe back in May 2018. It is a low profile, nitinol, self-expanding device with little radial strength and high flexibility in order to adapt to the anatomy of the VSD without exerting any pressure to the adjacent structures. The device is made of 2 discs united at its waist that has a polytetrafluoroethylene membrane. The right disc is simple while the left one has 1 cone attached to it similar the devices that are used to close the ductus arteriosus (figure 1). Each disc has a screw so it can be anchored to the delivery system in such a way that it can be implanted via antegrade (venous) and retrograde (arterial) access. The device comes in several sizes from 5 mm to 14 mm. It is suitable for different VSDs of different sizes, and anatomies (figure 1). The specific sheaths of the delivery system—5-Fr to 7-Fr—are also very flexible, which reduces pressure to the cardiac conduction system during the device implantation maneuvers. Also, it can be implanted through a 7-Fr or 8-Fr guide catheter.
Figure 1. KONAR-MF device (Lifetech, China) with the table of measures available. Data from the device instructions for use.
This is the early experience of 2 Spanish centers using this new device for the closure of VSD.
METHODS
Retrospective review of patients treated with the VSD KONAR-MF occluder device at 2 Spanish centers: Hospital Universitario Ramón y Cajal, Madrid, and Hospital Universitario La Fe, Valencia from February 2020—date of the first implantation procedure in our country—through September 2021. Patients were selected if they had suitable anatomies for percutaneous closure, that is, proper distance to the aortic valve (> 2 mm), lack of posterior prolongation (enough distance to the tricuspid valve), and proportionate size of the devices available. Since this was a short retrospective review, no control group was included.
The patients’ demographic, clinical, and anthropometric data were collected, as well as the echocardiographic anatomy of the defect, the hemodynamic variables of the procedure, and the immediate complications or at the follow-up.
Definitions
Residual shunt was defined as the presence of flow on the color Doppler echocardiography around the device. Flow was categorized into mild (1 mm to 2 mm), moderate (2 mm to 4 mm), or severe (> 4 mm). The presence of flow inside the device was called intradevice shunt and was considered less significant compared to mild shunt.
Complications were categorized as minor or major:
-
– Major complications: death, potentially fatal adverse events, events requiring surgery (embolization, myocardial perforation, vascular rupture, severe residual shunt, severe hemolysis, valvular damage, persistent AVB).
-
– Minor complications: complications that solve spontaneously or with medical therapy and don’t have fatal outcomes (issues with vascular access, mild hemolysis solved with medical therapy, complete transient AVB or other conduction abnormalities that do not require pacemaker implantation, fever, neurapraxias, etc.)
Device implantation was considered successful in the absence of major complications, and severe residual shunt within the next 24 hours.
Description of the procedure
Previous diagnostic cardiac catheterization, and transesophageal echocardiography (TEE) were performed in all the patients. The patients referred for closure had hemodynamic repercussions due to VSD (left ventricular dilatation). Also, the presence of a Qp/Qs ratio ≥ 1.5 was confirmed through a cardiac catheterization performed under general anesthesia while the patient remained intubated.
The size of the device was determined based on the measures of VSD obtained on the TEE, and left ventriculography. The device was 1 mm to 3 mm larger than the defect (figure 2).
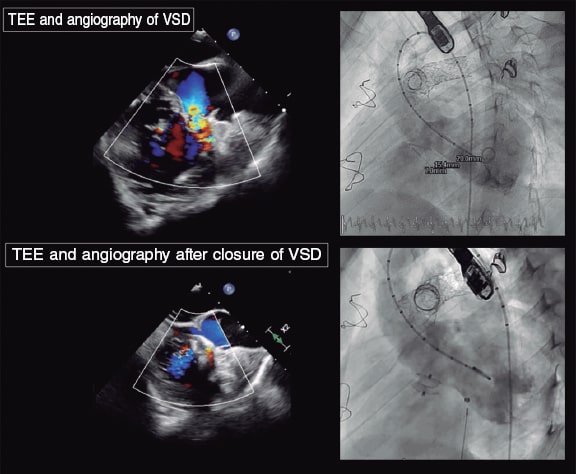
Figure 2. Ventricular septal defect (VSD) in a patch in a patient with tetralogy of Fallot. The upper images reveal the presence of the defect both on the transesophageal echocardiography (TEE), and the angiography. The lower images—also from TEE and angiography—reveal the defect being closed after KONAR-MF device implantation (Lifetech, China).
The VSD probing technique, and the device positioning and delivery are not substantially different compared to those used in other device occluders widely discussed in the medical literature.6-9 The interventional procedure was performed under TEE guidance, and the device was released after being properly deployed without severe residual shunt.
