Abstract
Introduction and objectives: Hemodynamically unstable patients with acute pulmonary embolism (PE) are eligible for systemic thrombolysis (ST). However, catheter-directed therapy (CDT) and surgical thrombectomy (SUT) can also be considered with less clinical evidence. Limited information exists regarding the best reperfusion therapy in this setting. Our objective was to perform a descriptive analysis of different reperfusion therapies in acute pulmonary embolism and determine their safety and efficacy profile.
Methods: Retrospective analysis from a prospective single-centre registry of patients admitted with a diagnosis of PE from 2006 through 2021 who required reperfusion therapy. We analyzed the in-hospital outcomes and at 14-day follow up.
Results: A total of 50 out of 399 patients admitted with a diagnosis of PE received reperfusion therapies and were included in our analysis. Mean age, 64.5 (53-72), 46% female. This was the reperfusion strategy applied: ST (44%), CDT (42%) and SUT (14%). All patients had right ventricular dilatation and high troponin levels. The overall in-hospital mortality was 18%. Major and minor bleeding rates among the different reperfusion methods were 9.0% vs 4.7% vs 57.4%; P = .001), and 18.1% vs 9.5% vs 14.2%; P = NS), respectively. The 14-day follow-up showed that only CDT and SUT reduced the pulmonary artery systolic pressure while ST and CDT were associated with a reduced right ventricular diameter and an improved right ventricular function.
Conclusions: High mortality rates were found in this population with acute PE. No differences were seen regarding effectiveness seen among the different reperfusion strategies used. CDT and SUT may be considered as alternative reperfusion methods especially if ST is contraindicated.
Keywords: Pulmonary embolism. Systemic thrombolysis. Catheter-directed therapy. Reperfusion therapy. Surgical thrombectomy.
RESUMEN
Introducción y objetivos: Los pacientes con tromboembolia pulmonar (TEP) aguda hemodinámicamente inestables son candidatos para recibir trombolisis sistémica (TS); sin embargo, el tratamiento guiado por catéter (TGC) y la trombectomía quirúrgica (TQ) también se pueden considerar, aunque con menor nivel de evidencia. Existe información limitada respecto a cuál es el mejor método de reperfusión en esta población. El objetivo es realizar un análisis descriptivo de las distintas terapias de reperfusión en la TEP aguda y determinar su efectividad y seguridad
Métodos: Análisis retrospectivo de un registro prospectivo unicéntrico de pacientes ingresados con TEP aguda entre los años 2006 y 2021, que requirieron tratamiento de reperfusión. Analizamos la evolución intrahospitalaria y en el seguimiento a 14 días.
Resultados: De 399 pacientes con TEP, 50 recibieron tratamiento de reperfusión y fueron incluidos en el análisis. La edad media era de 64,5 años (rango: 53-72) y el 46% eran mujeres. Los métodos de reperfusión fueron TS en el 44%, TGC en el 42% y TQ en el 14%. Todos presentaron dilatación del ventrículo derecho y elevación de las troponinas. La mortalidad intrahospitalaria fue del 18%. Las tasas de sangrado mayor en los grupos de TS, TGC y TQ fueron del 9,0%, el 4,7% y el 57,4% (p = 0,001), y las de sangrado menor fueron del 18,1%, el 9,5% y el 14,2% (p no significativa), respectivamente. Durante el seguimiento a 14 días, solo el TGC y la TQ lograron una reducción de la presión sistólica en la arteria pulmonar, y con la TS y la TGC hubo una reducción de los diámetros del ventrículo derecho y una mejoría de su función.
Conclusiones: En esta población de pacientes con TEP aguda encontramos altas tasas de mortalidad intrahospitalaria. No se observaron diferencias en términos de efectividad entre los distintos tratamientos de reperfusión. El TGC y la TQ podrían considerarse métodos de reperfusión alternativos, en especial cuando la TS está contraindicada.
Palabras clave: Tromboembolia pulmonar. Tratamiento de reperfusión. Tratamiento guiado por catéter. Trombolisis sistémica. Trombectomía quirúrgica.
Abbreviations CGT: catheter-guided therapy. PASP: pulmonary artery systolic pressure. PTE: pulmonary thromboembolism. RV: right ventricle. ST: systemic thrombolysis. SUT: surgical thrombectomy.
