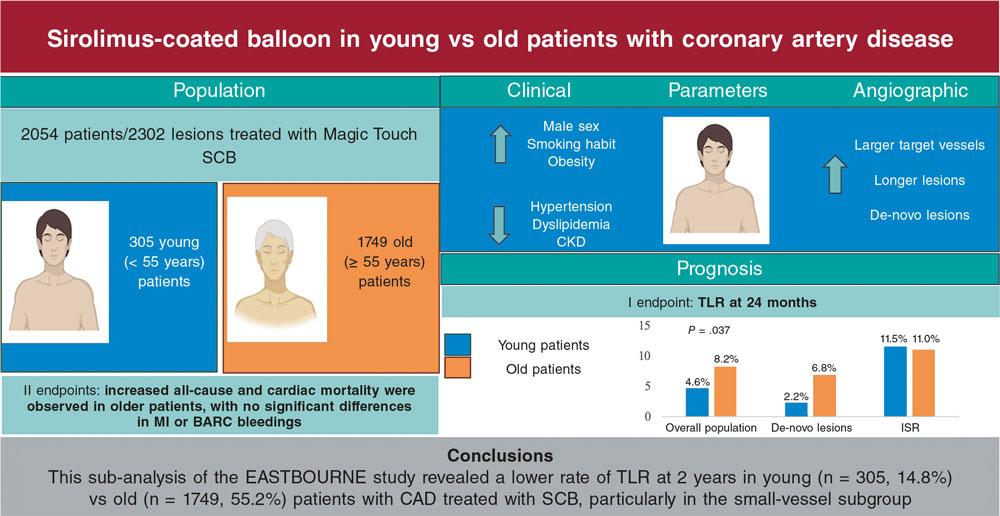
ABSTRACT
Introduction and objectives: Endomyocardial biopsy (EMB) is an established diagnostic tool in myocardial disease. However, this technique may carry major complications. We present the diagnostic and safety results of our experience in EMB in the non-transplant setting. We also present the results after the implementation of a technical and safety protocol developed at our center.
Methods: We retrospectively analyzed the data of all EMBs conducted in non-transplant patients from September 2004 through July 2018. We compared the diagnostic yield and rate of major complications of EMB in two different periods: before and after implementing the protocol.
Results: We included 204 EMBs performed in 190 patients. The most frequent indications were the evaluation of ventricular dysfunction or suspected myocarditis (51.5%) and the evaluation of restrictive cardiomyopathy or suspected infiltrative disease (44.6%). One hundred and seventy-two EMBs were performed in the right cardiac chambers (84.3%) and 30 EMBs in the left cardiac chambers (14.7%). The specimens were taken from both ventricles on 2 cases only. Definite diagnosis was reached in 52% of the cases. After the implementation of the protocol, the diagnostic yield significantly improved (42.5% vs 58.1%; P = .030) and the rate of major complications decreased (from 7.5% to 3.2%; P = .167), with a statistically significant lower rate of cardiac perforation (6.3% vs 0.8%; P = .025).
Conclusions: The EMB is a diagnostic tool with a great potential in patients with suspected cardiomyopathy. Our experience shows that a technical and safety protocol can help decrease the rate of complications and improve the diagnostic yield of EMB.
Keywords: Endomyocardial biopsy. EMB. Cardiomyopathy. Myocarditis. Amyloidosis. Electroanatomical map.
RESUMEN
Introducción y objetivos: La biopsia endomiocárdica (BEM) es una técnica diagnóstica fundamental en el diagnóstico de distintas miocardiopatías, pero no está exenta de posibles complicaciones. Se presentan los resultados en términos de rentabilidad diagnóstica y seguridad de la serie de BEM realizadas en corazón no trasplantado en nuestro hospital, así como las consecuencias de la implementación de un protocol de actuación y seguridad en BEM desarrollado en nuestro centro.
