ABSTRACT
Introduction and objectives: Left atrial appendage occlusion (LAAO) can be an efficient treatment to prevent strokes in patients who suffer from atrial fibrillation, especially those at risk of bleeding. A non-negligible number of patients treated with LAAO develop device-related thrombosis (DRT) after device implantation. Our study aimed to identify the key blood flow characteristics leading to DRT using patient-specific flow simulations.
Methods: Patients treated with LAAO between 2014 and 2019 at a single center with preoperative and follow-up computerized tomography images and ultrasound imaging (US) were used to create patient-specific flow simulations. Amulet LAAO devices were implanted in the study patients. Flow simulations were blindly assessed to discard the presence of DRT in the follow-up imaging.
Results: A total of 6 patients were processed in this pivotal study, half of them with DRT at the follow-up according to the imaging analysis. After a comprehensive analysis of the simulations, the most relevant in silico indices associated with DRT were the presence of stagnant blood flow, recirculation with low flow velocities (< 0.20 m/s) next to the device surface, and regions with high flow complexity combined with low wall shear stress.
Conclusions: Patient-specific flow simulations of LAAO were successfully used to predict blood flow patterns with different device configurations. The results show the potential of the present modelling and simulation approach to recommend optimal settings capable of minimizing the risk of DRT.
Keywords: Device-related thrombosis. Flow simulation. Left atrial appendage occlusion. Patient-specific.
RESUMEN
Introducción y objetivos: El cierre de la orejuela izquierda (COI) puede ser una alternativa de tratamiento eficaz para prevenir eventos cardiovasculares en pacientes con fibrilación auricular, en especial en aquellos con alto riesgo de sangrado. Sin embargo, algunos de estos pacientes en los que se realiza COI desarrollan trombosis relacionada con el dispositivo (TRD). Este estudio presenta las características del flujo sanguíneo que son clave en la formación de TDR, a partir de simulaciones personalizadas para cada paciente.
Métodos: Para crear las simulaciones personalizadas se incluyeron en el estudio pacientes intervenidos de COI entre 2014 y 2019 en un único centro, de quienes se disponía de imágenes de tomografía computarizada previas al procedimiento y de seguimiento, así como de control ecocardiográfico. Para el COI se utilizaron los dispositivos Amulet. Las simulaciones se analizaron de forma ciega al diagnóstico de TRD.
Resultados: En total se estudiaron 6 pacientes, de los que la mitad presentaban TRD según las imágenes del seguimiento clínico. Tras analizar los resultados de las simulaciones, los índices hemodinámicos asociados con TRD fueron la presencia de flujo estancado, las recirculaciones de sangre a velocidades bajas (< 0,20 m/s) cerca de la superficie del dispositivo y las regiones con alta complejidad de flujo y baja tensión de cizallamiento en la pared.
Conclusiones: Las simulaciones de flujo personalizadas en pacientes con COI predijeron correctamente el diagnóstico clínico de TRD en todos los casos analizados. Los resultados obtenidos demuestran el potencial de los modelos personalizados para recomendar configuraciones óptimas del dispositivo y minimizar el riesgo de TRD.
Palabras clave: Trombosis relacionada con el dispositivo. Simulaciones de flujo. Cierre de la orejuela izquierda. Personalización del paciente.
Abbreviations
AF: atrial fibrillation. CT: computed tomography. DRT: device-related thrombosis. ECAP: endothelial cell activation potential. LAAO: left atrial appendage occlusion.
INTRODUCTION
Former randomized trials have shown that percutaneous left atrial appendage occlusion (LAAO) can be an efficient strategy to predict cardioembolic events in selected patients with non-valvular atrial fibrillation (AF) as an alternative to lifelong oral anticoagulation (OAC).1,2 However, device-related thrombosis (DRT) has become a major concern due to its incidence rate (2% to 5%)3 and the increased rate of associated strokes.4 Despite the use of different antithrombotic therapies, the rate of DRT has not changed.5 Arguably, adding or intensifying anticoagulant therapy has proven to be capable of reducing effectively the thrombotic burden in patients diagnosed with DRT.6 However, in these high-risk patients, intensive antithrombotic therapies may translate into a higher risk of bleeding. Therefore, identifying the predictors of DRT appears to be essential to individualize suitable antithrombotic treatments post-LAAO and identify those patients who would need a closer follow-up.