Follow-up after closure of ventricular septal defect
Follow-up visits were conducted 1 month, 6 months, and 1 year after closure. After that time, depending on the patient’s baseline condition and clinical situation, follow-up was conducted every 6 or 12 months. Anamnesis, physical examination, electrocardiogram, and echocardiography were performed in these visits. Blood tests were also added to the mix in cases of suspected hemolysis. In the presence of any other symptoms or pathological findings in any of the tests performed, additional studies were conducted like Holter, ergometry or further imaging modalities.
Ethical aspects
In compliance with the current legislation, and since this was a retrospective case review it was not necessary to obtain the patients’ informed consent or approval by the ethics committees of the participant centers.
RESULTS
From February 2020 through June 2021, a total of 7 consecutive procedures of VSD closure were performed at the 2 centers using the KONAR-MF device by successfully implanting this device in 6 out of the 7 patients. Table 1 shows the overall description of the patients. Cases were restrictive defects (native or postoperative) with echocardiographic data of hemodynamic repercussion (left ventricular dilatation) without clinical translation in patients > 8 years.
Table 1. General description of patients treated with percutaneous closure of VSD
| Patient | Sex | Age (years) | Weight (kg) | Qp/Qs ratio | Anatomy | Size of VSD on the TEE (LV/RD) | Size of VSD on the angiography (LV/RV) | Device | X-ray imaging time (min) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 8 | 29.3 | 1.53 | PM | 6/4 | 6/5 | 7/5 | 21.2 |
| 2 | F | 14 | 57.2 | 1.71 | PM | ND | 8/4.5 | 8/6 | 50.4 |
| 3 | M | 19 | 59 | 1.5 | PR | 10/7 | 11/8 | 12/10 | 27 |
| 4 | F | 26 | 64 | 2.08 | PR | 10/7 | 11/8 | 12/10 | 42.3 |
| 5 | M | 9 | 23 | 1.58 | PM | 8/5 | 4/2 | 6/4 | 143 |
| 6 | M | 16 | 54 | 2.25 | PR | 9/8 | 11/8 | 12/10 | 25 |
| 7 | M | 13 | 51.2 | 1.66 | PM | 7/4 | 7/5 | 8/6 | 22 |
|
F, feminine; LV, left ventricle; M, masculine; NA, not available; PM, perimembranous; PR, postoperative residual; Qp, pulmonary cardiac output; Qs, systemic cardiac output; RV, right ventricle; TEE, transesophageal echocardiography; VSD, ventricular septal defect. |
|||||||||
The anatomy of VSD was:
-
– Native perimembranonus VSD in 4 patients (2 with aneurysmal tissue that partially closed the VSD) without associated disease in 3 patients while the fourth had been treated of coarctation of aorta.
-
– Residual VSD residual after repair of tetralogy of Fallot in 3 patients.
The size of the VSD measured on the TEE and angiography was consistent in all the cases except for 1 with a small VSD covered by an aneurysm.
In all the patients, vascular approach was attempted via femoral access (artery and vein); in 6 of them closure was performed via antegrade access, and in 1 patient via retrograde access. Retrograde access was attempted in 1 patient in whom the proper positioning of the right disc could not fully achieved. Finally, closure was successfully completed from the right ventricle, but with longer x-ray image and procedural times. The median x-ray image time was 27 minutes [IQR, 22-50].
There were no immediate complications in any of the cases reported except for 1 embolization in a small VSD with aneurysmal tissue (case #5) where the size of the VSD measured on the TEE and the angiography did not properly correlate. The device embolized to the left pulmonary artery and was retrieved percutaneously through a bailout procedure. The patient was treated with VSD elective surgery a few months later. No hemolysis or vascular complications were reported in the series with a maximum follow-up time of 17 months (median follow-up, 1.2 months; IQR, 0.9-15.5).
Immediate residual shunt was seen in 5 out of the 6 successfully closed VSDs. Two of the patients showed mild intradevice shunt that closed spontaneously within the first 24 hours; in another 2 patients the shunt disappeared 1 month after the procedure, and in the fifth case moderate residual shunt persisted 1 month after the procedure.
All patients were discharged from the hospital within the first 48 hours after the procedure. Three out of the 7 patients were already on acetylsalicylic acid due to their underlying condition while in the remaining 3 with successful closures, treatment with acetylsalicylic acid was started before discharge. Antibiotic prophylaxis was advised for 6 months after closing the residual shunt.