INTRODUCTION
Acute pulmonary thromboembolism (PTE) is the third leading cause of cardiovascular mortality, and most deaths are due to acute right heart failure following the obstruction of flow into the pulmonary arteries. The in-hospital mortality rate in patients whose first sign at presentation is hemodynamic instability is 30%. However, it can be as high as 50% in some registries, and 10 times higher compared to stable patients.1
In the registries of patients with PTE there is a group of intermediate risk patients with a higher mortality rate compared to that recorded in clinical trials involving a wide spectrum of individuals including a subgroup with a high mortality rate and similar to that of patients with high-risk PTE. Identifying this high-risk population and selecting those patients who may benefit from some reperfusion method is tremendously challenging for the treating heart team.2-4 Similarly, PTE is associated with other complications at the follow-up like risk of recurrence of thromboembolic events and those associated with a reduced functional capacity or the eventual development of chronic thromboembolic pulmonary hypertension.5-6
There is consensus on the management of reperfusion in patients with high-risk PTE while in intermediate-risk patients, treatment is controversial and could only be indicated in patients with hemodynamic impairment on anticoagulant therapy.7-9 Currently, the reperfusion strategy recommended as the first option in this group of patients is systemic thrombolysis (ST) despite the risk of bleeding involved.7
Although evidence is scarce on this regard, in selected cases, catheter-guided therapy (CGT), and surgical thrombectomy (SUT) could be associated with a lower rate of hemorrhagic complications with similar efficacy.10-13
To this date, no studies have been conducted comparing such reperfusion strategies, so it is unknown which one of them is safer and more effective. The objective of this study is to conduct a descriptive analysis on the different reperfusion mechanisms in acute PTE and determine their effectiveness and safety profiles.
METHODS
A single-center, retrospective, observational cohort study was conducted with patients admitted with PTE who required reperfusion treatment. Data were collected from the prospective registry of a teaching hospital between 2006 and 2021. Reperfusion methods studied were ST, CGT, and SUT.
Patients received reperfusion treatment based on the heart team criterion, evidence, and national and international recommendations available at that time. Patients with overt shock due to right ventricular (RV) failure, and patients without shock with RV dilatation, myocardial damage, and clinical signs indicative of early instability despite parenteral anticoagulant treatment in therapeutic doses were considered eligible to receive reperfusion treatment. Added to this, to be considered eligible for reperfusion treatment, patients should also present with, at least, 2 of the following: persistent tachycardia with a heart rate > 110 bpm or < 60 bpm, systolic arterial pressure < 100 mmHg, elevated lactic acid levels, oxygen saturation < 90%, shock index (heart rate/systolic arterial pressure) > 1 or high thrombotic load (modified Miller score > 22). We should mention that patients with moderate-to-severe RV dysfunction or thrombus in transit were considered eligible for reperfusion treatment on an emergency basis.
ST was preferred in young patients with low risk of bleeding and without absolute or relative contraindications for the use of fibrinolytic agents. In patients with higher risk of bleeding (old age, malignant neoplasm, elevated RIETE or HAS-BLED scores), absolute or relative contraindications for systemic thrombolytic therapy or presence of central thrombi, CGT was considered more suitable. SUT was preferred for patients with suspected subacute PTE (symptoms of >15-day evolution, pulmonary artery systolic pressure [PASP] > 60 mmHg or RV hypertrophy), severe RV dysfunction requiring circulatory support or thrombus in transit.
Streptokinase was the fibrinolytic agent used between 2006 and 2010, and only alteplase has been used ever since. From 2008 CGT started being used as an alternative reperfusion method. Regarding the devices used for CGT, from 2008 through 2017, pigtail-type catheters for thrombus fragmentation and multipurpose catheters for manual thrombus aspiration were being used; the Penumbra System (Penumbra, United States) has been used since 2017, and since 2020 the Angio-Jet device (Boston Scientific, United States) has been available as well.
Data was collected from each patient’s baseline characteristics (age, sex, cardiovascular risk factors, past medical history, comorbidities), clinical status when acute PTE was diagnosed (arterial pressure, heart rate, hemodynamic stability, PESI score, thrombotic load), bleeding risk scores (HAS-BLED, RIETE), lab test parameters (ultra-sensitive cardiac T troponin, and lactic acid), and echocardiographic data on the RV size and function and PASP.
RV dilatation was assessed as a dichotomic variable and defined as normal with diastolic diameters < 41 mm, and dilated if ≥ 41 mm or else a RV/LV ratio > 0.9 as seen on the echocardiography or coronary computed tomography angiography.
RV function was also assessed as a dichotomic variable and defined as normal if the tricuspid annular plane systolic excursion was ≥ 16 mm and reduced if < 16 mm or in the presence of hypokinesia of the RV free wall as seen on the echocardiography.
PASP was assessed quantitatively by measuring the trans-tricuspid pressure gradient, and pressure to the right atrium derived from the inferior vena cava or its collapsibility as seen on the echocardiography.
Physician-investigators obtained this data from the patients’ electronic health records and stored it in an encrypted database authorized by the center research ethics committee.
The privacy of patients in the registry was guaranteed because names or initials were not stored in the database, and access to it was limited to the lead investigator only.
The study of the patients’ clinical progression while hospitalized included in-hospital mortality, the need for mechanical ventilation, major and minor bleeding according to the categorization established by the Bleeding Academic Research Consortium,14 and a composite of in-hospital mortality and major bleeding. The echocardiographic parameters studied before reperfusion and at 14 days were the RV function, the presence of RV dilatation, and the value of PASP.
