ABSTRACT
Introduction and objectives: Transcatheter aortic valve implantation (TAVI) was first introduced in 2007 as an alternative to open heart surgery to treat patients with severe symptomatic aortic stenosis (sSAS) with various indication expansions since that date. Recently, the PARTNER 3 study (Placement of aortic transcatheter valve) demonstrated clinical benefits with TAVI with the SAPIEN 3 valve vs surgical aortic valve replacement (SAVR) in selected low surgical mortality risk patients. We reviewed data from the PARTNER 3 and economic data from Spain to assess the cost-effectiveness ratio of TAVI vs SAVR in patients with sSAS and low surgical mortality risk.
Methods: A 2-stage model was used to estimate direct healthcare costs and health-related quality of life data regarding TAVI with the SAPIEN 3 valve and SAVR. Early adverse events associated with TAVI from the PARTNER 3 were fed into a Markov model that captured longer-term outcomes after TAVI or SAVR.
Results: TAVI with SAPIEN 3 improved quality-adjusted life years per patient (+ 1.00) with an increase in costs vs SAVR (€6971 per patient). This meant an incremental cost-effectiveness ratio/quality-adjusted life year of €6952 per patient. The results were robust with TAVI with the SAPIEN 3 valve remaining cost-effective across several sensitivity analyses.
Conclusions: TAVI with the SAPIEN 3 valve is cost effective compared to SAVR in patients with sSAS and low surgical mortality risk. These findings can inform policymakers to facilitate policy development in Spain on intervention selection in this patient population.
Keywords: Spain. Transcatheter aortic valve replacement. Heart procedures and surgeries. Prosthetic heart valve. Surgical aortic valve replacement. Cost-benefit analysis. Aortic stenosis. Low-risk.
RESUMEN
Introducción y objetivos: El implante percutáneo de válvula aórtica (TAVI) se introdujo en 2007 como una alternativa a la cirugía a corazón abierto para tratar a pacientes con estenosis aórtica grave sintomática, y desde entonces han aumentado las indicaciones autorizadas. Recientemente, el Placement of Aortic Transcatheter Valve Study (PARTNER) 3 ha demostrado beneficios clínicos con el TAVI con la válvula SAPIEN 3 frente al reemplazo quirúrgico de válvula aórtica (RVAo) en pacientes seleccionados con bajo riesgo de mortalidad quirúrgica. Utilizando los datos del PARTNER 3 junto con datos económicos de España, se evaluó la relación coste-efectividad del TAVI en comparación con el RVAo en pacientes con estenosis aórtica grave sintomática con bajo riesgo de mortalidad quirúrgica.
Métodos: Se utilizó un modelo en dos etapas para estimar los costes directos sanitarios y los datos de calidad de vida relacionados con la salud para TAVI con la válvula SAPIEN 3 y RVAo. Los eventos adversos tempranos relacionados con TAVI del PARTNER 3 se incluyeron en un modelo de Markov, que capturó los resultados a más largo plazo tras TAVI o RVAo.
Resultados: El TAVI con SAPIEN 3 mejoró los años de vida ajustados por calidad por paciente (+1,00), con un aumento en el coste frente al RVAo de 6.971 € por paciente. Esto representó una ratio coste-efectividad incremental por año de vida ganado ajustado por calidad de 6.952€ por paciente. Los resultados fueron robustos en los diversos análisis de sensibilidad realizados, en los que el TAVI con SAPIEN 3 se mantiene como una opción coste-efectiva.
Conclusiones: El TAVI con SAPIEN 3 es coste-efectivo en comparación con el RVAo en pacientes con estenosis aórtica grave sintomática con bajo riesgo de mortalidad quirúrgica. Estos resultados pueden informar a los decisores políticos en España para facilitar el desarrollo de políticas sobre la selección de opciones terapéuticas en esta población de pacientes.
Palabras clave: España. Implante percutáneo de válvula aórtica. Cirugía cardiaca. Prótesis valvular cardiaca. Reemplazo quirúrgico de válvula aórtica. Análisis coste-beneficio. Análisis coste-efectividad. Estenosis aórtica. Bajo riesgo.
Abbreviations
ICER: incremental cost-effectiveness ratio. QALYs: quality-adjusted life years. SAVR: surgical aortic valve replacement. sSAS: severe symptomatic aortic stenosis. TAVI: transcatheter aortic valve implantation.