Regarding the clinical course, in 5 out of the 6 patients with successful device implantation and without previous ECG alterations, no conduction abnormalities were seen after closing the VSD. However, 1 case of progression into long-term preexisting postoperative AVB was reported that required pacemaker implantation. This was the case of a patient with repaired tetralogy of Fallot (case #3) who—before the percutaneous closure of the VSD—had advanced AVB of several years of evolution without an indication for pacemaker implantation. Fourteen months after the procedure, the patient required percutaneous pacemaker implantation because data on atrioventricular conduction worsened in Holter, ergometry, and electrophysiological studies.
DISCUSSION
This is a small and heterogeneous series of occluded VSDs with the KONAR-MF device with a short follow-up too. However, we wanted to share our case since this was the first experience in our country using a device that has joined the therapeutic arsenal of occluder devices available for the percutaneous treatment of VSD. Using this device was technically easy and reproducible from the interventional cardiology standpoint. Also, the echocardiographic visualization of the device was rather good from the imaging standpoint (figure 2).
The percutaneous occlusion of VSD with the KONAR-MF device is feasible and effective with complete closure of VSD rates 1 month after implantation of up to 98%,9-12 which has been associated with the possibility of oversizing the device vs the VSD without damaging any adjacent structures given its flexibility.9-12 In our series of a single patient with residual shunt vs 5 patients without it, the rate of occlusion was 83% 1 month after implantation.
Compared to other devices, the advantages attributed to this device are its flexibility and adaptability to the patient’s anatomy, both favorable to minimize complications and increase the efficacy of occlusion. Also, other advantages are the possibility of implanting this device from the aortic side shortening procedural time.
Although, to this date, literature is scarce and only limited to early series of cases, the experience is growing, particularly in Asia.9-14 It has been used in a wide array of clinical scenarios and patients including breastfed babies13 proving effective and safe overall. However, as it occurs with all invasive procedures, it is not stranger to major complications being embolization the most common of all.10,11
The rates of success and major complications (embolization, AVB, and hemolysis) reported with this device are similar—or somehow lower—compared to those reported with other VSD closure devices.11,15 However, the rates of immediate closure are higher compared to those reported with other devices, which would—theoretically speaking—minimize the risk of complications like hemolysis or endocarditis.11,15
Our results are consistent with the series published to this date without cases of hemolysis being reported. The serious complications reported were 1 embolization, and 1 AVB at the follow-up. In our series, embolization was attributed to the fact that a small device was selected as a consequence of the mismatch reported between the VSD size measured on the TEE and on the angiography. The presence of aneurysmal tissue when trying to measure the defect properly was seen as a setback. Future cases should examine the TEE-angiography correlation when measuring the size of VSD.
The medical literature reports 2 cases of permanent AVB (another transient AVB was reported during the procedure contraindicating implantation13): 1 early AVB in the series of Tanidir et al.10 of 98 patients—a rate of AVB of 1%—plus another case deferred for a week14 that made Leong et al.14 review the rate of AVB described in the medical literature with what they referred to as «new» devices. In our sample no cases of rhythm disorders were reported after the procedure was performed in 5 out of 6 cases. However, it is relevant that in a patient with previous advanced AVB, disease progression was reported, which led to pacemaker implantation after closing the defect. Since this patient had tetralogy of Fallot, the device was implanted in a patch without prior direct compression on the cardiac conduction system. Also, this patient had a hemodynamic disorder with right ventricular overload due to acute respiratory failure, and significant stenosis of pulmonary arteries. Given this baseline situation, the worsening AVB cannot be fully attributed to the device although it cannot be discarded either. In any case, the previous presence of conduction abnormalities should be a warning of possible worsening after percutaneous closure of a VSD.
The results of our series should be interpreted in the context of its own limitations (small number of patients and short follow-up period). Although both the versatility of the device and the successful outcomes are encouraging, the presence of serious complications requires a careful approach. Therefore, larger studies with more cases and longer mid-term follow-ups are required to confirm the device safety profile.
CONCLUSIONS
The percutaneous closure of the VSD with the KONAR-MF device emerges as a proper alternative to occlusion with other devices. Also, it is a feasible alternative to surgery for some patients. Also, it stands as an effective occlusion technique in selected defects being the right anatomical assessment of the VSD one of the keys for success. The rates of complete closure and complications of this early sample should improve with more cases, experience, and longer follow-ups. As it occurs with other devices, implanting this device is associated with complications like AVB, and embolization.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
M. Álvarez-Fuente collected the patients’ data and drafted the manuscript. J.I. Carrasco collected the patients’ data and was involved in the review process of the manuscript. B. Insa drafted the manuscript. M. Toledano was involved in the review process of manuscript. E. Peiró participated in the review process of the manuscript. J.P. Sandoval participated as an advisor in the process of drafting the manuscript, as well as the manuscript final review process. M.J. del Cerro drafted the manuscript.