The informed consent forms of all the patients were collected for the use of data with academic, statistical, and scientific purposes in the healthcare setting. The protocol was assessed and approved by our hospital Bioethics Research Committee (resolution no. 19-041).
Statistical analysis
This was a single-center, retrospective, observational, cohort study. For quantitative variable description, the mean ± standard deviation or median and interquartile range (IQR) 25-75 were used based on their distribution. Qualitative variables were expressed as frequency and percentage. Regarding the bivariate analysis of the 3 strategies the ANOVA technique with Bonferroni correction was used for continuous variables with equal variances while the Kruskal Wallis test was used for different variances. The chi-square test was used for dichotomic variables. Student t test and the Kruskal Wallis test were used for paired sample analysis based on distribution while the dichotomic variables were studied using McNemar test. P values < .05 were considered statistically significant. Analysis was conducted using the Stata/SE v13.0 statistical software package (StataCorp, United States).
RESULTS
A total of 50 patients out of the 399 with PTE received reperfusion treatment and were included in our analysis. No patient was excluded. The patients’ mean age was 64.5 years [53-72], and 46% were women. ST was indicated in 44% of the patients while CGT in 42%, and SUT in 14%. Three patients from the ST group required bailout CGT. The ST group (mean age, 53.5 years [50-68]) was younger compared to the CGT and SUT groups (69 [59-72] and 71 years [60-79]), respectively; P = .02. The remaining baseline characteristics were similar (table 1).
Table 1. Baseline characteristics of the population
| Variable | Overall (N = 50) | ST (N = 22) | CGT (N = 21) | SUT (N = 7) | P |
|---|---|---|---|---|---|
| Clinical characteristics | |||||
| Masculine sex, % | 54 (27) | 50 (11) | 61.9 (13) | 42.8 (3) | .61 |
| Age, years (range) | 64.5 (53-72) | 53.5 (50-68) | 69 (59-72) | 71 (60-79) | .022 |
| Dyslipidemia, % | 36 (18) | 36.6 (8) | 42.8 (9) | 14.2 (1) | .4 |
| Current smoking, % | 38 (19) | 22.7 (5) | 52.3 (11) | 42.8 (3) | .13 |
| Arterial hypertension, % | 54 (27) | 45.4 (10) | 57.1 (12) | 71.4 (5) | .46 |
| Diabetes mellitus | 18 (9) | 22.7 (5) | 19.5 (4) | 0 (0) | .40 |
| Ischemic heart disease, % | 10 (5) | 4.5 (1) | 9.5 (2) | 28.5 (2) | .18 |
| Heart failure, % | 4 (2) | 0 (0) | 4.7 (1) | 14.2 (1) | .24 |
| COPD, % | 2 (1) | 0 (0) | 4.7 (1) | 0 (0) | .51 |
| Malignant neoplasm, % | 26 (13) | 18.1 (4) | 38.1 (8) | 14.2 (1) | .25 |
| HAS BLEED > 4, % | 10 (5) | 9.0 (2) | 4.76 (1) | 28.5 (2) | .19 |
| RIETE | 1.5 (0-3) | 0 (0-1.5) | 1.5 (1-4) | 3 (1-5) | .08 |
| High risk ESC, % | 14 (7) | 13.6 (3) | 19.0 (4) | 0 (0) | .46 |
| SAP, mmHg | 120.5 (110-140) | 121 (111-140) | 118 (100-130) | 135 (111-143) | .94 |
| DAP, mmHg | 78 (65-87) | 80.5 (60-100) | 76 (65-80) | 91 (75-101) | .60 |
| HR, bpm | 110 (99-116) | 110 (100-128) | 110 (100-111) | 105 (85-130) | .44 |
| Absolute contraindication for ST, % | 19 (4) | 0 (0) | |||
| Relative contraindication for ST, % | 28 (6) | 15 (1) | |||
| PESI | |||||
| Very high, % | 28 (14) | 13.6 (3) | 52.3 (11) | 0 (0) | |
| High, % | 30 (15) | 31.8 (7) | 23.8 (5) | 42.8 (3) | |
| Intermediate, % | 22 (11) | 18.1 (4) | 19.0 (4) | 42.8 (3) | |
| Low, % | 16 (8) | 31.8 (7) | 0 (0) | 14.2 (1) | |
| Very low, % | 4 (2) | 4.5 (1) | 4.7 (1) | 0 (0) | |
| Echocardiographic parameters | |||||
| RV dysfunction, % | 88.0 (44) | 77.2 (17) | 95.2 (20) | 100 (7) | .11 |
| RV dilatation, % | 100 (50) | 100 (22) | 100 (21) | 100 (7) | NS |
| PASP | 55 (45-61.5) | 45 (45-58) | 56.5 (49-67.5) | 60 (51-60) | .17 |
| PTE location | |||||
| Multiple-subsegmental, % | 1.3 (5) | 13.6 (3) | 10 (2) | 0 (0) | |
| Pulmonary artery trunk, % | 14.2 (7) | 9.0 (2) | 5 (1) | 57.1 (4) | |
| Both branches, % | 57.1 (28) | 59.0 (13) | 65 (13) | 28.5 (2) | |
| 1 branch only, % | 18.3 (9) | 18.1 (4) | 20 (4) | 14.2 (1) | |
| Laboratory parameters | |||||