Several clinical variables (age, AF at time of implantation, congestive heart failure, CHA2DS2-VASc score) have been associated with a higher risk of DRT mainly due to their impact on hypercoagulability.7 Other factors such as peri-device leaks and uncovered pulmonary venous ridge have been suggested as potential factors for DRT, although the data published on this regard are still controversial.8 Remarkably, only scarce data have been reported on the impact of blood stasis around the device, although the characteristics of blood flow largely influence thrombus formation.9-11
Acquiring reliable imaging data characterizing the complex 4D behaviour of blood flow patterns in the left atrium is a challenge. However, patient-specific computational models of the heart, also known as ‘Digital Twin’ models are emerging as a valuable technology in clinics to back up clinical decisions and contribute to interventional planning, diagnosis, and device optimization.12-14 Several studies analysing blood flow patterns in the LA and LAA with flow-related computational models have been proposed,15,16 but most of them have been applied to a very limited set of patient-specific clinical data without follow-up. Furthermore, only a couple of studies have considered LAAO implantation.11,17 As a matter of fact, only 1 study has analyzed the direct impact of flow dynamics on the generation of DRT with computational models.11
This manuscript is a proof-of-concept study that describes our early experience evaluating a computational workflow to assess the risk of DRT through personalized flow simulations after LAAO implantation. Our objective was to identify patients who would need closer follow-ups after the intervention due to a higher risk of DRT.
METHODS
General overview
We developed a computational methodology to build patient-specific models and drew personalized in silico indices from clinical data standardly available in patients treated with LAAO implantation. Figure 1 shows a scheme of the proposed methodological workflow. To test this workflow, a retrospective, single-centre study was performed including 6 patients (3 with DRT, and 3 without DRT, respectively) referred for LAAO implantation, post-implantation cardiac computerized tomographic (CT) imaging of the whole atrium, and ultrasound imaging (US) of the mitral valve (MV). The study protocol was approved by the Hospital de la Santa Creu i Sant Pau ethics committee, and all patients gave their informed consent.
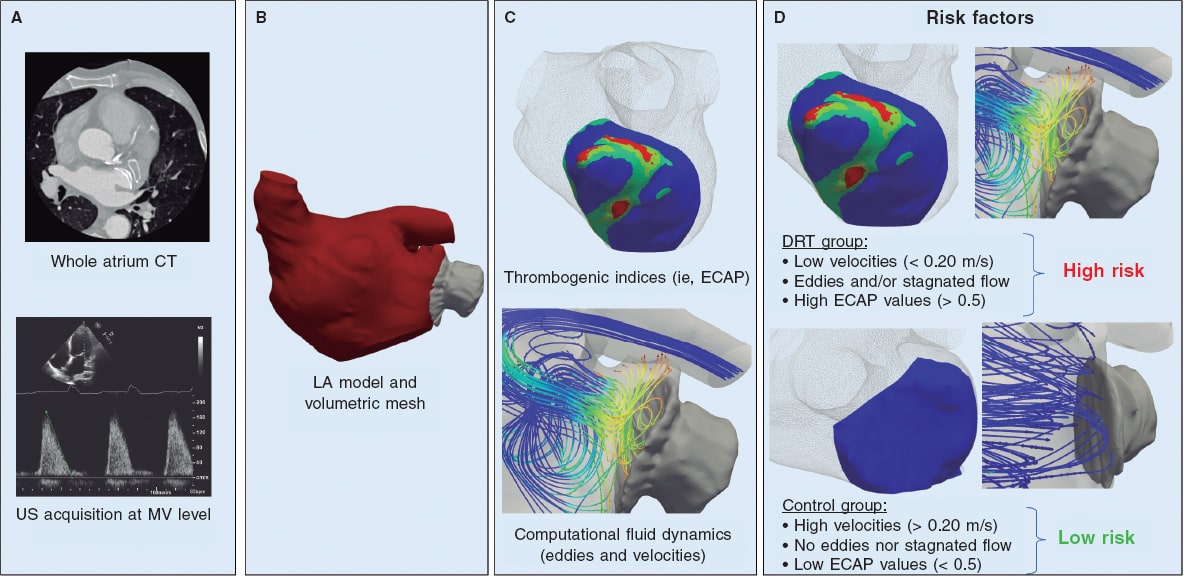
Figure 1. Scheme of the patient-specific computational workflow to predict the risk of device-related thrombus (DRT) formation after left atrial appendage occluder (LAAO) implantation. A: computerized tomography (CT) scan acquisition of whole left atrium (LA) and ultrasound (US) study with Doppler measurements at mitral valve (MV) level. B: 3D LA segmentation and model generation where finite volume analysis was performed. C: blood flow velocities and in silico indices like endothelial cell activation potential (ECAP) estimated from personalized computational fluid dynamics simulations. D: the risk factors predicting the presence of DRT were low velocities (< 0.20 m/s), and stagnated flow next to the device surface as well as high ECAP values (indicative of complex blood flow patterns and low wall shear stress).
Clinical data
CT images were acquired at least twice between months 1 and 3, and between months 3 and 6, respectively after LAAO implantation. A prospective cardiac-gated computed tomography angiography was performed with a Phillips Brilliance iCT scanner (Philips Healthcare, The Netherlands). A biphasic contrast injection protocol was used: 40 cc of iodinated material (Iomeprol 350 mg/mL, Bracco, Italy) were infused through an 18-gauge cubital catheter at a rate of 5 mL/s followed by a saline flush of 40 mL.