INTRODUCTION
Aortic stenosis affects nearly 3% of adults aged > 65 years.1 It often has an initial asymptomatic latent period, but as the disease becomes worse, signs of heart failure, angina, or syncope become evident.1,2 Aortic valve replacement is recommended for most symptomatic patients with echocardiographic evidence of significant aortic stenosis as well as for some asymptomatic patients.1,2
Since the first transcatheter aortic valve implantation (TAVI) was used as a treatment option for severe symptomatic aortic stenosis (sSAS) almost 20 years ago, clinical trial evidence has further increased and continued to validate its use.3 In 2013, TAVI became the treatment of choice for inoperable patients with sSAS, and high surgical mortality risk patients. More recently, this treatment choice has expanded to include patients of intermediate/low surgical mortality risk.4,5
The very recent Placement of aortic transcatheter valve study (PARTNER 3) is among the growing body of robust clinical trial evidence. This is a pivotal, multicenter, randomized, and controlled study in patients with sSAS of low surgical mortality risk.6,7 In PARTNER 3, treatment outcomes with surgical aortic valve replacement (SAVR) were compared to TAVI with the SAPIEN 3 transcatheter heart valve via transfemoral access.6,7 Compared to SAVR, TAVI with the SAPIEN 3 valve reduced the composite endpoint of death, stroke or rehospitalization after 1 and 2 years.6,7 In view of these positive clinical developments, the European Society of Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS) guidelines now recommend SAVR in younger, low-risk patients, while TAVI is now the treatment of choice in older patients. Also, it can be considered in all other patients with sSAS following careful evaluation of individual clinical, anatomical, and procedural characteristics by the heart team.5
There are no treatment guidelines specific to Spain describing the use of TAVI, but the Spanish Society of Cardiology, as a member of the ESC, endorses the ESC guidelines, and healthcare professionals in Spain follow these ESC guidelines.5 Irrespective of these guidelines, TAVI adoption in Spain remains low compared to other European countries. Despite a higher level of infrastructure available,8 defined as the number of TAVI centres available per million population, there is still significant variability among regions regarding TAVI implantation rates in Spain.9 In 2021, nearly 5000 patients benefited from this transformative minimally invasive technology in Spain. In a recent publication,10 the annual number of TAVI candidates for Spain was estimated at 15 783 patients including low-risk patients. Considering this together with the increasingly evident clinical benefits of TAVI in patients with sSAS, it is important to evaluate the cost-effectiveness ratio of using TAVI vs SAVR for the low surgical risk sSAS patient group for whom TAVI is now advised in recent guidelines.5 Furthermore, compared to SAVR, transfemoral TAVI with the SAPIEN 3 valve has proven cost-effective in the high-and-intermediate-risk population in Spain.11 This further accentuates the need for evidence on the cost-effectiveness ratio of TAVI with the SAPIEN 3 valve in the low surgical risk population of patients with sSAS in Spain. Therefore, the objective of this article is to review the PARTNER 3 data and the economic data from Spain to assess the cost-effectiveness ratio of using TAVI vs SAVR in patients with sSAS and low surgical mortality risk.
METHODS
A cost-utility analysis was developed using methodology validated for the French12 and Italian13 population to estimate changes in both direct healthcare costs and health-related quality of life with the use of TAVI with the SAPIEN 3 valve compared to SAVR in patients with sSAS and low surgical mortality risk (Society of Thoracic Surgeons < 4%) from the perspective of the Spanish National Health System. Costs were measured in 2021 euros and benefits in quality-adjusted life years (QALYs) gained. The incremental cost-effectiveness ratio (ICER) was calculated by dividing the difference in costs between the 2 treatment groups by the difference in QALYs. Consistent with previous studies,11,14 an incremental cost-effectiveness ratio of < €30 000 per QALY gained was used as the willingness-to-pay (WTP) threshold of acceptable cost-effectiveness.
Model structure
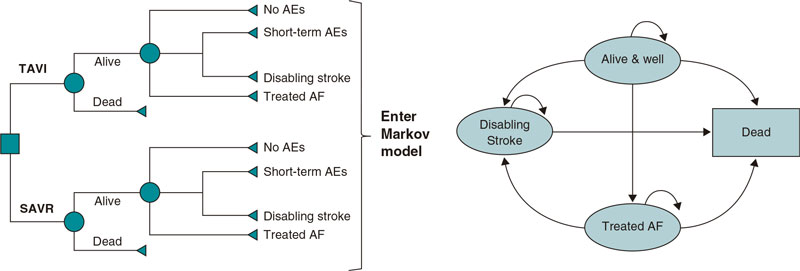
Details of the 2-stage model structure have been previously described for the French population.12 In brief, early adverse events associated with TAVI were first captured using the 30-day early adverse events dataset from the PARTNER 3 study6 in a decision tree (figure 1A); subsequently, these data were fed into a Markov model that included 4 distinct health states (‘alive and well’, ‘treated atrial fibrillation [AF]’, ‘disabling stroke’, and ‘dead’) to capture longer-term outcomes of patients after TAVI or SAVR (figure 1B). The model was considered appropriate for the Spanish setting by all authors based on their clinical and health-economic expertise.
Figure 1. Central illustration. The cost-effectiveness model had 2 stages: a) early AEs from the PARTNER 3 trial were captured in a decision tree, which fed into b) a Markov model that captured longer-term outcomes of patients categorized into 4 different health states: ‘Alive and well’= patients who have undergone the procedure and survived with only short-term or no AEs; patients in this health state can transition to ‘disabling stroke’, ‘AF’ or ‘dead’ at any time during the model timespan. ‘Treated AF’= patients who have undergone the procedure and survived, but developed AF requiring specific treatment; this can either occur within the first 30 days or during the rest of the model timespan, and patients in this health state can transition to ‘disabling stroke’ or ‘dead’ at any time during the model timespan. ‘Disabling stroke’ = patients who have undergone the procedure and survived, but had a disabling stroke; this can either occur within the first 30 days or during the rest of the timespan of the model, and patients in this health state can only transition into the ‘dead’ state at any time during the model timespan. ‘Dead’ = this is the absorbing state in the model: all patients in the model are at risk of dying due to general all-cause mortality; patients with treated AF and stroke are at an increased risk of dying.