| high sensitivity cardiac troponin T, pg/mL | 48.5 (27.5-142) | 31 (24-88) | 64 (33-196) | 88 (36-153) | .02 |
| Lactic acid, mEq/L | 2.1 (1.5-3) | 2 (1.1-2.5) | .63 | ||
| Procedural data | |||||
| Procedural time, m | 97.7 | 209.1 | |||
| Thrombus aspiration, % | 95 (20) | ||||
| Thrombus fragmentation, % | 52 (11) | ||||
| Local thrombolytic agents, % | 38 (8) | ||||
| Doses of thrombolytic agents | |||||
| Alteplase | 100 mg (60%) | 31.1 +- 11.9 | |||
| Streptokinase | 2 000 000 (40%) | ||||
|
bpm, beats per minute; CGT, catheter-guided therapy; COPD, chronic obstructive pulmonary disease; DAP, diastolic arterial pressure; ESC, European Society of Cardiology; HR, heart rate; NS, non-significant; PASP, pulmonary artery systolic pressure: PTE, pulmonary thromboembolism; RV, right ventricle; SAP, systolic arterial pressure; ST, systemic thrombolysis; SUT, surgical thrombectomy. |
|||||
All patients had RV dilatation and elevated cardiac T troponin levels (mean, 48.5 pg/mL). The group that received ST had higher mean troponin levels around 31 pg/mL [24-48], which was not as high compared to the CGT and SUT groups with 64pg/mL [33-196], and 88 pg/mL [36-153], respectively (P = .02). A total of 88% of the patients had RV dysfunction (ST, 77.2%; CGT, 95.2%; ST, 100%; non-significant P value). A total of 14% had high-risk PTE according to the ESC classification from 2019 (ST, 13.6%; CGT, 19%; SUT, 0%; non-significant P value). In 14.2% of the patients the pulmonary artery was compromised while in 75.4% of the patients 1 or the 2 main branches were compromised. The mean systolic arterial pressure was 120.5 mmHg, and the average heart rate 110 bpm with no inter-group differences being reported. A total of 58% of the population had high or very high PESI scores. PASP was high in all the patients for an average 55 mmHg [45-61.5] with no inter-group differences being reported (ST, 45 mmHg [45-58]; CGT, 56.5 mmHg [49-67,5]; SUT, 60mmHg [51-60]). Lactic acid levels were 2.1 mmol/L on average with no inter-group differences being reported (ST, 2 mmol/L [1.1-2.5]; CGT, 2.5 mmol/L [1.5-7.0]; SUT, 2.1 mmol/L [2-2.5]).
Alteplase was used in 60% of the patients from the ST group (mean dose, 100 mg) while streptokinase was used in the remaining 40% (mean dose, 2.0 million IU).
Within the CGT group, thrombus aspiration was performed in 95% of the patients, thrombus fragmentation in 52%, and local thrombolytic agents were used in 38%. Regarding the type of catheter used for CGT, we should mention that between 2008 and 2016 pigtail-type catheters were used for thrombus fragmentation and multipurpose catheters for thrombus aspiration in 10 patients; between 2017 and 2020 Penumbra catheters were used in 10 patients, and from 2020 to 2021 the Angio-jet catheters was used in 1 patient. Mean procedural time was 97.7 minutes and the fibrinolytic agent used in CGT was alteplase in 100% (mean dose, 31.1 mg ± 11.9 mg).
A total of 47% of the patients from the CGT group had absolute or relative contraindications to receive ST vs 15% of the patients from the SUT group. Similarly, the SUT mean procedural time was 209.1 minutes.
The mean length of stay was 10 days [7-18], which was longer for SUT (22 days [15-34]) compared to the other 2 groups (ST, 8.5 [7-15]; CGT, 10 days [6.5-15]; P = .02).
A total of 40% of the population required mechanical ventilation that was more widely used in the SUT group (100%) compared to the other 2 groups (ST, 18.1%; CGT, 42.8%; P = .0002).
Minor bleeding occurred in 14% of the population with no inter-group differences being reported while major bleeding occurred in 14% of the population, more often in the SUT group (57.4%) compared to the other 2 groups (ST, 9%,; CGT, 4,7%; P = .001). The only intracranial bleeding even reported occurred in 1 patient who received ST; the major bleeding events occurred in the SUT group were due to transfusion need and low hemoglobin count without need for reintervention. The only major bleeding event occurred in the CGT group was due to transfusion need after the intervention.