The bolus-tracking method was used for the arterial phase images being the region of interest on the ascending aorta with a 100 HU threshold. A volumetric scan from heart to diaphragm (14 cm to 16 cm) was acquired. Cardiac phase reconstruction was performed at 30% to 40% of the interval between the QRS complex. Digital image post-processing and reconstruction were performed using the Brilliance Workstation to assess the LAAO device positioning and presence/location of the DRT (defined as a CT hypodensity on the device left atrial extremity): a) unexplained by imaging artefacts, b) inconsistent with normal healing, c) visible in multiple CT planes, and d) in contact with the device. Patient data were anonymized prior to any computational processing. None of the patients showed leaks after assessing the CT following the methodology and the definition provided by Linder et al.18
A 2D Doppler echocardiography was performed within 7 days from CT follow-up acquisition. Transmitral flow velocities as seen on the pulsed-wave Doppler echocardiography were recorded from the apical 4-chamber view with the Doppler samples placed between the tips of the mitral leaflets. Four out of the 6 patients were diagnosed with permanent AF and 2 with paroxysmal AF. One patient from the latter group was in sinus rhythm when the US images were acquired. Since these patients did not have A waves, the measurement of velocity curves corresponded to the mean of the measurements of their E waves over 3 or 5 beats.
3D model construction and simulation experiments
A personalized geometrical model of the whole left atrium was constructed for each patient from the CT images through semi-automatic segmentation. The Slicer 4.10.1 software was used. The geometry and position of the LAAO device implanted were also extracted from the post-procedural CT scans. The thrombi were segmented as a part of the LA. Therefore, the modeller was blind and did not know whether there was a thrombus when he received the 3D model segmented. The segmented regions were then built on 3D mesh models for computational fluid dynamics simulations. The velocity curves at the mitral valve were obtained from the Doppler ultrasound imposing patient-specific boundary conditions in terms of outflow during the left atria flow simulations. All simulations used a generic pressure wave from a patient with AF at pulmonary vein level. The movement of the mitral valve annular plane was defined according to the medical literature available,19 and distributed through the whole LA thanks to a dynamic mesh approach. Flow simulations were performed using the computational fluid dynamics solver Ansys Fluent 19 R3 (ANSYS Inc, United States). Post-processing and visualization of simulation results were performed using ParaView 5.4.1 (Sandia National Laboratories, Kitware Inc., Los Alamos National Laboratory, United States). More details on the 3D construction modelling and computer simulation pipeline are shown on the supplementary data.
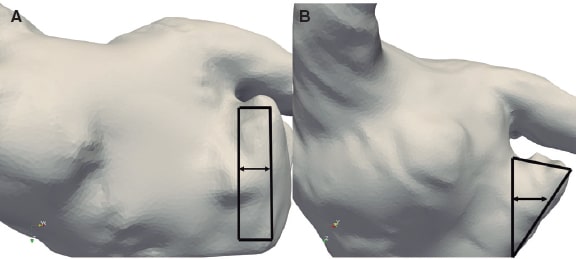
Patient-specific flow simulations allowed the local analyses of the following in silico indices: a) the presence of swirling flows (eg, eddies) or stagnated flow next to the device surface; b) the velocity magnitude averaged over the whole cardiac cycle within the area outlined between the pulmonary ridge and the device surface (see figure 2); low velocity values (< 0.20 m/s) were defined as a strong indicator of thrombus formation20; and c) regions with complex flow patterns and low wall shear stress were characterized using the endothelial cell activation potential (ECAP) index21 (ECAP > 0.5).
Figure 2. Two examples: patient #2 (A) and patient #6 (B): region (black rectangle and triangle, respectively) where velocities are estimated from flow simulation between the perpendicular line towards the pulmonary ridge and the device edge.
RESULTS
Patient characteristics
Six patients treated with LAAO with the Amulet device were selected from the overall LAAO database based on the availability of complete CT imaging at the follow-up including the whole atrium anatomy and an echocardiography study with mitral flow analysis. Three cases with diagnosed DRT and 3 controls (without DRT) were included for patient-specific computational fluid dynamics analyses.
The indication for LAA closure was motivated by a history of major bleeding in 5 patients and high bleeding risk in the remaining one. The patients’ baseline characteristics are shown on table 1 and table 2 (DRT and control groups, respectively). Antithrombotic treatment post-LAAO was prescribed for a minimum of 3 months (table 1 and table 2). No cardioembolic strokes occurred during a minimum clinical follow-up of 12 months.