Reproduced from Gilard M, et al. Value Health 202112 under the terms of the creative commons licence.44 AE, adverse event; AF, atrial fibrillation; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Given that sSAS requires life-long valve replacement, a lifetime timespan of 50 years was selected for the cost-utility analysis with a discounting factor per year of 3% applied for both future costs and benefits following the recommendations set for Spain.15 This timespan was chosen to reflect all potential consequences to individuals with sSAS over their lifetime. Healthcare costs and health-related quality of life was measured using QALYs.
Model inputs
Study overview
The model was informed by the PARTNER 3 study population, which excluded patients with clinical frailty, bicuspid aortic valves or other anatomical features that increased the risk of complications associated with either surgery or TAVI. In the PARTNER 3, 1000 patients were enrolled, 503 of whom were randomized to TAVI and 497 to SAVR, with ‘as treated’ groups of 496 and 454 patients, respectively.6 The primary endpoint was a composite of all-cause mortality, stroke or rehospitalization 1 year after the procedure.
Clinical events
Probabilities of clinical events used in the model (table 1 of the supplementary data) were based on a decision tree that captured all early adverse events experienced up to 30 days after the procedure as reported in the PARTNER 3. Monthly transition probabilities among the Markov model health states were estimated. Regarding the transition from ‘alive and well’ to ‘treated AF’, data from the PARTNER 3 on new-onset treated AF between 30 days and 1 year were used.6 Other literature sources provided a more realistic estimate of the remaining 2 transitions due to the scarcity of these events reported in the PARTNER 3. Burden of stroke data in Spain (Stroke Alliance for Europe)16 were used for the transition from ‘alive and well’ to ‘disabling stroke’, and data from a systematic review/meta-analysis involving 104 eligible cohort studies were used for the transition from ‘treated AF’ to ‘disabling stroke’.17 Myocardial infarction, transient ischemic attack, and severe or life-threatening bleeding were captured as intercurrent events between 30 days and 1 year from PARTNER 3 data.6 Other relevant events like rehospitalization rates using data from the PARTNER 3,6 and reintervention rates due to valve deterioration (data up to 2 years from the PARTNER 3)6,7 and from 3 years onwards from a study on 20-year outcomes of pericardial aortic tissue valve bioprosthesis18 were also considered (table 1 of the supplementary data). In the base case, the same reintervention rate was used for both the TAVI and SAVR arms; this simplifying assumption allowed better use of the available data. In scenario #1, higher reintervention rates were assumed for TAVI with the SAPIEN 3 valve compared to SAVR based on data from the PARTNER 2 at 5 years19 while in scenario #2, an increased risk of stroke was assumed, which was consistent with the PARTNER 3 outcomes.
Table 1. Base case results with acute and lifetime costs
| Summary results | TAVI with SAPIEN 3 | SAVR | Incremental |
|---|---|---|---|
| Cost per patient | € 39 052 | € 32 081 | € 6971 |
| Life year gained (undiscounted) | 14.08 | 13.22 | 0.86 |
| Median survival (years) | 16.50 | 14.50 | 2.00 |
| QALYs per patient | 8.66 | 7.66 | 1.00 |
| Incremental cost effectiveness ratio (ICER) | € 6952 | ||
| Incremental net monetary benefit (NMB) | € 23 111 | ||
| Incremental net health benefit (NHB) | 0.77 | ||
| Acute phase cost (first hospitalization and rehabilitation) | |||
| Index hospitalization | € 24 781 | € 13 779 | € 11 003 |
| Rehabilitation | € 114 | € 461 | -€ 347 |
| Pacemaker implantation | € 506 | € 311 | € 195 |
| Acute phase costs | € 25 401 | € 14 550 | € 10 656 |
| Additional costs at 1 year | |||
| MI | € 181 | € 92 | € 89 |
| Pacemaker implantation complication costs | € 38 | € 23 | € 15 |
| Hospitalization costs | € 212 | € 316 | -€ 104 |
| Reintervention costs | € 117 | € 147 | -€ 30 |
| Alive and well health state costs | € 1 258 | € 844 | € 415 |
| Treated AF health state costs | € 48 | € 376 | -€ 328 |
| Disabling stroke costs | € 11 | € 221 | -€ 210 |
| Death costs | € 0 | € 0 | € 0 |
| Overall cost at 1 year | € 27 267 | € 16 570 | € 10 698 |
| Additional lifetime costs | |||
| Pacemaker implantation complication costs | € 433 | € 251 | € 182 |
| Hospitalization costs | € 374 | € 353 | € 21 |
| Reintervention costs | € 4464 | € 4941 | -€ 477 |
| Alive & well health state costs | € 4120 | € 2590 | € 1530 |
| Treated AF health state costs | € 970 | € 3963 | -€ 2993 |
| Disabling stroke costs | € 1424 | € 3414 | -€ 1990 |
| Additional lifetime costs | € 11 785 | € 15 512 | -€ 3727 |
| Total lifetime costs | € 39 052 | € 32 081 | € 6971 |
|
AF, atrial fibrillation; MI, myocardial infarction; QALY, quality-adjusted life-year. |
|||
Survival extrapolation
There were 2 options regarding survival extrapolation. In option #1, transition probabilities were taken from the literature (relative risk of death with AF of 1.517; and relative risk of death with disabling stroke of 2.0520). In option #2, parametric survival fitting was performed based on Kaplan-Meier data from the PARTNER 3. A total of 3 parametric distributions were used (Weibull, Exponential, Gompertz) and adjusted to the survival of the overall Spanish population. Therefore, in the base case, survival estimates were based on transition probabilities due to immaturity of survival data from the trial. Annual mortality risk for ‘alive and well’, and other relative risks for other health states are shown on table 2 of the supplementary data. Option #2 (parametric survival analysis) was explored using alternative hazard ratios (HR) in scenario #3: HR, 0.75 from the PARTNER 3 at 2 years adjusted to Spanish population overall mortality. An additional scenario #4 removed any survival benefit with the SAPIEN 3 valve (HR, 1).