The in-hospital mortality rate was 18% (ST, 9%; CGT, 28.5%; SUT, 14.2%; non-significant P value), and except for 1 death due to cancer occurred in the CGT group, all deaths reported were due to cardiogenic shock following right ventricular failure. The rate of occurrence of the composite “in-hospital mortality and major bleeding” (table 2) was 28% (ST, 13.6%; CGT, 33.3%%; SUT, 57.4%; non-significant P value).
Table 2. In-hospital clinical outcomes
| Variables | Overall (N = 50) | ST (N = 22) | CGT (N = 21) | SUT (N = 7) | P |
|---|---|---|---|---|---|
| Length of stay, days (range) | 10 (7-18) | 8.5 (7-15) | 10 (6.5-15) | 22 (15-34) | .02 |
| Ventilatory support, % | 40 (20) | 18.1 (4) | 42.8 (9) | 100 (7) | .0002 |
| Minor bleeding (BARC < 3), % | 14 (7) | 18.1 (4) | 9.5 (2) | 14.2 (1) | .72 |
| Major bleeding (BARC ≥ 3), % | 14.0 (7) | 9 (2) | 4.7 (1) | 57.4 (4) | .001 |
| In-hospital mortality, % | 18 (9) | 9.0 (2) | 28.5 (6) | 14.2 (1) | .25 |
| Composite of in-hospital mortality and major bleeding, % | 28.0 (14) | 13.6 (3) | 33.3 (7) | 57.1 (4) | .064 |
|
BARC, Bleeding Academic Research Consortium; CGT, catheter-guided therapy; ST, systemic thrombolysis; SUT, surgical thrombectomy. |
|||||
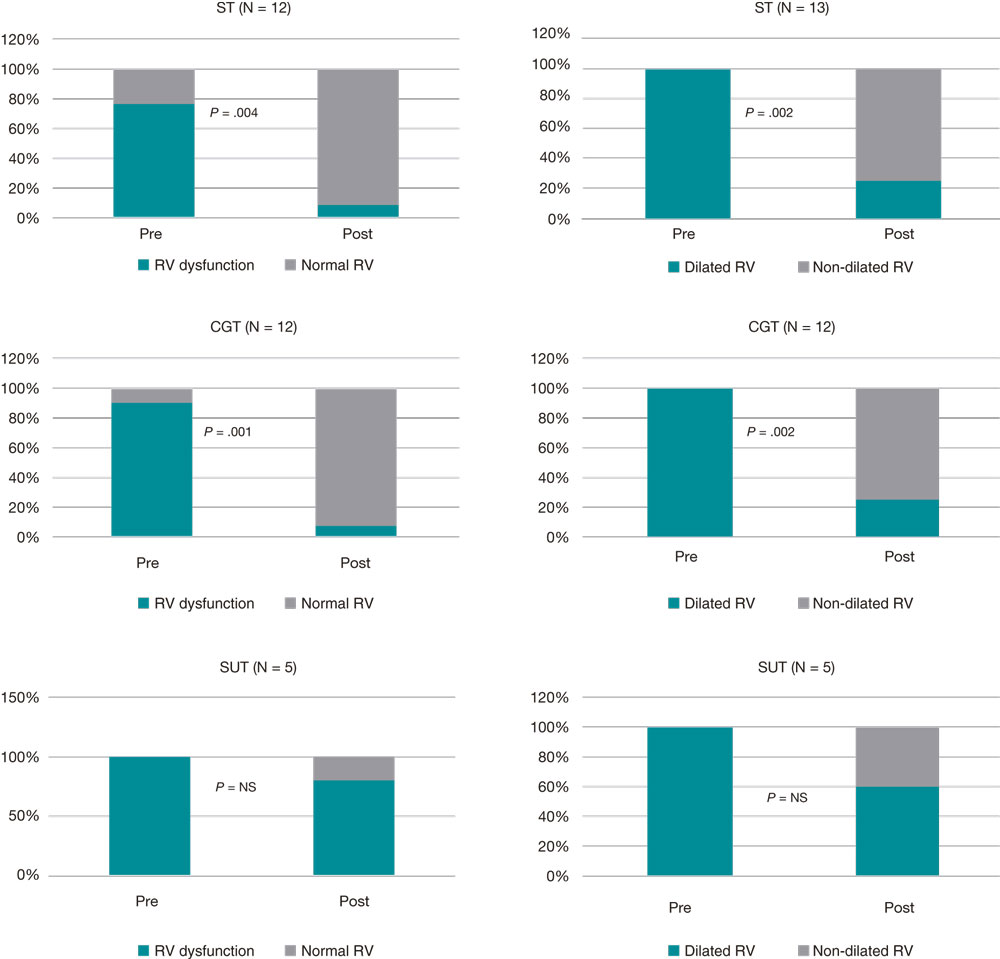
A total of 42% of the patients were lost to follow-up after 14 days, which limits the validity of the findings reported after hospital discharge. In the population that was able to complete the study, normal RV diameters were reported in 70% of the patients from the ST group (P = .002), 75% of the patients from the CGT group (P = .002) and 40% of the patients from the SUT group (non-significant P value). Also, normal RVs were reported in 92% of the patients from the ST group (P = .004), 92% of the patients from the CGT group (P = .001), and 20% of the patients from the SUT group (non-significant P) as shown on figure 1.