Table 1. Characteristics of patients with device-related thrombus
| Patient #1 | Patient #2 | Patient #3 | |
|---|---|---|---|
| Age, years | 83 | 86 | 75 |
| Sex | Male | Male | Male |
| LVEF, % | 68% | 47% | 62% |
| Indication for LAAO | Intracranial bleeding | GI bleeding | High bleeding risk |
| Creatinine, mmol/L | 121 | 122 | 71 |
| Atrial fibrillation | Permanent | Permanent | Permanent |
| Diabetes | No | No | No |
| Current smoker | No | No | No |
| Arterial hypertension | Yes | Yes | No |
| History of stroke/TIA | Yes | No | No |
| CHA2DS2VASc score | 6 | 4 | 3 |
| HAS-BLED score | 4 | 1 | 4 |
| Device size, mm | 31 | 28 | 22 |
| Time after LAAO, CT thrombus detection, in weeks | 12 | 22 | 15 |
| Therapy at time of thrombus detection | Clopidogrel | No treatment | No treatment |
|
CHA2DS2VASc score (congestive heart failure, hypertension, age ≥ 75, diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism, vascular disease, age 65 74, sex); CT, computerized tomography; GI, gastrointestinal; HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/alcohol concomitantly); LAAO, left atrial appendage occlusion; LVEF, left ventricular ejection fraction; TIA, transient ischemic attack. |
|||
Table 2. Characteristics of patients without device-related thrombus (control group)
| Patient #4 | Patient #5 | Patient #6 | |
|---|---|---|---|
| Age, years | 66 | 64 | 65 |
| Sex | Male | Male | Male |
| LVEF, % | 77 | 29 | 29 |
| Indication for LAAO | GI bleeding | GI bleeding | GI bleeding |
| Creatinine, mmol/L | 75 | 170 | 147 |
| Atrial fibrillation | Permanent | Paroxysmal | Paroxysmal |
| Diabetes | No | Yes | No |
| Current smoker | No | Yes | No |
| Arterial hypertension | Yes | Yes | Yes |
| History of stroke/TIA | No | No | No |
| CHA2DS2VASc score | 3 | 4 | 4 |
| HAS-BLED score | 3 | 2 | 3 |
| Device size, mm | 28 | 28 | 22 |
| Time after LAAO, CT performed in weeks | 25 | 5 | 38 |
| Therapy at time of thrombus detection | DAPT | Acenocumarol | Acetylsalicylic acid |
|
CHA2DS2VASc score (congestive heart failure, hypertension, age ≥ 75, diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism, vascular disease, age 65 74, sex); CT, computerized tomography; GI, gastrointestinal; HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/alcohol concomitantly); LAAO, left atrial appendage occlusion; LVEF, left ventricular ejection fraction; TIA, transient ischemic attack. |
|||
Analysis of the simulated flows to predict the risk of DRT
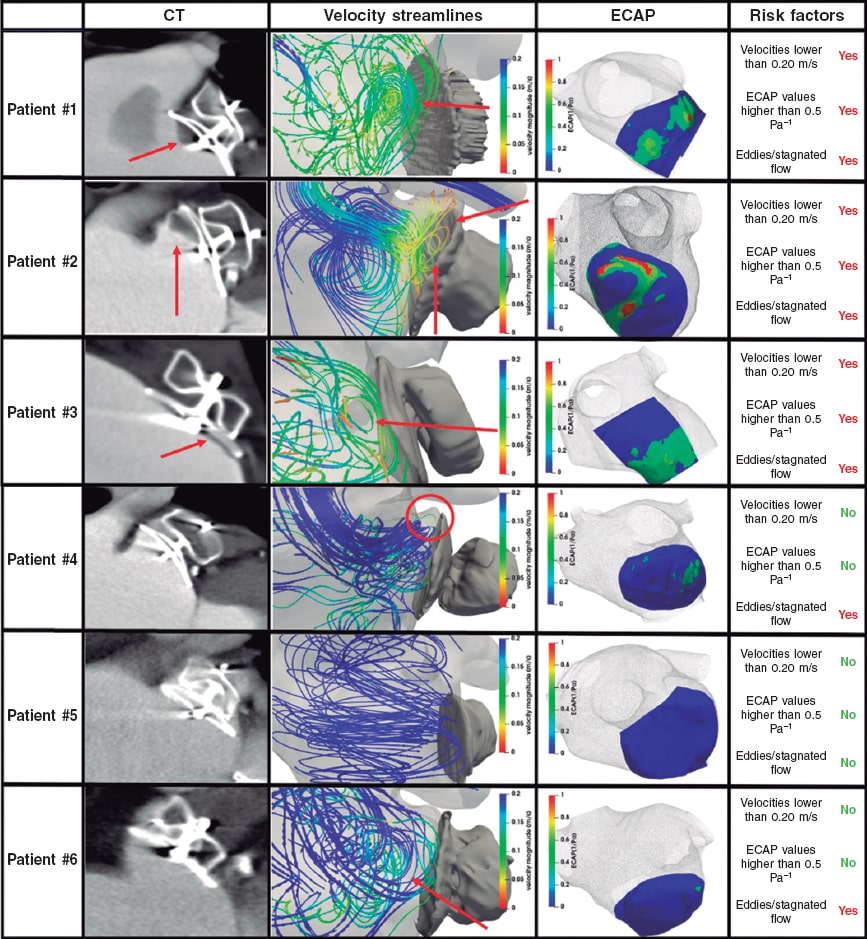
Swirling flows or eddies (figure 3, column 2, red markers) due to blood stagnation and recirculation near the LAAO device surface were found in all patients except for patient #5. The areas with flow recirculation in the simulations matched exactly the location where thrombi were found in the post-CT follow-ups (figure 3, column 1) of patients with DRT. In addition, the magnitude of blood velocities near the device surface, averaged over the whole beat, were different in the DRT group compared to the control cases: around 0.15 m/s for the DRT group, and > 0.20 m/s for the control cases. Table 3 shows the estimate average blood velocities at systole, diastole, and over the whole cardiac cycle. These velocities were generally higher during ventricular diastole. Remarkably, patient #5 who suffered severe mitral regurgitation had particularly high flow velocities (2-3 m/s during the E wave), according to simulations (0.87 m/s on average) in the LAAO region and Doppler data. On the contrary, patient #2 had the smallest average velocity value at 0.10 m/s.