Health utilities
There were 2 options for determining utility decrement: option #1 used utility decrements by health state from the literature adjusted by age and Spanish population standards.21 This was the preferred option because there were very few corresponding events in the PARTNER 3, and estimates from the literature were deemed realistic. The age and population standards adjusted utility decrements were 0.16 for AF22 and 0.42 for disabling stroke.23 Option #2 used treatment options from the PARTNER 3 and was explored within scenario #5. The utility decrement for option #2 was individually extracted from the PARTNER 3 at baseline, after 30 days, 6 months and 1 year, and then converted to Spanish health utilities.24
Cost inputs
Costs associated with TAVI and SAVR (procedure, complications, and long-term) are shown on table 3 of the supplementary data. Base case procedure cost information was drawn from the SERGAS.25 We should mention that the SERGAS fee includes market valve and ancillary material price. Also, personnel costs were additionally estimated on a per hour price basis for different professionals. Costs corresponding to complications and health states were drawn from the literature and diagnosis-related groups (DRG). The breakdown of TAVI and SAVR procedure costs are shown on table 4 of the supplementary data. The micro-cost elements are informed from the study conducted by Bayón et al.26 and updated to reflect current TAVI practice in Spanish low-risk patients with sSAS. As costs vary depending on the Spanish region at stake, 3 additional scenarios: 6A, 6B, and 6C were explored using cost information adjusted to reflect current clinical practice in Murcia, Huelva, and Basque regions. Furthermore, a scenario #7 was included to account for early adverse events costs at 30 days.
Model outputs
Key outputs of the model were the overall per-patient costs and QALYs in each arm and ICER.
Sensitivity analyses
To evaluate uncertainty, 1-way deterministic sensitivity analyses were performed by varying inputs using confidence intervals and ranges from the literature when available, and plausible ranges when data were unavailable (table 5 of the supplementary data). Multiple parameters were changed and the impact on the results explored. Overall parameter uncertainty was addressed using a probabilistic sensitivity analysis (PSA) (table 6 of the supplementary data). Several scenario analyses were conducted to explore the impact of major structural assumptions as shown on table 7 of the supplementary data. All analyses were performed using Microsoft Excel (Microsoft Corporation, United States).
RESULTS
Base case
TAVI with SAPIEN 3 improved QALYs per patient (+ 1.0) with higher costs compared to SAVR of approximately €6971 per patient. This represented an ICER of €6952 per QALY, which is lower compared to the WTP threshold of €30 000/QALY that is commonly referenced in the Spanish setting. Base case results over a 50-year timespan are shown on table 1. Further examination of the breakdown of costs for TAVI vs SAVR revealed that although initial procedural costs in the model were higher with TAVI, costs associated with ‘disabling stroke’ and ‘treated AF’ were somehow lower (table 1, and figure 1 of the supplementary data).
Deterministic sensitivity analyses
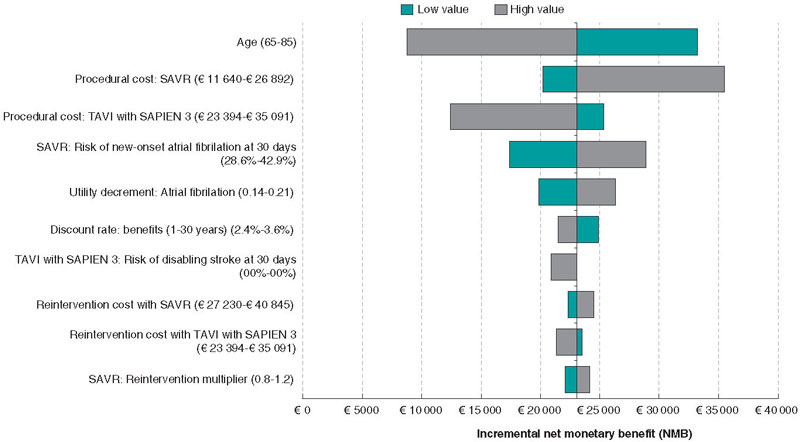
Univariate sensitivity analyses are displayed in the Tornado diagram (figure 2). SAPIEN 3 TAVI remained cost-effective regardless of any plausible changes to individual model parameters (note: the 20 parameters with the greatest influence on the model are shown on the diagram). The model was most sensitive to age, SAVR procedural costs, and risk of disabling stroke at 30 days with TAVI.
Figure 2. Tornado diagram showing the 20 parameters with greatest influence on the model (deterministic sensitivity analysis).
