To the Editor,
Over the last few years we have become aware of the adverse impact of tricuspid regurgitation on morbidity (worse quality of life, hospital admissions…) and mortality with the corresponding increase in the number of interventions performed on the tricuspid valve both surgically and percutaneously.1,2 From the surgical point of view, the most widely used technique for the management of tricuspid valve disease is repair with an annuloplasty to reduce the size of the ring and facilitate leaflet coaptation, usually with incomplete rings, in an attempt to spare the septal conduction system. Short-term results are satisfactory in most cases but according to the series published so far, up to 25% of the patients show moderate or severe regurgitation at 5 years. Overall, we are talking about patients of great complexity, multiple comorbidities, and several prior cardiac surgeries with the corresponding surgical risk, which is why the development of percutaneous coronary intervention techniques may be a great ally.
Currently the treatment of tricuspid valve dysfunction through valve-in-valve procedures is the percutaneous treatment of the tricuspid valve for which we have more and most successful experience. The percutaneous implantation of valves in dysfunctional rings are usually procedures with a series of difficulties due to the great heterogeneity of size, shape or rigidity of the rings. In many cases there are incomplete rings, which complicates the correct adaptability of the valve and often triggers the appearance of paravalvular regurgitation. Therefore, the correct planning of the case is required. For that purpose, imaging modalities are essential to obtain adequate results. However, these are frequently “compassionate use” procedures with a reported experience of just a few isolated cases or small series.3-5
We present the initial experience of 2 different centers with 2 cases of tricuspid percutaneous implantation with 2 of the most widely used bioprosthetic valves currently available for the treatment of tricuspid valve disease both aortic and pulmonary: the Edwards Sapiens XT valve (Edwards Lifesciences, Irvine, California, United States) and the Melody valve (Medtronic, Minneapolis, United States).