Figure 3. Results of the computational modelling analysis to predict device-related thrombogenesis (DRT). CT column: computerized tomography (CT) scan, red arrows indicate where the thrombus was found at the follow-up imaging analysis. Column with velocity streamlines: simulated blood flow patterns colored by velocity magnitude (blue, high values; red, low values), red arrows indicative of stagnated flow or recirculation. ECAP column: map of the endothelial cell activation potential (ECAP) in silico index near the device surface indicative of high and low DRT risk (red and blue, respectively). Column of risk factors: list of simulation-based risk factors for developing DRT.
Table 3. Blood flow velocities and ECAP near the device surface
| Vel-syst (m/s) | Vel-diast (m/s) | Vel-whole (m/s) | Max-ECAP (Pa–1) | Mean-ECAP (Pa–1) | |
|---|---|---|---|---|---|
| Patient #1 (DRT) | 0.07 ± 0.02 | 0.27 ± 0.08 | 0.16 ± 0.11* | 1.23* | 0.23 ± 0.14 |
| Patient #2 (DRT) | 0.11 ± 0.03 | 0.09 ± 0.02 | 0.10 ± 0.03* | 1.50* | 0.24 ± 0.26 |
| Patient #3 (DRT) | 0.10 ± 0.03 | 0.19 ± 0.07 | 0.14 ± 0.07* | 0.61* | 0.25 ± 0.11 |
| Patient #4 (control) | 0.13 ± 0.03 | 0.24 ± 0.13 | 0.20 ± 0.12 | 0.45 | 0.10 ± 0.08 |
| Patient #5 (control) | 0.28 ± 0.10 | 1.6 ± 0.77 | 0.87 ± 0.83 | 0.07 | 0.01 ± 0.01 |
| Patient #6 (control) | 0.08 ± 0.02 | 0.37 ± 0.17 | 0.29 ± 0.19 | 0.30 | 0.03 ± 0.04 |
|
diast, diastole; ECAP, endothelial cell activation potential; max, maximum; syst, systole; vel, velocity (average ± standard deviation); whole, whole cardiac cycle. * Velocity values averaged across the whole cardiac cycle < 0.20 m/s and ECAP values were > 0.5 since they are indicators of high risk of device-related thrombogenesis. |
|||||
ECAP values ≥ 0.5 Pa–1 were found near the device surface in all patients with DRT (see table 3 for peak and average ECAP values). The peak ECAP values in patients #1 and #2 (figure 3, column 3, red areas) allowed us to clearly locate the specific areas where simulations predicted the formation of DRT, which was later compared to the post-CT imaging analyses. For instance, the spatial location of the ECAP highest values in patient #2 (figure 3, row 2) on the device upper region next to the pulmonary ridge matches the location of the thrombus in the follow-up images. However, the ECAP map of patient #1 suggested an inferior thrombogenic area whereas the real thrombus also formed on the device upper region. In patient #3, ECAP values were more homogeneously distributed over the entire device surface, yet the peak values were still found where the thrombus was formed (lower device region). Regarding the control group, ECAP results were very low except for those of patient #4. In any case, the threshold of 0.5 was not reached, and the follow-up confirmed that this patient did not develop DRT.