Métodos: Se revisaron de forma retrospectiva todas las BEM en corazón no trasplantado realizadas desde septiembre de 2004 hasta julio de 2018. Se comparó la rentabilidad diagnóstica y seguridad en dos etapas: antes y después de la puesta en marcha del protocolo.
Resultados: Se incluyeron 204 BEM realizadas en 190 pacientes. La indicación más frecuente fue el estudio de disfunción ventricular o sospecha de miocarditis (51,5%), seguida de estudio de miocardiopatía restrictiva o infiltrativa (44,6%). Se realizaron 172 BEM en cavidades derechas (84,3%) y 30 en cavidades izquierdas (14,7%); solo en 2 de los procedimientos se tomaron muestras de ambos ventrículos. La BEM permitió el diagnóstico definitivo en el 52% de los casos. Tras la implementación del protocolo se observó una mejoría en la rentabilidad diagnóstica (42,5 frente a 58,1%; p = 0,030) y una disminución en la tasa de complicaciones mayores (del 7,5% al 3,2%; p = 0,167), con una reducción estadísticamente significativa en la tasa de perforaciones cardiacas (6,3 frente a 0,8%; p = 0,025).
Conclusiones: La BEM es una técnica con un gran potencial diagnóstico en pacientes con sospecha de miocardiopatía. Aunque puede presentar complicaciones potencialmente graves, la puesta en marcha de un protocol de actuación y seguridad se asocia a una reducción en la tasa de complicaciones y a una mejoría en la rentabilidad diagnóstica.
Palabras clave: Biopsia endomiocárdica. BEM. Miocardiopatías. Miocarditis. Amiloidosis. Mapa electroanatómico.
Abbreviations: EMB: endomyocardial biopsy.
INTRODUCTION
Endomyocardial biopsy (EMB) is a key diagnostic tool to monitor rejection in individuals with a heart transplant1 and also for the diagnosis of different cardiomyopathies.2-4 Since this technique was born over a century ago,5,6 several important advances have been made to improve its diagnostic yield and minimize the risk of complications for the patient. However, EMB-induced major complications, though rare, can be serious.7
Our goal was to present the results in terms of diagnostic yield and safety of an EMB series in non-transplanted hearts conducted at our hospital, a national reference center in the diagnosis and management of cardiomyopathies with a huge experience in heart transplants and, consequently, in the monitoring of rejection through EMB in cardiac transplants. Also, we aimed to describe the consequences that implementing a plan of action and safety has on the rate of complications and diagnostic yield of this technique.
METHODS
All EMB procedures conducted in non-transplanted hearts were retrospectively included from September 2004 through July 2018. The demographical and physiological data, relevant echocardiographic parameters (left ventricular ejection fraction and interventricular septum maximum thickness) and times associated with the procedure were all studied.
The main indications for conducting an EMB in a non-transplanted heart were taken into consideration according to the recom- mendations published by the American Heart Association/European Society of Cardiology back in 2007.2,3 In an attempt to facilitate the analysis of data, the indication for the procedure was coded into 4 categories: 1) study of unexplained ventricular dysfunction or suspicion of myocarditis; 2) suspicion of infiltrating disease or restrictive cardiomyopathy; 3) study of ventricular arrhythmias and 4) cardiac tumors. All the histopathological studies extracted from all specimens were reviewed in all the cases (both before and after the protocol) by the same highly-experienced anatomical pathologist in the study of EMBs. The specimens were not reviewed retrospectively in this study again. Instead, the initial 2-stage diagnosis was maintained.
Also, the rate of major complications such as the ones shown in formerly published studies was taken into consideration: mortality, perforation with cardiac tamponade, sustained ventricular arrhythmias with hemodynamic instability, complete atrioventricu- lar block requiring pacemaker, stroke, acute myocardial infarction and appearance of severe valve regurgitation.8-10 The main characteristics of the procedures conducted before and after implementing a plan of action and safety were compared including the rate of major complications and diagnostic yield of the EMB in both periods of time.