Figure 1. Presence of RV dilatation and dysfunction after reperfusion. CGT, catheter-guided therapy; NS, non-significant; RV, right ventricle; ST, systemic thrombolysis; SUT, surgical thrombectomy.
A significant reduction of PASP was reported after reperfusion therapy both in the CGT group and in the SUT group (table 3).
Table 3. Values of pulmonary artery systolic pressure at admission and at 14 days
| Strategy | PASP at admission (mmHg) | PASP at 14 days (mmHg) | Difference (mmHg) | P |
|---|---|---|---|---|
| ST (N = 11) | 46.8 ±18.7 | 36.7 ± 23.7 | 10.0 ± 15.1 | .051 |
| CGT (N = 12) | 58.83 ± 12.6 | 31.3 ± 10.89 | 27.5 ± 15.2 | .0001 |
| SUT (N = 5) | 56.2 ±-9.47 | 35 ± 7.9 | 21.2 ± 15.3 | .036 |
|
CGT, catheter-guided therapy; PASP, pulmonary artery systolic pressure; ST, systemic thrombolysis; SUT, surgical thrombectomy. |
||||
DISCUSSION
Our registry included a population of patients with acute PTE who required reperfusion treatment and whose in-hospital mortality rate was 18%, which is indicative that it was a population with high morbidity and mortality rates compared to the one seen in randomized clinical trials.15-20 One of the reasons that explains this phenomenon is that, unlike registries, randomized clinical trials often include younger, less severe, and less complex patients with fewer comorbidities.
Although the current clinical guidelines recommend ST as the first reperfusion treatment, in our population, only 44% of the patients received ST, the remaining 42% received CGT, and 14% SUT. The high rate of reperfusion with CGT reported in our registry is consistent with that reported by other high-volume centers in the United States where it is used in nearly 11% to 29% of intermediate-high or very high-risk PTEs. In such registries there is a clear tendency towards a wider use of CGT replacing ST that was used in 5.6% of the patients only.21-23 This phenomenon occurs in the context of low compliance to the ST recommendations made in this population. An example of this is the CONAREC XX registry where almost half of the patients with hemodynamic instability did not receive ST although it was the first option recommended by the clinical practice guidelines.24 The reasons behind this are still unclear. However, the risk of major bleeding reported, including intracranial bleeding, associated with the use of systemic thrombolytic agents could partially explain it. It is well known that registries include patients who are often left out of the clinical trials like the elderly, patients with active cancers, postoperative patients, and critically ill patients who often have a higher risk of bleeding, and contraindications for the use of fibrinolytic agents as our study confirmed where almost half of the patients from the CGT group had some contraindication for the use fibrinolytic agents.
One of the theoretical benefits of CGT or SUT over ST is the possible lower rate of severe or fatal bleeding. In a meta-analysis that only included prospective studies of a total of 566 patients treated with CGT, the rate of major bleeding was 5.8% (33 patients), similar to that of our registry.25 Although these rates are lower compared to those seen on trials on ST (rate of major bleeding, 11.5%; intracranial hemorrhage, 2% to 3%), no studies have been published to this date comparing CGT and ST.26-28
Although in our registry no statistically significant differences were seen regarding the rates of major bleeding between CGT and ST, we should mention that the population of patients treated with CGT was 10 years older, and almost half of them had contraindications for systemic fibrinolytic agents. This suggests that the population with the highest risk of bleeding could benefit from this reperfusion strategy. Also, no intracranial bleeding was reported in patients who received CGT. Although SUT was not associated with more major bleeding events, these were due to transfusion needs and a low hemoglobin count without further need for a new intervention; also, the low number of cases reported in this group does not allow us to draw any definitive conclusions on this matter.
Currently, there is no solid evidence demonstrating the benefit of CGT in the in-hospital “hard” clinical endpoints like in-hospital mortality and hemodynamic instability or in long-term results like PTE recurrence, development of chronic thromboembolic pulmonary hypertension, and improved quality of life.
When in-hospital mortality was compared among the different reperfusion treatments, no statistically significant differences were seen (ST, 9%; CGT, 28.5%; SUT, 14.2%; non-significant P value). However, patients from the CGT and SUT groups were older, had a higher risk of bleeding, and elevated troponin levels, which means that they were higher-risk patients. This is indicative that these strategies could be particularly beneficial in this population. We should mention that comparisons between groups and conclusions have a limited value, mainly because of the usual selection bias found in the registries, and the small number of patients and events included (possible beta error).