Probabilistic sensitivity analysis
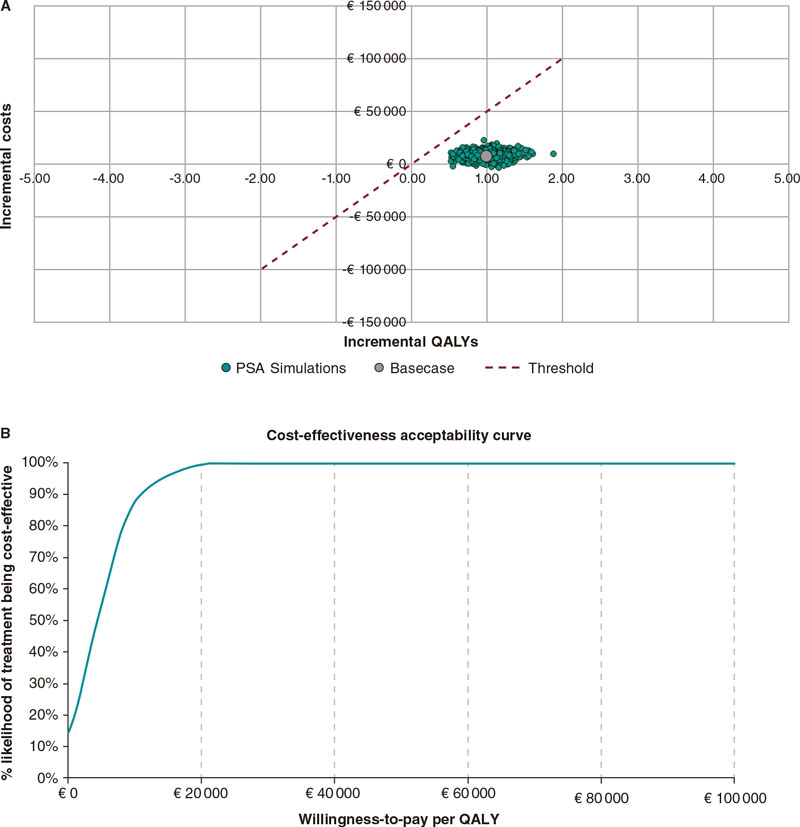
The results of the PSA confirm the results of the base case analysis. At the conventional WTP threshold of €30 000/QALY, TAVI with SAPIEN 3 remains cost-effective compared to SAVR in 100% of the simulations run in the model (figure 3A). In addition, the cost-effectiveness acceptability curve indicates that SAPIEN 3 TAVI has a 99.9% probability of treatment being cost-effective with a €30 000/QALY WTP threshold (figure 3B). PSA assumptions are shown on table 5 of the supplementary data.
Figure 3. Probabilistic sensitivity analysis: A: cost-effectiveness scatter plot; and B: cost-effectiveness acceptability curve. PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life years.
Scenario analysis
A series of different scenario analyses were conducted to assess the impact of changing various assumptions on the results of the model and the model robustness. TAVI with the SAPIEN 3 valve remains cost-effective compared to SAVR across most of the tested scenarios (table 6 of the supplementary data) including those with different timespans (10, 15, 20, and 30 years). The results from the scenario analyses demonstrate the comparative robustness of the model reported.
DISCUSSION
This analysis suggests that TAVI with the SAPIEN 3 vavle is likely to be a cost-effective valve replacement option for patients with sSAS and low surgical mortality risk in Spain. TAVI with the SAPIEN 3 valve showed an improvement in QALYs (+ 1.0) associated with slightly increased costs compared to SAVR (approximately €6971 per patient). The ICER benefits for TAVI with the SAPIEN 3 shown in this model represent a highly cost-effective intervention (ICER/QALY €6 952) in the Spanish system with a WTP threshold of €30 000/QALY. Uncertainty was assessed using various sensitivity analyses, and the results appeared robust.
The findings of the current study are supported by other cost-effectiveness studies that show that TAVI with SAPIEN 3 is either dominant or cost-effective in patients of low risk surgical mortality risk.27-31 The Spanish findings are also consistent with cost-effectiveness analyses of TAVI with SAPIEN 3 vs SAVR in France12 and Italy13 using the same model structure.
The current analysis is important because TAVI provides patients with a minimally invasive treatment option and a lower risk of complications and/or rehospitalization plus improved recovery rates and quality of life gains. From a provider perspective, TAVI also brings efficiencies by limiting healthcare resource use, reducing postoperative complications, and shortening hospital stays (including Intensive care unit [ICU] beds).32 Shortening the hospital stay allows more patients to be treated in the same hospital, an important element for a health system in high demand and with long waiting lists. These efficiencies also lead to a reduced risk of infections and contamination,33 which was much welcomed during the recent COVID-19 pandemic. Finally, TAVI reduces the recovery period to normal activity that may not be accounted for in this analysis. Indirect benefits like volunteering, grandchild support or less caregiving support most likely would increase even further the overall benefits of this technology.34
The results of this analysis could also enable greater access to TAVI for Spanish patients with sSAS. Recent studies demonstrate the efficacy and safety profile of transfemoral TAVI in Spain.9 This together with the recent European guidelines suggests that the number of TAVIs will increase, thus rendering many low surgical risk patients with sSAS eligible for TAVI. Moreover, with time, TAVI will likely become simplified even further with shorter admission times;35 this should lead to lower TAVI costs in the future. In this regard, the results of this study could inform policy makers on the management of patients with sSAS in Spain.