Plan of action and safety
Back in February 2013, a plan for action and safety was implemented at our center in an attempt to improve the safety of EMBs and diagnose early whatever complications that may arise. These are the landmarks of this plan:
-
Designation of a coordination group for the EMB program in the non-transplant setting including hemodynamic cardiologists, specialists in cardiomyopathies and advanced heart failure, and pathologists.
-
Planning the procedure together with the cardiologist who prescribed the test taking the indication and characteristics of the patient into consideration to be able to determine the location of the EMB and the access route. The location of the EMB (right ventricle, left ventricle or both) is basically determined by the gadolinium enhancement pattern on the cardiac magnetic resonance imaging. In highly selected cases with patchy uptake pattern or a prior negative EMB, the electroanatomic mapping-guided EMB is preferred.
-
Delivery of the informed consent document by the prescribing cardiologist and explanation to the patient of all potential benefits and risks involved in this procedure.
-
Management of perioperative antiaggregant and antiplatelet drugs by the prescribing cardiologist.
-
– Antiaggregant action: most EMBs can be conducted without the need to withdraw antiaggregant drugs with acetylsalicylic acid. However, if withdrawal is required, it should occur 7 days in advance. For patients treated with clopidogrel and ticagrelor, it should occur 5 days in advance and for those treated with prasugrel, withdrawal should occur 7 days in advance.
-
– Antiplatelet action: each patient’s thromboembolic risk is taken into consideration. In patients on dicumarinic treatment, bridge therapy is implemented only in those with high thromboembolic risk, being the drug withdrawn 5 days prior to the procedure and treatment with low molecular weight heparin prescribed 3 days prior to the procedure. In patients on direct-acting anticoagulants, the drug is withdrawn between 24 and 72 hours in advance depending on renal clearance. Bridge therapy is not required.
-
– The moment when antiaggregant or antiplatelet drugs are reintroduced is always determined taking into consideration each patient’s individual hemorrhagic and thromboembolic risk.
-
-
Conducting or otherwise supervising the procedure should always be the sole responsibility of the interventional cardiologist with the most experience in performing EMBs in non-transplant settings.
-
Conducting a transthoracic echocardiography prior to the procedure in order to confirm the lack of pericardial effusion, define cardiac anatomy (size of the interventricular septum and cavities, location of papillary muscles, etc.), and determine the presence and degree of possible valve regurgitation.
-
The pericardiocentesis working team should be prepared before starting the procedure.
-
Monitorization of vital signs and electrocardiogram during the entire procedure.
-
Acquisition of at least 3 good quality specimens from every previously projected location, and confirmation of the position of the bioptome using x-ray imaging and contrast injection before every take.
-
Transportation of the specimens preserved in formaldehyde at 4% or a specific preservation medium as per the pathologist instructions.
-
Conducting the transthoracic echocardiography immediately after performing the last biopsy or on suspicion of complications during the procedure and monitoring the presence or increase of pericardial effusion or other mechanical complications such as valvular regurgitation. At times (ie, on suspicion of inflammatory or infiltrative cardiomyopathy with segmental damage based on prior imaging modalities), it may be useful to perform an electrocardiogram during the procedure in order to be more precise on the location of the specific segment to be biopsized.
-
Hemodynamic and electrocardiogram monitoring for at least 6-8 hours at the diagnostics hemodynamics unit in day-hospital care or, in the case of patients already hospitalized, at the intensive care unit, paying special attention to the appearance of any possible complications of vascular access.
-
In the presence of pericardial effusion following the EMB and clinical or echocardiographic data of cardiac tamponade, a pericardiocentesis for drainage purposes should be attempted at the cath. lab. In most cases, a drainage catheter is inserted and then removed when the amount of drainage fluid is nearly nonexistent and the pericardial effusion has been resolved. In the presence of progressive effusion or hemodynamic instability despite the pericardiocentesis procedure, urgent surgery is indicated to drain the pericardial effusion and repair cardiac perforation.