CONFLICTS OF INTEREST
None reported.
WHAT IS KNOWN ABOUT THE TOPIC?
- Currently, the percutaneous closure of VSD is starting to become routine in PCI-capable centers specialized in congenital heart disease. However, this technique still cannot be compared to or even replace surgery. Numerous devices for the closure of VSD have been developed. However, not a single one has been found to perform this procedure with enough efficacy and safety.
WHAT DOES THIS STUDY ADD?
- This is the early experience using a new device to close VSDs with results that are promising enough to think that the interventional procedures performed with it are a reliable alternative to the surgical closure of VSD.
REFERENCES
1. Lindinger A, Schwedler G, Hense HW. Prevalence of congenital heart defects in newborns in Germany:results of the first registration year of the PAN Study (July 2006 to June 2007). Klin Padiatr. 2010;222:321-326.
2. Zhao LJ, Han B, Zhang JJ, et al. Postprocedural outcomes and risk factors for arrhythmias following transcatheter closure of congenital perimembranous ventricular septal defect:a single-center retrospective study. Chin Med J (Engl). 2017;130:516-521.
3. Ergün S, GençSB, Yildiz O, et al. Risk factors for major adverse events after surgical closure of ventricular septal defect in patients less than 1 year of age:a single-center retrospective. Braz J Cardiovasc Surg. 2019;34:335-343.
4. Saurav A, Kaushik M, Mahesh Alla V, et al. Comparison of percutaneous device closure versus surgical closure of peri-membranous ventricular septal defects:a systematic review and metaanalysis. Catheter Cardiovasc Interv. 2015;86:1048-1056.
5. Haas NA, Kock L, Bertram H, et al. Interventional VSD-Closure with the Nit-Occlud((R)) Le VSD-Coil in 110 patients:early and midterm results of the EUREVECO-Registry. Pediatr Cardiol. 2017;38:215-227.
6. Huang X-S, Luo Z-R, Chen Q, et al. A Comparative Study of Perventricular and Percutaneous Device Closure Treatments for Isolated Ventricular Septal Defect:A Chinese Single-Institution Experience. Braz J Cardiovasc Surg. 2019;34:344-351.
7. Nguyen HL, Phan QT, Doan DD, et al. Percutaneous closure of perimembranous ventricular septal defect using patent ductus arteriosus occluders. PLoS One. 2018;13:e0206535.
8. Solana-Gracia R, Mendoza Soto A, Carrasco Moreno JI, et al. Spanish registry of percutaneous VSD closure with NitOcclud Le VSD Coil device:lessons learned after more than a hundred implants. Rev Esp Cardiol. 2021;74:591-601.
9. Haddad RN, Daou LS, Saliba ZS. Percutaneous closure of restrictive-type perimembranous ventricular septal defect using the new KONAR multifunctional occluder:Midterm outcomes of the first Middle-Eastern experience. Catheter Cardiovasc Interv. 2020;1;96:E295-E302.
10. Tanidir IC, Baspinar O, Saygi M, et al. Use of Lifetech™KONAR-MF, a device for both perimembranous and muscular ventricular septal defects:A multicentre study. Int J Cardiol. 2020;1;310:43-50.
11. Sadiq M, Qureshi AU, Younas M, et al. Percutaneous closure of ventricular septal defect using LifeTechTM KONAR-MF VSD Occluder:initial and short-term multi-institutional results. Cardiol Young. 2021;28:1-7.
12. Schubert S, Kelm M, Koneti NR, et al. First European experience of percutaneous closure of ventricular septal defects using a new CE-marked VSD occluder. EuroIntervention. 2019;12;15:e242-e243.
13. Damsky-Barbosa J, Alonso J, Ferrín L, et al. Endovascular VSD Closure with Lifetech KONAR-Multifunctional Occluder - Novel Device. J Struct Heart Dis. 2019;5:237-247.
14. Leong MC, Alwi M. Complete atrio-ventricular heart block, a not to be forgotten complication in transcatheter closure of perimembranous ventricular septal defect –a case report and review of literature. Cardiol Young. 2021;31:2031-2034.
15. Santhanam H, Yang L, Chen Z, et al. A meta-analysis of transcatheter device closure of perimembranous ventricular septal defect. Int J Cardiol. 2018;254:75-83.