The first case is a 19-year-old female carrier of a heart transplant of 10-year duration with tricuspid annuloplasty with a dysfunctional, incomplete Medtronic 25 ring, and clinical signs of congestive heart failure refractory to medical treatment, and advanced renal failure. She was considered a very high-risk patient for reintervention, which is why percutaneous treatment was decided. In this case, the ring was measured using a 3D transesophageal echocardiogram to minimize the use of contrast while the use of a planning CT scan was discarded (figure 1A,B). Due to the size of the ring, it was decided to implant a Melody bioprosthetic valve using the 22 mm Ensemble balloon delivery system that was dilated using a 24 mm-balloon and remained in good ring apposition without significant residual periprosthetic regurgitation (figure 1C-F).
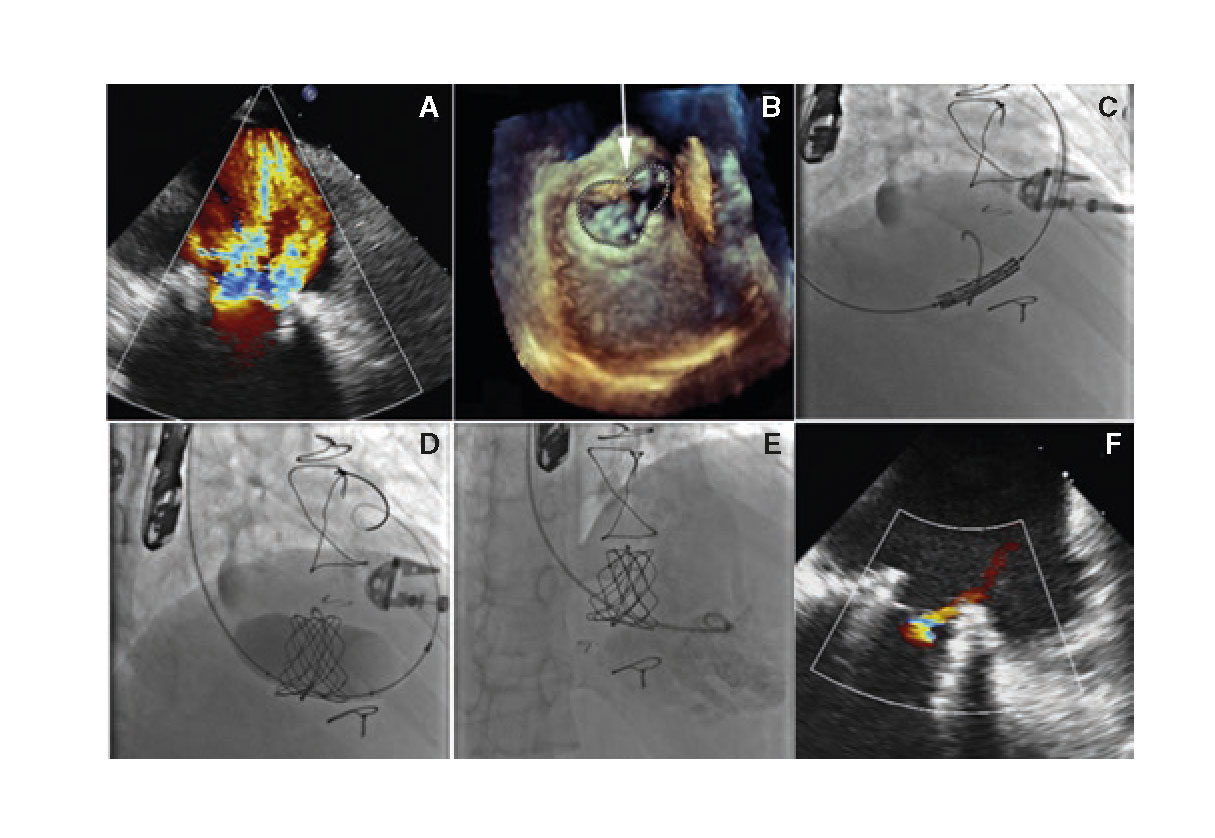
Figure 1. A: transesophageal echocardiogram (TEE) showing severe tricuspid regurgitation. B: measurement of the ring using 3D TEE. C: tricuspid valve-in-ring implantation using the Melody valve. D: post-dilation of the valve using a 24 mm-balloon. E and F: right ventriculography and TEE with correct valve apposition and without significant regurgitation.
The second case is a 53-year-old woman with multiple comorbidities (pulmonary emphysema, peripheral vasculopathy), congenital heart disease operated 25 years ago (closure of interventricular communication [IVC], ductus, and correction of partially anomalous pulmonary venous drainage), reintervention the following year due to IVC patch dehiscence, new heart surgery a year ago due to IVC patch endocarditis and tricuspid valve abscess with IVC closure and tricuspid annuloplasty using the Carpentier- Edwards Physio 32 incomplete ring and resection of tricuspid septal tissue. In the immediate postoperative period, severe tricuspid regurgitation was found but reintervention was disregarded due to its high surgical risk (figure 2A).
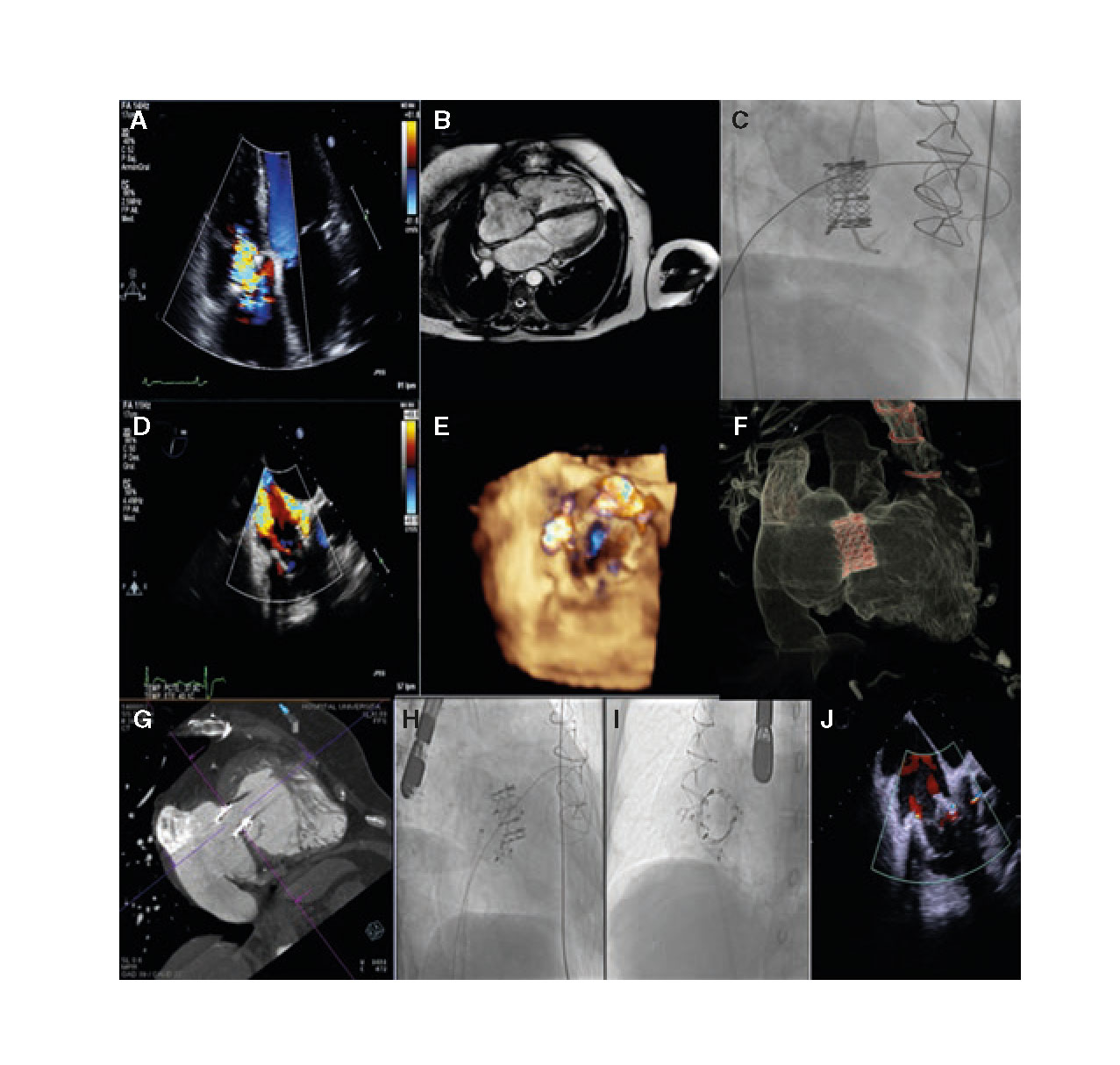
Figure 2. A: transthoracic echocardiogram showing severe tricuspid regurgitation. B: CT scan prior to valve implantation. C: implantation of the Edwards bioprosthetic valve using the Safari guidewire. D and E: severe residual tricuspid regurgitation due to 2 perivalvular leak. F and G: CT scan prior to the closure of paravalvular leaks. H: closure of posterior inferior leak. I: closure of septal leak. J: final outcome without significant tricuspid regurgitation.
A percutaneous valve-in-ring implantation was proposed as an alternative procedure and, in a second procedure, the closure of all paravalvular defects that would have likely occurred after the implantation since we are dealing with an incomplete ring (posterior region) added to the resection of the native valve septal tissue in the previous surgery (figure 2B). The diameter of the ring was measured using a CT scan to decide the size and type of bioprosthetic valve.