Description of the procedure
The description of the procedure is shown at in the supplementary data and in the figure 1.
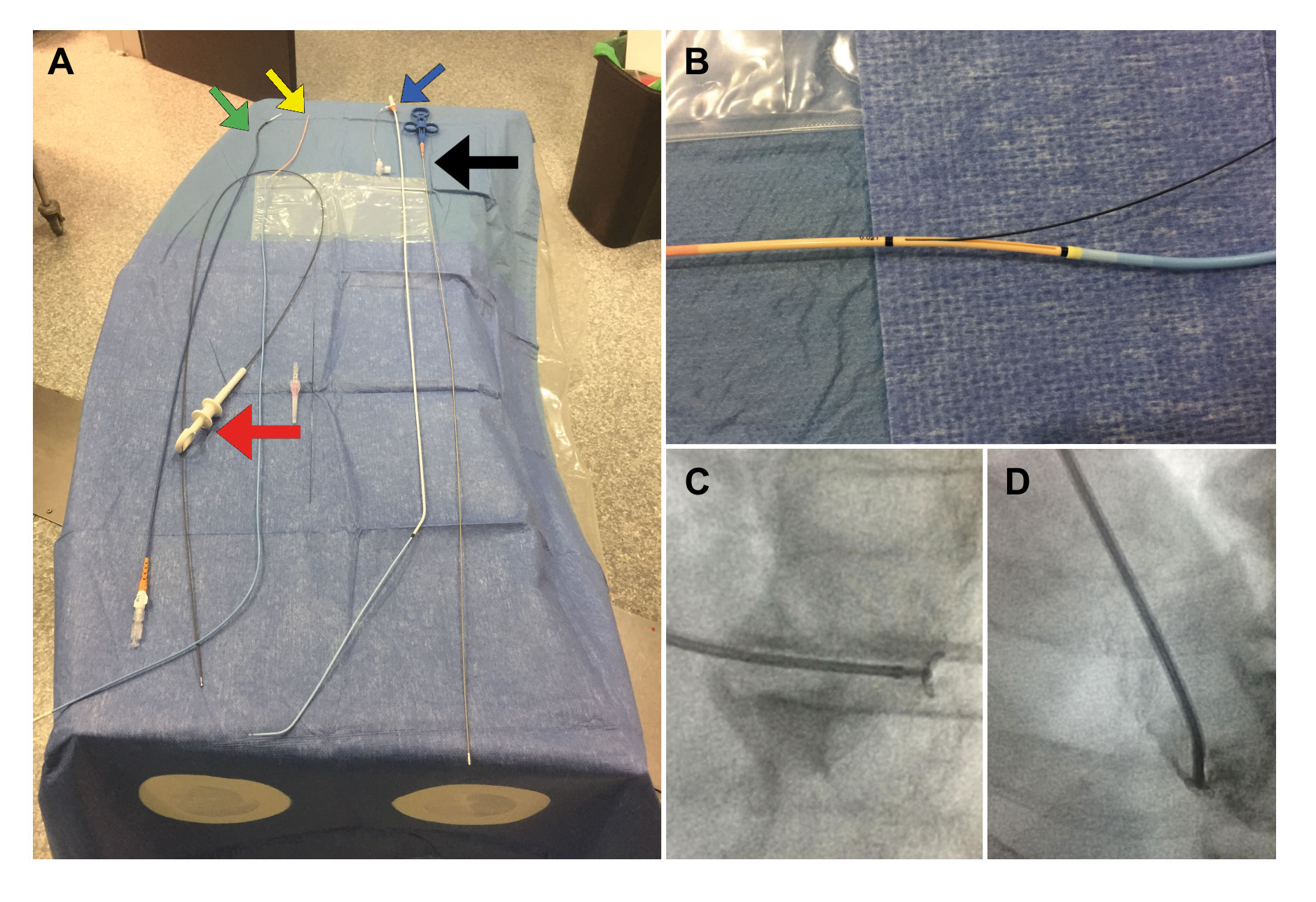
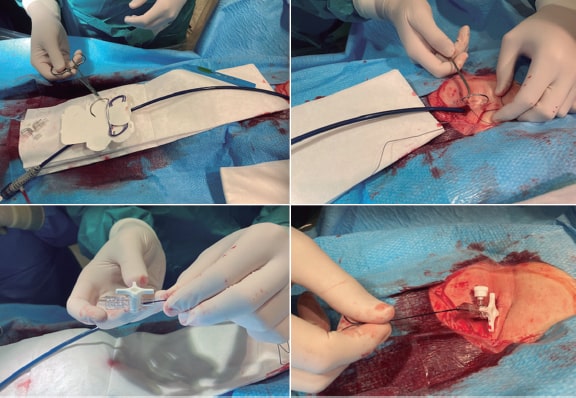
Figure 1. A: Material used to perform EMBs at our center. On the right side, the bioptome (black arrow) and the sheath and the 7-Fr multipurpose catheter (blue arrow) used in right-side EMBs. On the left side, the Endojaw biopsy forceps (red arrow) and 2 of the sheathless systems used for radial access in left-side EMBs: the JR4 7.5-Fr 100-cm-long guidewire catheter (green arrow) and the 7-Fr Railway access system (yellow arrow). B: the latter system described is shown while mounted on a multipurpose catheter at exchange port level. C: open EMB forceps directed towards the left ventricular posterolateral wall for specimen acquisition purposes (left anterior oblique 30° and cranial 15°). D: clockwise rotation in the same projection to direct the guidewire catheter towards the septum using an IV contrast to verify its position.
Statistical analysis
Qualitative variables are expressed as percentages and continuous variables as mean ± standard deviation as the measure of dispersion. The chi-square test was used to compare qualitative variables ant the Student t-test was used for independent sample comparison purposes.
The statistical software package SPSS 21 (SPSS, Inc.; Chicago, Illinois, United States) was used for statistical analysis purposes. P values < .05 were considered statistically significant.
RESULTS
From September 2004 through July 2018, 204 EMBs were performed in the non-transplant setting in 190 patients (12 with 2 EMBs and 1 with 3 EMBs). After implementing the aforemen- tioned plan, all EMBs were performed under the direct supervision of an experienced interventional cardiologist or by the cardiologist himself. A total of 172 EMBs were performed in right cavities (84.3%) and 30 in left cavities (14.7%), whereas in only 2 procedures specimens