Limitations
This study comes with certain limitations. The first pertains to certain model inputs and assumptions made. In this model, hospitalization data were based on 1- and 2-year data from the PARTNER 3 study with the assumption that this rate remained constant over the model timespan after 2 years. The impact of this assumption is unknown because individuals from both treatment arms in the model remained at risk of hospitalization. The rate of reinterventions was assumed to remain constant after 22 years; the impact of this assumption on modelled outcomes was deemed minimal based on the expectation that nearly 11% of patients would still be alive in the model after this point in time with limited need for reintervention. Despite of this, uncertainty on the longer-term durability of the TAVI device and subsequent reintervention rates in younger patients cannot be disregarded. Disutilities were not included for any intercurrent events because you can run the risk of counting them twice with the health state utilities being applied to patients in the ‘treated AF’ and ‘disabling stroke’ states. This was a conservative assumption because, apart from pacemaker complications, the rates of intercurrent events are generally lower for TAVI with SAPIEN 3 compared to SAVR.6
A second limitation of this study is the generalizability of the results. Conclusions cannot be generalised to the overall population with aortic stenosis because, among others, patients with unfavourable coronary anatomy were excluded from the PARTNER 3 study. Moreover, caution should be observed when trying to generalize any findings from this model to populations outside Spain.
Finally, we should mention that procedural costs across different regions of Spain are heterogenous. In this study, we use publicly available cost data from a region in Spain and our approach is conservative as we additionally account for current practice. We also conducted multiple scenario analyses with other available cost data sets.
CONCLUSIONS
Data from the PARTNER 3 suggested that the use of TAVI with the SAPIEN 3 valve was more favorable, on the clinical level, compared to SAVR in patients with sSAS and low surgical mortality risk. The results of this cost-effectiveness model indicate that, in Spain, TAVI could provide a cost-effective option over SAVR for this population with an estimated ICER/QALY value well below the national threshold. The model appeared to be robust with uncertainty assessed by various sensitivity analyses. The results of this cost-effectiveness analysis can support policy makers and healthcare budget holders to optimize the management of Spanish patients with sSAS.
FUNDING
Edwards Lifesciences SA, Switzerland provided funding for the economic assessment and was involved in the analysis as well as in the drafting of this manuscript.
AUTHORS’ CONTRIBUTIONS
J.M. Vázquez participated in economic data mining, model validation, and manuscript review. E Pinar in economic data mining, and model validation. J. Zamorano participated in data mining, and model validation. J. Burgos participated in data mining and model validation. J. Díaz participated in data mining, and model validation. B. García del Blanco participated in data mining, and model validation. A. Sarmah in data collection and analysis, result preparation, and manuscript drafting and review. P. Candolfi participated in cost analysis and manuscript drafting. J Shore was involved in model development and manuscript review. M. Green participated in model development, and manuscript drafting.
CONFLICTS OF INTEREST
J.M. Vázquez Rodríguez declared department research or training grants from Edwards Lifesciences, Medtronic, and Boston Scientific, and personal advisory fees from Medtronic, and Boston Scientific. J.L. Zamorano declared research grants from Abbott, and Medtronic to the Institution, and speaker fees from Edwards Lifesciences, Bayer, Novo Nordisk, and Daiichi Sankyo. J. Moreu Burgos declared having received fees from Biosensors, Boston Scientific, Cardiva, Edwards Lifesciences, and Medtronic. A. Sarmah declared to be an employee of Edwards Lifesciences and hold stock options. P. Candolfi declared to be an employee of Edwards Lifesciences and hold stock option. J. Shore declared consultancy fees to the employer for developing the economic model. M. Green declared consultancy fees to the employer for developing the economic model. E. Pinar, J.F. Díaz-Fernández, and B. García del Blanco declared no conflicts of interest whatsoever.
ACKNOWLEDGEMENTS
Writing support was provided by Zenith Healthcare Communications Ltd (Chester, United Kingdom), and funded by Edwards Lifesciences.
WHAT IS KNOWN ABOUT THE TOPIC?
- Recent clinical trial evidence confirms the clinical benefits of TAVI with the SAPIEN 3 valve for a low surgical risk population compared to SAVR. Furthermore, following favorable recent updates in the American and European guidelines, TAVI can now be considered as a treatment option in low surgical risk patients with sSAS. Regarding the economic evidence, however, TAVI with the SAPIEN 3 valve has proven cost-effective compared to SAVR only in high and intermediate risk patients with sSAS in Spain.
WHAT DOES THIS STUDY ADD?
- Data from the PARTNER 3 suggested that the use of TAVI with the SAPIEN 3 valve was more clinically favorable compared to SAVR in patients with sSAS and low surgical mortality risk. The results of this robust, cost-effectiveness analysis indicate that, in Spain, TAVI could provide a cost-effective option over SAVR for this population with an estimated ICER/QALY value well below the national threshold. Data from the PARTNER 3 together with data from this cost-effectiveness analysis can support policy makers and healthcare budget holders to optimize the management of Spanish patients with sSAS.
REFERENCES
1. Grimard BH, Larson JM. Aortic Stenosis: Diagnosis and Treatment. Am Fam Physician. 2016;93:371-378.
2. Marquis-Gravel G, Redfors B, Leon MB, Généreux P. Medical treatment of Aortic Stenosis. Circulation. 2016;134:1766-1784.