To this date, the evidence on the efficacy of CGT to treat acute PTE is based on surrogate endpoints like the RV/LV ratio, PASP, and the thrombotic load using Miller score.16-19 The use of CGT in patients with PTE would revert RV dilatation more rapidly compared to anticoagulation alone.23 Based on the scientific evidence available, we can see that both ST and CGT reduced the RV size and function significantly, an essential endpoint since the leading cause of death in patients with acute PTE is shock due to RV failure. Also, reperfusion treatments could be useful to reduce PASP at the follow-up, and eventually reduce the risk of chronic thromboembolic pulmonary hypertension. Still, no definitive conclusions can be drawn because of the patients lost, and the lack of long-term follow-up. More multicenter, prospective registries with long follow-ups are needed to strengthen the evidence available and determine whether reperfusion in the management of patients with acute PTE could have implications in the rate of chronic thromboembolic pulmonary hypertension, which sits at around 4% in most registries.
In our own opinion and considering the current evidence available, ST is still the first option in patients with acute PTE who require reperfusion treatment while CGT and SUT should be indicated in the presence of contraindications for systemic thrombolytic agents. It is essential to define whether invasive strategies could be an alternative to ST in patients with high risk of bleeding. It seems obvious that randomized clinical trials with control groups are needed to compare the different reperfusion strategies available to treat acute PTE that should include “hard” endpoints within their efficacy and safety endpoints always bearing in mind that these results don’t look anything like the real world.
However, there are numerous limitations that should be dealt with when facing this type of studies in the real world. That’s why the evidence collected from ideally multicenter prospective registries with clear inclusion and exclusion criteria and standard and reproducible reperfusion techniques would be highly valuable.29
Limitations
As it occurs in other registries, our study was observational and the indication for reperfusion and the method used were left to the heart team and were backed by the international recommendations effective at the time. The significant selection and inclusion bias and the low number of patients included in our study limits group comparison. Also, there was a considerable number of patients who were lost to follow-up, which limits even more the conclusions of the elements studied after hospital discharge.
Similarly, we should mention that the extended period of patient recruitment of the registry is associated with significant changes within the same reperfusion strategy (type and dose of fibrinolytic agents administered, catheters used, and coadjuvant therapy). The results obtained may not be generalized to other less complex centers, use of other fibrinolytic agents or less experience in the use of CGT, and SUT.
CONCLUSIONS
In this population of patients with acute PTE we found high rates of in-hospital mortality. Although the study has several limitations and biases regarding patient selection, no differences were seen regarding effectiveness among the different reperfusion treatments used. Both ST and CGT reduced the RV diameter significantly and improved the RV function after reperfusion with similar rates of bleeding. CGT and SUT could be considered alternative reperfusion methods in selected cases, and especially when ST is contraindicated or there is a high risk of bleeding.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
M. Iwanowski, J. A. Bilbao, and J. M. Bononino: study design, data curation, analysis and interpretation of data, draft of the manuscript, and final approval of the latest version of the manuscript. H. E. Fernandez, and S. J. Baratta: study design, critical review of the manuscript, final approval of the latest version of the manuscript. R. E. Melchiori: study design, analysis and interpretation of data, draft of the manuscript, final approval of the latest version of the manuscript. N. A. Torres: study design, data curation, draft of the manuscript. R. A. Costantini, J. C. Santucci, and G. N. Vaccarino: critical review of the manuscript, and final approval of the latest version of the manuscript. S. N. Márquez Herrero, P. M. Rubio, E. M. Spaini, G. M. García Juárez, and M. Bivort Haiek: data curation, analysis and interpretation of data, and draft of the manuscript.
CONFLICTS OF INTEREST
None reported.
WHAT IS KNOWN ABOUT THE TOPIC?
- Patients with acute PTE and hemodynamic instability require reperfusion treatment because they are associated with high mortality and morbidity rates.
- CGT is associated with significant improvements in surrogate endpoints. However no significant reductions have been reported regarding the mortality rate.
- No randomized clinical trials have been conducted comparing ST, CGT, and SUT
WHAT DOES THIS STUDY ADD?
- Real-world patients who require reperfusion treatment have high mortality and morbidity rates that are higher compared to those seen in randomized clinical trials.
- No significant differences were found regarding the effectiveness of the different reperfusion treatments studied. Both CGT and ST reduce the RV size significantly and improve the RV function after reperfusion.
- Our study provides information on the feasibility, effectiveness, and safety of the different reperfusion methods available in an Argentinian teaching hospital where evidence is even more limited.
REFERENCES
1. Wendelboe AM, Raskob GE. Global burden of thrombosis: epidemiologic aspects. Circ Res. 2016;118:1340-1347.
2. Vanni S, Jimenez D, Nazerian P, et al. Short-term clinical outcome of normotensive patients with acute PE and high plasma lactate. Thorax. 2015;70:333338.