were taken from both ventricles. When it comes to right-side EMBs, the most widely used vascular access was the femoral vein (88.4%) followed by the cephalic or the basilic vein (9.9%) and the right internal jugular vein (1.7%). In the case of left-side EMBs, over half of them were performed through the radial artery (56.7%) and the rest (43.3%) through the femoral artery. One of the cases of biventricular EMB required femoral vein puncture and transseptal access, and another one, arterial and femoral vein puncture separately. In 47.5% of the cases, the EMB was performed in isolation and in the remaining cases in association with another procedure (right catheterization, coronary angiography, and even intra-aortic balloon pump counterpulsation implantation in one patient). Also, it should be mentioned that three of the procedures were electroanatomic mapping-guided EMBs.
Procedural characteristics and diagnostic yield
Table 1 shows the main procedural characteristics by comparing both stages: before and after the implementation of the plan of action and safety. Overall, a definitive anatomopathological diagnosis was achieved in 52.0% of the cases. It is important to stress out that even though the indications were not significantly different in one stage compared to the other, the diagnostic yield improved significantly after the implementation of the plan (42.5% vs 58.1%; P = .030), basically to the detriment of a greater diagnostic yield in cases of ventricular dysfunction or suspicion of myocarditis (28.2% vs 53.0%; P = .013). Also, there was a significant increase in the number of specimens obtained and the number of left-side EMBs. There was a significant reduction in procedural time with no differences in the x-ray imaging time, although it is true that this difference may have been related with the fact that the isolated EMB (without an associated procedure) was less common before than after the implementation of the plan (33.8% vs 56.5%; P = .004).