3. Cribier A. Development of Transcatheter Aortic Valve Implantation (TAVI): A 20-year Odyssey. Arch Cardiovasc Dis. 2012;105:146-152.
4. Falk, V, Baumgartner H, Bax, JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg. 2017;52:616-664.
5. Beyersdorf F, Vahanian A, Milojevic M, et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur J Cardiothorac Surg. 2021;60:727-800.
6. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter Aortic-Valve Replacement With a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med 2019;380:1695-1705.
7. Leon MB, Mack MJ, Hahn RT, et al. Outcomes 2 Years After Transcatheter Aortic Valve Replacement in Patients at Low Surgical Risk. J Am Coll Cardiol. 2021;77:1149-1161.
8. Ali N, Faour A, Rawlins J, et al. ‘Valve for Life’: tackling the deficit in transcatheter treatment of heart valve disease in the UK. Open Heart. 2021;8: e001547.
9. Íñiguez-Romo A, Javier Zueco-Gil J, Álvarez-Bartolomé M, et al. Outcomes of transcatheter aortic valve implantation in Spain through the Activity Registry of Specialized Health Care. REC Interv Cardiol. 2022;4:123-131.
10. Durko, AP, Osnabrugge RL, Van Mieghem NM, et al. Annual number of candidates for transcatheter aortic valve implantation per country: current estimates and future projections. Eur Heart J. 2018;39:2635-2642.
11. Pinar E, García de Lara J, Hurtado J, et al. Análisis coste-efectividad del implante percutáneo de válvula aórtica SAPIEN 3 en pacientes con estenosis aórtica grave sintomática. Rev Esp Cardiol. 2021;75:325-333.
12. Gilard M, Eltchaninoff H, Lung B, et al. Cost-Effectiveness Analysis of SAPIEN 3 Transcatheter Aortic Valve Implantation Procedure Compared with Surgery in Patients with Severe Aortic Stenosis at Low Risk of Surgical Mortality in France. Value Health. 2022;25:605-613.
13. Mennini FS, Meucci F, Gabriele Pesarini G, et al. Cost-effectiveness of transcatheter aortic valve implantation vs surgical aortic valve replacement in low surgical risk aortic stenosis patients. Int J Cardiol. 2022;357:26-32.
14. Sacristán JA, Oliva J, Del Llano J, Prieto L, Pinto JL. What is an efficient health technology in Spain? Gac Sanit. 2002;16:334-343.
15. Lopez-Bastida J, Oliva J, Antoñanzas F, et al. Spanish recommendations on economic evaluation of health technologies. Eur J Health Econ. 2010;11:513-20.
16. SAFE 2017. The Burden of Stroke in Spain. Available online: https://www.safestroke.eu/wp-content/uploads/2017/12/SAFE_STROKE_SPAIN.pdf. Acessed 22 May 2022.
17. Odutayo A, Wong CX, Hsiao AJ, et al. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ. 2016;354:i4482.
18. Bourguignon T, Bouquiaux-Stablo A-L, Candolfi P, et al. Very long-term outcomes of the Carpentier-Edwards Perimount valve in aortic position. Ann Thorac Surg. 2015;99:831-837.
19. Makkar RR, Thourani VH, Mack MJ, et al. Five-Year Outcomes of Transcatheter or Surgical Aortic-Valve Replacement. N Engl J Med. 2020;382:799-809.
20. de Andrés-Nogales F, Álvarez M, de Miquel MÁ, et al. Cost-effectiveness of mechanical thrombectomy using stent retriever after intravenous tissue plasminogen activator compared with intravenous tissue plasminogen activator alone in the treatment of acute ischaemic stroke due to large vessel occlusion in Spain. Eur Stroke J. 2017;2:272-284.
21. Szende A, Janssen B, Cabases J, editors. Self-Reported Population Health: An International Perspective based on EQ-5D. Dordrecht (NL): Springer; 2014. p. 30.
22. Oyagüez I, Suárez C, López-Sendón JL, et al. Cost-Effectiveness Analysis of Apixaban Vs Edoxaban in Patients with Atrial Fibrillation for Stroke Prevention. Pharmacoecon Open. 2020;4:485-497.
23. Lopez-Bastida J, Oliva Moreno J, Worbes Cerezo M, et al. Social and economic costs and health-related quality of life in stroke survivors in the Canary Islands, Spain. BMC Health Serv Res. 2012;12:315.
24. Ramos-Goñi JM, Craig BM, Oppe M, et al. Handling Data Quality Issues to Estimate the Spanish EQ-5D-5L Value Set Using a Hybrid Interval Regression Approach. Value Health. 2018;21:596-604.
25. SERGAS 2014. DECRETO 56/2014, de 30 de abril, por el que se establecen las tarifas de los servicios sanitarios prestados en los centros dependien-tes del Servicio Gallego de Salud y en las fundaciones públicas sanitarias. DOG 96. 2014. Available online: https://www.xunta.gal/dog/Publicados/2014/20140521/AnuncioC3K1-140514-0001_es.html. Accessed 22 May 2022.