DISCUSSION
Early diagnosis, and even prediction, of DRT seems essential to reduce further complications after LAAO implantation like stroke or systemic embolism. It would also contribute to individualize optimal antithrombotic therapies, on which there is still not consensus in the medical community. In this study, the combination of several in silico indices successfully predicted the presence or lack of DRT in all simulated cases (3 controls and 3 patients with DRT diagnosed with follow-up CT imaging). The computational pipeline developed basically required the 3D reconstruction of the whole LA anatomy, obtained with regular cardiac CT imaging acquisition plus a standard US study, already routinely acquired for LAAO candidates, which allowed us to define patient-specific boundary conditions (such as the mitral flow velocity profile from Doppler data). Each patient-specific simulation extended for 48 hours on average. These requirements make the proposed tool particularly suitable for clinical use, and the estimates indicated that an early diagnosis of DRT within 72 hours following device implantation is possible.
This is the first study to validate the ECAP index with a clear clinical endpoint and assess its performance in patients treated with LAAO. The ECAP index could differentiate between DRT and non-DRT cases based on the characteristics of flow complexity. However, it could not robustly predict the exact location of thrombus formation in all of the cases as it wrongly suggested the formation of an inferior thrombus in patient #1 in whom a superior thrombus was identified clinically. Even though the cohort was small, the results suggested that if thrombotic risk after LAAO needs to be studied, the ECAP index alone is not enough, and needs to be combined with other variables.
The velocity results obtained in our in silico analysis are consistent with studies on low velocities with thrombus formation since they could favour the stagnation of flow and consequently trigger the inflammatory process.11,20 Velocity results during systole were more similar among the patients compared to results during diastole that varied more. Point velocity measurement was allocated near the device surface, and it was very close to the MV. Therefore, in general, during ventricular systole when the valve is closed, the velocities in that region tend to be low and differences are difficult to see. Once the MV is open the velocities increase. Also, the mean velocity of the entire cardiac cycle (table 3, column 3) showed that patients who developed DRT had lower velocities in all the beats compared to the control group. However, the process through which blood stasis triggers the inflammatory cascade is not fully understood. For instance, the spatial proximity of the left atrial appendage to the MV makes blood flow into the LAA be quite dependent on the dynamics of the MV as it occurred with patient #5. The unusual hemodynamic behaviour of this patient (eg, very high blood velocities) was due to mitral regurgitation, an effect that was captured by the simulations thanks to the patient-specific US-based boundary conditions. Remarkably, these observations are consistent with studies that hypothesize about a certain degree of protection against flow stagnation and thrombus formation in patients with mitral regurgitation due to a better blood washout of the LAA.22
The areas of flow recirculation at low velocities could indicate potential regions with risk of thrombus formation, but its precise localization depends on the patient’s LA anatomy and the LAAO device final deployment. Our flow simulations revealed the device upper region with an uncovered pulmonary ridge (PR) as the preferred area for eddies as shown on figure 3. This finding was consistent with the literature available on pulmonary ridge uncovering and a higher risk of DRT: 82% of DRT cases with uncovered left upper pulmonary venous ridge.4,8,11 However, we showed that pulmonary ridge uncovering could increase the risk of DRT only if flow velocities are low and the whole pulmonary ridge area cannot be properly washed out. Therefore, covering the pulmonary ridge, which is often not possible due to anatomical constraints (eg, proximity of a circumflex to other structures) would only be critical if blood flow velocities are not high enough.
Limitations
The main limitations of our study were the reduced number of patients to confirm our simulation-based factors, the different anticoagulant therapies used in the DRT group, the differences reported between the acquisition time of the follow-up CT imaging among the different patients, the lack of a unified protocol at the follow-up, and the differences seen between the stroke risk stratification scores. The need for follow-up CT images of the entire left atria and patient-specific MV velocity profiles as seen on the echocardiography, both essential to run flow simulations, confirmed that only a few patients were eligible for this study. Hence, our computational fluid dynamics-based descriptors for DRT prediction should be viewed as novel potential biomarker candidates accessible through digital twin technologies. Fortunately, CT imaging is increasingly becoming accepted as a key technology for LAAO planning and follow-up analysis,23 thus facilitating more extensive studies in the next future. More specifically, the requirement of having a whole LA CT image at the follow-up for the computational model would not substantially change the clinical protocol in centers with access to this imaging technique. Also, the examination can be performed within 72 hours after LAAO implantation. Additionally, in the near future, the constant improvements in spatial and temporal resolution of echocardiographies would make it possible to build patient-specific models based on 3D reconstructions of the LA anatomy from these images. Hence, achieving a larger cohort of prospective cases is possible and will allow more rigorous analyses and validations of the candidate factors and thresholds (in both velocities and the ECAP index) to confirm the performance of the proposed in silico indices to predict the risk of DRT after LAAO implantation.