Although it never reached statistical significance (P = .083), diagnostic yield was different for each and every indication. It was greater in cases of suspicion of restrictive or infiltrative cardiomyopathy and cardiac tumors. Table 2 shows the anatomical pathology diagnosis in each and every indication.
The left-side and biventricular EMB diagnostic yield was similar compared to the right-side EMB diagnostic yield (56.3% vs 51.2%; P = .384). It should be mentioned that, in left-side EMBs, the most common indication was the study of ventricular dysfunction or suspicion of myocarditis (70% of the cases). However, this indication was less common in right-side EMBs (48.9% of the cases).
All electroanatomic mapping-guided EMBs were performed after the implementation of the plan. The definitive anatomopatho- logical diagnosis was achieved in 2 of the 3 EMBs (one case of myocarditis and one case of enteroviral cardiomyopathy). Also, specific therapy was prescribed in both cases.
Table 1. Baseline characteristics and EMB procedural characteristics. Overall and comparisons before and after the implementation of the plan of action and safety
| Total (n = 204) | Before the implementation (n = 80) | After the implementation (n = 124) | P | |
|---|---|---|---|---|
| Main characteristics | ||||
| Age (years) | 52.1 ± 17.1 | 50.4 ± 16.5 | 53.2 ± 17.4 | .252 |
| Males (%) | 60.3 | 55.0 | 63.7 | .214 |
| LVEF (%) | 44.2 ± 17.2 | 48.1 ± 18.9 | 42.5 ± 16.2 | .060 |
| IVS (mm) | 12.8 ± 4.5 | 12.7 ± 4.5 | 12.8 ± 4.5 | .927 |
| BSA (m2) | 1.83 ± 0.21 | 1.81 ± 0.23 | 1.84 ± 0.20 | .632 |
| Number of valid specimens | 3.6 ± 1.4 | 3.0 ± 1.2 | 4.0 ± 1.4 | < .001 |
| Duration of the procedure (min) | 43.3 ± 19.9 | 47.8 ± 22.5 | 41.1 ± 18.2 | .038 |
| X-ray imaging time (min) | 12.1 ± 7.1 | 12.6 ± 6.3 | 11.9 ± 7.6 | .516 |
| Indications | .698 | |||
| Study of unexplained ventricular dysfunction or myocarditis | 105 (51.5%) | 39 (48.8%) | 66 (53.2%) | |
| RCM or suspicion of infiltrative cardiomyopathy | 91 (44.6%) | 36 (45.0%) | 55 (44.4%) | |
| Ventricular arrhythmias | 5 (2.5%) | 3 (3.7%) | 2 (1.6%) | |
| Tumors | 3 (1.4%) | 2 (2.5%) | 1 (0.8%) | |
| Location of the EMB | .003 | |||
| Right ventricle only | 172 (84.3%) | 76 (95.0%) | 96 (77.4%) | |
| Left ventricle only | 30 (14.7%) | 4 (5.0%) | 26 (21.0%) | |
| Biventricular | 2 (1.0%) | 0 | 2 (1.6%) | |
| 52.0 | 42.5 | 58.1 | .030 | |
|
BSA, body surface area; EMB, endomyocardial biopsy; IVS, interventricular septum; LVEF, left ventricular ejection fraction; RCM, restrictive cardiomyopathy. |
||||
Safety and major complications
In our series, there were 10 major complications that amounted to a 4.9% overall rate. No patient died. All complications occurred while performing the EMB in the right cavities, except for two cases of transient ischemic attack that occurred while performing two left-side EMBs. Table 3 shows all major complications and their progression.