3. Kreit JW. The impact of right ventricular dysfunction on the prognosis and therapy of normotensive patients with pulmonary embolism. Chest. 2004;125:1539-1545.
4. Becattini C, Agnelli G. Predictors of mortality from pulmonary embolism and their influence on clinical management. Thromb Haemost. 2008;100:747-751.
5. Lubberts B, Paulino Pereira NR, Kabrhel C, David JK, DiGiovanni CW. What is the effect of venous thromboembolism and related complications on patient reported health-related quality of life? A meta-analysis. Thromb Haemost. 2016;116:417-431.
6. Kahn SR, Comerota AJ, Cushman M, et al. The postthrombotic syndrome: evidence-based prevention, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2014;130:1636-1661.
7. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603.
8. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic Therapy for VTE Disease. CHEST Guideline and Expert Panel Report. Chest. 2016; 149:315-352.
9. Ubaldini J, Bilbao J, Bonorino J, et al. Consenso de Enfermedad Tromboembólica Aguda. Rev Argent Cardiol. 2016;84:74-91.
10. Giri J, Sista AK, Weinberg I, et al. Interventional Therapies for Acute Pulmonary Embolism: Current Status and Principles for the Development of Novel Evidence. A Scientific Statement from the American Heart Association. Circulation. 2019;140:e774-e801.
11. Qi Min W, Liang Wan C, Dao Zhong C, et al. Clinical outcomes of acute pulmonary embolectomy as the first-line treatment for massive and submassive pulmonary embolism: a single-centre study in China. J Cardiothorac Surg. 2020;15:321-327.
12. Lehnert P, Moller CH, Mortensen J, et al. Surgical embolectomy compared to thrombolysis in acute pulmonary embolism: morbidity and mortality. Eur J Cardio-Thorac Surg. 2017;2:354-361.
13. Kalra R, Bajaj NS, Arora P, et al. Surgical embolectomy for acute pulmonary embolism: systematic review and comprehensive meta-analyses. Ann Thorac Surg. 2017;103:982-990.
14. Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736-2747.
15. Kasper W, Konstantinides S, Geibel A, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol. 1997;30:1165-1171.
16. Piazza G, Hohlfelder B, Jaff MR, et al. SEATTLE II Investigators. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. JACC Cardiovasc Interv. 2015;8:1382-1392.
17. Kucher N, Boekstegers P, Müller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014;129:479-486.
18. Tapson VF, Sterling K, Jones N, et al. A Randomized Trial of the Optimum Duration of Acoustic Pulse Thrombolysis Procedure in Acute Intermediate-Risk Pulmonary Embolism: the OPTALYSE PE trial. JACC Cardiovasc Interv. 2018;11:1401-1410.
19. Tu T, Toma C, Tapson VF, et al. FLARE Investigators. A prospective, single-arm, multicenter trial of catheter-directed mechanical thrombectomy for intermediate-risk acute pulmonary embolism: the FLARE study. JACC Cardiovasc Interv. 2019;12:859-869.
20. Kuo WT, Banerjee A, Kim PS, et al. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT): initial results from a prospective multicenter registry. Chest. 2015;148:667-673.
21. Kabrhel C, Rosovsky R, Channick R, et al. A multidisciplinary pulmonary embolism response team: initial 30-month experience with a novel approach to delivery of care to patients with submassive and massive pulmonary embolism. Chest. 2016;150:384-393.
22. Sista AK, Friedman OA, Dou E, et al. A pulmonary embolism response team’s initial 20 month experience treating 87 patients with submassive and massive pulmonary embolism. Vasc Med. 2018;23:65-71.
23. Reza N, Dudzinski DM. Pulmonary embolism response teams. Curr Treat Options Cardiovasc Med. 2015;17:387.
24. Cigalini I, Igolnikof D, Scatularo C, et al. Tromboembolismo pulmonar agudo en la Argentina. Registro CONAREC XX. Rev Argent Cardiol. 2019;87:137-145.
25. Kuo WT, Gould MK, Louie JD, Rosenberg JK, Sze DY, Hofmann LV. Catheter-directed therapy for the treatment of massive pulmonary embolism: systematic review and meta-analysis of modern techniques. J Vasc Interv Radiol. 2009;20:1431-1440.
26. Meyer G, Vicaut E, Danays T, et al., for the PEITHO investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014; 370:1402-1411.
27. Fiumara K, Kucher N, Fanikos J, Goldhaber SZ. Predictors of major hemorrhage following fibrinolysis for acute pulmonary embolism. Am J Cardiol. 2006;97:127-129.
28. Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA. 2014;311:2414-2421.
29. Piazza G. Trailblazing in pulmonary embolism research: the importance of extending beyond randomized controlled trials. Eur Heart J Acute Cardiovasc Care. 2021;10:237-239.
* Corresponding author:
E-mail address: mateo_iwanowski@hotmail.com (M. Iwanowski).