In a first procedure, the Edwards XT 29 bioprosthetic valve was implanted via femoral access with overpacing through the Safari guidewire with good ring apposition but 2 residual paravalvular leaks, 1 septal and 1 posterior (figure 2C-E). In a second procedure, and after performing a detailed planning, cardiac CT scan (figure 2F, G) and a rotational angiography, the percutaneous closure of all paravalvular defects was attempted using 2 Amplatzer Vascular Plug III devices with good final outcomes and residual mild tricuspid regurgitation (figure 2H-J).
In both patients, functional class improved and there were no hospital readmissions due to decompensation. With these cases we aim to illustrate that the percutaneous implantation of bioprosthetic valves not designed for this purpose is feasible for the management of dysfunctional tricuspid rings in patients non-eligible for surgical reintervention. In these cases, it is important to carry out detailed prior studies using multimodal imaging (3D echocardiogram, multislice CT scan, rotational angiography…) based on the availability and experience of each particular center, because tricuspid rings are usually asymmetric and often incomplete devices upon which the correct apposition of the valve can be difficult and with significant chances of perivalvular regurgitation that can be usually solved percutaneously.
REFERENCES
1. Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. 2004:43:405-409.
2. Antunes MJ, Barlow JB. Management of tricuspid valve regurgitation. Heart. 2007;93:271-276.
3. Kondo N, Dhuto T, McGarvey JR, et al. Melody valve in ring procedure for mitral valve replacement:feasibility in four annuloplasty types. Ann Thorac Surgery. 2012;93:783-788.
4. Bouleti C, Himbert D, Brochet E, et al. Transfemoral tricuspid valve in ring implantation using the Edwards Sapien XT valve:one-year-follow-up. Circ Cardiovasc. 2015;8:002225.
5. McElhinney D, Cabalka A, Aboulhosn J, et al. Transcatheter Tricuspid Valve-in-Valve Implantation for the Treatment of Dysfunctional Surgical Bioprosthetic Valves. Circulation. 2016;133:1582-1593.
Corresponding author: Unidad de Cardiología Intervencionista, Hospital Universitario de Cruces, Plaza Cruces s/n, 48903 Baracaldo, Vizcaya, Spain.
E-mail address: luisfg82@hotmail.com (L. Fernández González).