There is not a clear consensus on the optimal boundary conditions to model LA hemodynamics in a realistic way. On the one hand, we used a velocity profile as outlet in the mitral valve since such profile can be obtained from standard echocardiography images routinely acquired on LAAO candidates. On the other hand, a generic pressure waveform of a patient with AF from a former study was applied in the pulmonary veins as an inlet model while coping with the fact that patient-specific pressure measurements would require invasive catheterization, which is not usually performed in these patients. Similarly, in our study, the movement of the LA wall was extrapolated from the passive movement of the mitral valve annular plane, imposed by the left ventricle, which was extracted from the medical literature available. Whereas our patient-specific approximations of the boundary conditions uniquely allowed simulating mitral regurgitation effects, a more realistic left atrial wall dynamics may be extracted from temporal imaging sequences.
Simplifications are intrinsically associated with the concept of modelling, which was applied in the present study. Still, the integration of relevant patient-specific structural and functional information in our modelling and simulation workflow provided boundary conditions that were realistic enough to achieve accurate estimations of the risk of DRT after LAAO implantation.
CONCLUSIONS
In this proof-of-concept study we present a description of an in silico modelling workflow capable of integrating patient-specific data and simulating hemodynamics within the LA while predicting the risk of DRT after LAAO device implantation. The model was used to study 6 patients retrospectively: 3 patients with DRT and patients 3 without DRT. The simulations reproduced the flow dynamics inside the LA and showed that patients with DRT had low velocity blood flow recirculation with complex patterns next to the device surface. The combination of several in silico indices representing pro-thrombotic factors that cannot be measured in situ, in clinics, could detect differences and distinguish patients with DRT from those of the control group. Here we showed a first proof-of-concept study with in silico indices from personalized models capable of identifying potential complications of LAAO device implantation and individualizing follow-up therapies to minimize the rate of unfavorable clinical outcomes. Nevertheless, future studies should focus on validating the computational workflow developed in a larger cohort of cases.
FUNDING
This work was supported by the Spanish Ministry of Science, Innovation and Universities under the Retos I+D (RTI2018-101193-B-I00), the Maria de Maeztu Units of Excellence (MDM-2015-0502), the Formation of Doctors (PRE2018-084062) and the Recruitment of Talents (RYC-2015-18888) programmes.
AUTHORS’ CONTRIBUTIONS
J. Mill: study idea, methodology, investigation, and writing; V. Agudelo: study idea, methodology, and investigation; C. H. Li: data curation; J. Noailly: formal analysis, supervision, and methodology; X. Freixa, and D. Arzamendi: study idea, supervision, and validation; O. Camara: supervision, writing, validation, and study idea.
CONFLICTS OF INTEREST
D. Arzamendi has received personal grants for proctoring for Abbott, and Boston Scientific. X. Freixa has received personal grants for proctoring for Abbott, and Lifetech. The remaining authors have not declared any other conflicts of interest.
WHAT IS KNOWN ABOUT THE TOPIC?
- DRT has become a major concern, because of its incidence rate (2% to 5%) and the increased rate of associated strokes. Despite the use of different antithrombotic therapies, the rate of DRT has not changed.
- Following Virchow’s triad, 3 factors are thought to contribute to thrombus formation: hypercoagulability, endothelial injury, and blood stasis.
- Factors such as peri-device leaks and uncovered pulmonary venous ridge have been suggested as potential factors for DRT, but the data published are still controversial.
WHAT DOES THIS STUDY ADD?
- Patient-specific flow models correctly predicted the formation or lack of device-related thrombus after LAAO implantation in all studied cases.
- The most relevant in silico indices to predict DRT after LAAO implantation were the presence of flow stagnation, low velocity values next to the device surface, and the ratio between (high) flow complexity and (low) wall shear stress.
- Patients treated with LAAO implantation could have more individualized DRT risk assessments and follow-up antithrombotic therapies using personalized simulations built from the patient’s postoperative CT scans and ultrasound imaging.
REFERENCES
1. Reddy VY, Doshi SK, Kar S, et al. 5-Year Outcomes After Left Atrial Appendage Closure:From the PREVAIL and PROTECT AF Trials. J Am Coll Cardiol. 2017;70:2964-2975.
2. Holmes DR, Kar S, Price MJ, et al. Prospective Randomized Evaluation of the Watchman Left Atrial Appendage Closure Device in Patients With Atrial Fibrillation Versus Long-Term Warfarin Therapy:The PREVAIL Trial. J Am Coll Cardiol. 2014;64:1-12.
3. Fauchier L, Cinaud A, Brigadeau F, et al. Device-Related Thrombosis After Percutaneous Left Atrial Appendage Occlusion for Atrial Fibrillation. J Am Coll Cardiol. 2018;71:1528-1536.
4. Aminian A, Schmidt B, Mazzone P, et al. Incidence, Characterization, and Clinical Impact of Device-Related Thrombus Following Left Atrial Appendage Occlusion in the Prospective Global AMPLATZER Amulet Observational Study. JACC Cardiovasc Interv. 2019;12:1003-1014.