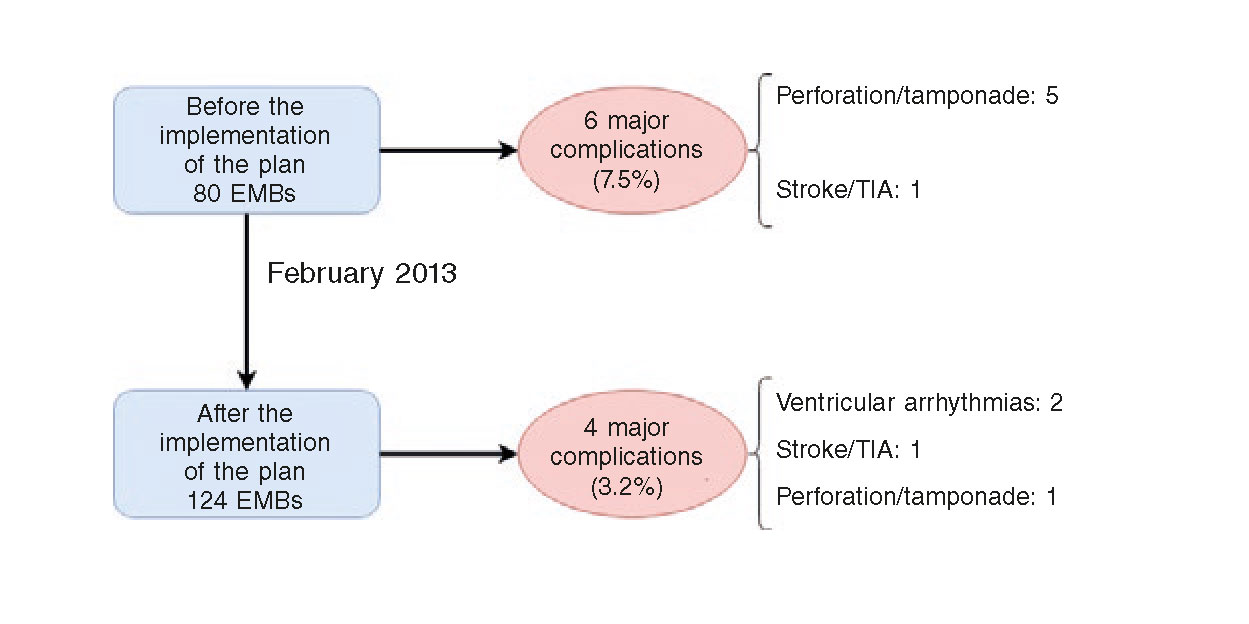
Figure 2 shows the main major complications that took place in our series before and after the implementation of the plan of action and safety. It is important to say that after the implementation of this plan the major complications were cut in half (from 7.5% to 3.2%), although this difference was not statistically significant (P = .167). This decrease was due to less cases of cardiac perforation with only one case being reported after the implementation of the plan (6.3% before vs 0.8% after the implementation of the plan; P = .025).
Figure 2. Major complications associated with the EMB before and after the implementation of the plan of action and safety. EMB, endomyocardial biopsy. TIA, transient ischemic attack.
DISCUSSION
Even though over the last few years we have not had any significant advances in the non-invasive diagnosis of acute rejection in heart transplant recipients11,12 or in the non-invasive diagnosis of different cardiomyopathies,13-16 the EMB is still the gold standard procedure to achieve a definitive diagnosis in these situations. The findings of the EMB can also have relevant prognostic implications. However, the diagnostic yield of this technique is not absolute and varies from one series published to the next (table 4).8,9,17-24 In our series it was impossible to achieve a definitive anatomopathological diagnosis in little over half the cases. It is interesting to see how the diagnostic yield of our series improved significantly after the implementation of the plan basically to the detriment of an improved diagnostic yield in cases of ventricular dysfunction or on suspicion of myocarditis. The advances made in immunohistochemical techniques and genomic detection methods, the planning of all cases by choosing the most suitable approach for each patient (based on the type of cardiomyopathy suspected), the experience accumulated, and the acquisition of a larger amount of specimens in every procedure are some of the reasons that would justify such a change.