26. Bayón Yusta JC, Gutiérrez Iglesias A, Mateos del Pino M, IIbarrola Gutiérrez MI, Gómez Inhiesto E, Acaiturri Ayesta MT. Análisis coste-efectividad del recambio valvular aórtico mediante prótesis valvular percutánea frente al tratamiento quirúrgico habitual. Ministerio de Sanidad, Servicios Sociales e Igualdad; Servicio de Evaluación de Tecnologías Sanitarias del País Vasco. Informes de Evaluación de Tecnologías Sanitarias: OSTEBA (2014). Available online: https://www.euskadi.eus/contenidos/informacion/biblioteca_central/es_9528/scp/215765.pdf Accessed 22 May 2022.
27. Tam DY, Azizi PM, Fremes SE, Chikwe J, Gaudino M, Wijeysundera HC. The Cost-Effectiveness of Transcatheter Aortic Valve Replacement in Low Surgical Risk Patients With Severe Aortic Stenosis. Eur Heart J Qual Care Clin Outcomes. 2021;7:556-563.
28. Zhou JY, Liew D, Duffy SJ, Walton A, Htun N, Stub D. Cost-Effectiveness of Transcatheter Vs Surgical Aortic Valve Replacement in Low-Risk Patients with Severe Aortic Stenosis. Heart Lung Circ. 2021;30:547-554.
29. Health Information and Quality Authority (HIQA) Health Technology Assessment of transcatheter aortic valve implantation (TAVI) in patients with severe symptomatic aortic stenosis at low and intermediate risk of surgical complications. 2019. Available online: https://www.hiqa.ie/sites/default/files/2019-12/TAVI_HTA.pdf. Accessed 22 May 2022.
30. Norwegian Institute of Public Health (NIPH) Transcatheter aortic valve implantation (TAVI) vs surgical aortic valve replacement (SAVR) for patients with severe aortic stenosis and low surgical risk and across surgical risk groups: a health technology assessment. 2021. Available online: https://www.fhi.no/en/publ/2021/TAVI-vs-SAVR-for-patients-with-severe-aortic-stenosis-and-low-surgical-risk-and-across-surgical-risk-groups/. Accessed 22 May 2022.
31. Haute Autorité de Santé (HAS) Traitement de la sténose aortique sévère symptomatique en France chez les patients à faible risqué chirurgical. 2021. Available online: https://www.has-sante.fr/upload/docs/application/pdf/2021-04/sapien3_9022021_avis_economique_vf2.pdf. Accessed 22 May 2022.
32. Valdebenito, M, Massalha E, Barbash IM et al. Transcatheter aortic valve implantation during the COVID-19 pandemic. Am J Cardiol. 2021;145:97-101.
33. Noad RL, Johnston N, McKinley A, et al. A pathway to earlier discharge following TAVI: Assessment of safety and resource utilization. Catheter Cardiovasc Interv. 2016;87:134-142.
34. Huygens SA, van der Kley F, Bekkers JA, Bogers AJJC, Tekkenberg JJM, Rutten-van Mölken MPMH. Beyond the clinical impact of aortic and pulmonary valve implantation: health-related quality of life, informal care and productivity. Eur J Cardiothorac Surg. 2019;55:751-759.
35. Martínez-Sellés M. Coste-efectividad del implante percutáneo de válvula aórtica en 2022. Rev Esp Cardiol. 2022;75(10):853.
36. Instituto Nacional de Estadística. National Life Tables Spain (2019) Available online: https://www.ine.es/dyngs/INEbase/en/operacion.htm?c=Estadistica_C&cid=1254736177004&menu=ultiDatos&idp=1254735573002. Accessed 22 May 2022.
37. de Andrés-Nogales F, Vivancos Mora J, Barriga Hernández FJ, et al. Use of healthcare resources and costs of acute cardioembolic stroke management in the Region of Madrid: The CODICE Study. Neurología. 2015;30:536-544.
38. Esquivias, GB, Albaladejo GE, Zamorano JL, et al. Cost-effectiveness analysis comparing apixaban and acenocoumarol in the prevention of stroke in patients with nonvalvular atrial fibrillation in Spain. Rev Esp Cardiol. 2015;68:680-690.
39. Rubio-Terrés, C, Graefenhain de Codeset R, Rubio-Rodríguez D, Evers T, Grau Cerrato SG. Cost-effectiveness analysis of rivaroxaban vs acenocoumarol in the prevention of stroke in patients with non-valvular atrial fibrillation in Spain. JHEOR. 2016;4:19-34.
40. Alvarez-Sabín J, Quintana M, Masiuan J, et al. Economic impact of patients admitted to stroke units in Spain. Eur J Health Econ. 2017;18:449-458.
41. Ministerio de Sanidad de España. GRD APR 190/191 (2019). Registro de Altas de los Hospitales Generales del Sistema Nacional de Salud. CMBD. Norma Estatal de Años Anteriores Available online: https://www.sanidad.gob.es/estadEstudios/estadisticas/cmbdAnteriores.htm. Accessed 22 May 2022.
42. Crespo C, Linhart M, Acosta J, et al. Optimisation of cardiac resynchronisation therapy device selection guided by cardiac magnetic resonance imaging: cost-effectiveness analysis. Eur J Prev Cardiol. 2020;27:622-632.
43. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016;374:1609-1620.
44. Attribution 4.0 International (CC BY 4.0). Available online: https://creativecommons.org/licenses/by/4.0/. Accessed 25 Aug 2022.
* Corresponding author.
@jmvazrod; @CorazonHuvr; @pepp183; @PascalCandolfi; @CIBER_CV