5. Boersma L V., Ince H, Kische S, et al. Evaluating Real-World Clinical Outcomes in Atrial Fibrillation Patients Receiving the WATCHMAN Left Atrial Appendage Closure Technology:Final 2-Year Outcome Data of the EWOLUTION Trial Focusing on History of Stroke and Hemorrhage. Circ Arrhythmia Electrophysiol. 2019;12:1-13.
6. Asmarats L, Cruz-González I, Nombela-Franco L, et al. Recurrence of Device-Related Thrombus After Percutaneous Left Atrial Appendage Closure. Circulation. 2019;140:1441-1443.
7. Sedaghat A, Schrickel J-W, AndriéR, Schueler R, Nickenig G, Hammerstingl C. Thrombus Formation After Left Atrial Appendage Occlusion With the Amplatzer Amulet Device. JACC Clin Electrophysiol. 2017;3:71-75.
8. Freixa X, Cepas-Guillen P, Flores-Umanzor E, et al. Impact of Pulmonary Ridge Coverage after Left Atrial Appendage Occlusion. EuroIntervention. 2021;16:e1288-e1294.
9. Freixa X, Chan JLK, Tzikas A, Garceau P, Basmadjian A, Ibrahim R. The AmplatzerTM Cardiac Plug 2 for left atrial appendage occlusion:novel features and first-in-man experience. EuroIntervention. 2013;8:1094-1098.
10. Cochet H, Iriart X, Sridi S, et al. Left atrial appendage patency and device-related thrombus after percutaneous left atrial appendage occlusion:a computed tomography study. Eur Hear J Cardiovasc Imaging. 2018;19:1351-1361.
11. Mill J, Olivares AL, Arzamendi D, et al. Impact of flow-dynamics on device related thrombosis after left atrial appendage occlusion. Can J Cardiol. 2020;36:968.e13-968.e14.
12. Ribeiro JM, Astudillo P, de Backer O, et al. Artificial Intelligence and Transcatheter Interventions for Structural Heart Disease:A glance at the (near) future. Trends in Cardiovascular Medicine. 2021. https://doi.org/10.1016/j.tcm.2021.02.002.
13. Detmer FJ, Mut F, Slawski M, Hirsch S, Bijlenga P, Cebral JR. Incorporating variability of patient inflow conditions into statistical models for aneurysm rupture assessment. Acta Neurochir (Wien). 2020;162:553-566.
14. Jorge Corral-Acero et al. The 'Digital Twin'to enable the vision of precision cardiology. Eur Heart J. 2020;41:4556–4564.
15. Otani T, Al-Issa A, Pourmorteza A, McVeigh ER, Wada S, Ashikaga H. A Computational Framework for Personalized Blood Flow Analysis in the Human Left Atrium. Ann Biomed Eng. 2016;44:3284-3294.
16. Masci A, Alessandrini M, Forti D, et al. A Patient-Specific Computational Fluid Dynamics Model of the Left Atrium in Atrial Fibrillation:Development and Initial Evaluation. In:Pop M, Wright GA, eds. Functional Imaging and Modelling of the Heart. Cham:Springer International Publishing;2017, 392-400.
17. Aguado AM, Olivares AL, Yagüe C, et al. In silico Optimization of Left Atrial Appendage Occluder Implantation Using Interactive and Modeling Tools. Front Physiol. 2019;10:1-26.
18. Lindner S, Behnes M, Wenke A, et al. Assessment of peri-device leaks after interventional left atrial appendage closure using standardized imaging by cardiac computed tomography angiography. Int J Cardiovasc Imaging. 2019;35:725-731.
19. Veronesi F, Corsi C, Sugeng L, et al. Quantification of Mitral Apparatus Dynamics in Functional and Ischemic Mitral Regurgitation Using Real-time 3-Dimensional Echocardiography. J Am Soc Echocardiogr. 2008;21:347-354.
20. Tamura H, Watanabe T, Hirono O, et al. Low Wall Velocity of Left Atrial Appendage Measured by Trans-Thoracic Echocardiography Predicts Thrombus Formation Caused by Atrial Appendage Dysfunction. J Am Soc Echocardiogr. 2010;23:545-552.e1.
21. Di Achille P, Tellides G, Figueroa CA, Humphrey JD. A haemodynamic predictor of intraluminal thrombus formation in abdominal aortic aneurysms. Proc R Soc A. 2014. http://dx.doi.org/10.1098/rspa.2014.0163.
22. Karatasakis GT. Influence of Mitral Regurgitation on Lefi Atrial Thrombus and Spontaneous Contrast With Rheumatic Valve Disease. Am J Cardiol. 1995;76:279-281.
23. Jaguszewski M, Manes C, Puippe G, et al. Cardiac CT and echocardiographic evaluation of peri-device flow after percutaneous left atrial appendage closure using the AMPLATZER cardiac plug device. Catheter Cardiovasc Interv. 2015;85:306-312.