The EMB rates from series published by high-volume centers indicate rates of major complications below 1% (table 4). In our series, the rate of complications is higher. The fact that the most common indication in our center was for the study of ventricular dysfunction could explain this, since this group of patients has a higher risk of complications.25 It is important to emphasize here that the implementation of the plan, added to the role played by the learning curve in this technique23,24 have cut the occurrence of major complications at our center in half revealing a rate of perforations below 1%. We believe that our results show a more realistic situation of EMBs currently performed in our setting. Therefore, we believe that this type of procedure should not be trivialized and should be performed at centers with enough experience and under the supervision and rules from a clear-cut plan of action of safety.
Table 2. Diagnostic yield in each and every EMB indication
| Indication for EMB | Diagnostic yield | Definitive anatomopathological diagnosis |
|---|---|---|
| Study of unexplained ventricular dysfunction or suspicion of myocarditis (n = 105) | Total: 43.8% Before the implementation: 28.2% After the implementation: 53.0% (P = .013) |
Myocarditis: 37 (35.2%) HCM: 4 (3.8%) Amyloidosis: 2 (1.9%) Cobalt toxicity: 2 (1.9%) Mitochondrial cardiomyopathy: 1 (1.0%) Undiagnosed: 61 (58.1%) |
| Suspicion of RCM or infiltration (n = 91) | Total: 61.5% Before the implementation: 58.3% After the implementation: 63.6% (P = .611) |
Amyloidosis: 44 (48.4%) HCM: 7 (7.7%) EMF: 2 (2.2%) Sarcoidosis: 1 (1.1%) Myocarditis: 1 (1.1%) Fabry: 1 (1.1%) Undiagnosed: 35 (38.5%) |
| Ventricular arrhythmias (n = 5) | Total: 40.0% | MCH: 1 (20.0%) Myocarditis: 1 (20.0%) Undiagnosed: 3 (60.0%) |
| Cardiac tumors (n = 3) | Total: 66.7% | Angiosarcoma: 2 (66.7%) Undiagnosed: 1 (33.3%) |
|
EMB, endomyocardial biopsy; EMF, endomyocardial fibrosis; HCM, hypertrophic cardiomyopathy. The overall diagnostic yield of the entire series is shown here as well as the comparison between the 2 stages (before and after the implementation of the plan of action and safety) in the 2 main indications. The definitive anatomopathological diagnosis of each indication is expressed as absolute numbers and percentages using brackets. |
||
Table 3. Summary of major complications in chronological order of appearance
| Patient | Date of the procedure | Age (years) | Sex | Indication for the EMB | Location | Final diagnosis | Complication | Treatment |
|---|---|---|---|---|---|---|---|---|
| 1 | June 2017 | 66 | Female | Study of ventricular dysfunction or suspicion of myocarditis | RV | Undiagnosed | Perforation with cardiac tamponade | Pericardiocentesis |
| 2 | November 2016 | 40 | Male | Study of ventricular dysfunction or suspicion of myocarditis | LV | Lymphocytic myocarditis | TIA | Did not need |
| 3 | June 2016 | 35 | Male | Suspicion of RCM or infiltrative cardiomyopathy | Biventricular (RV) | Sarcoidosis | SMVT during right-side EMB with hemodynamic instability | Electrical cardioversion |
| 4 | May 2015 | 71 | Male | Suspicion of RCM or infiltrative cardiomyopathy | RV | Amyloidosis | Ventricular arrhythmia leading to asystole | Transcutaneous cardiac pacing and IV atropine |
| 5 | January 2013 | 49 | Female | Study of ventricular dysfunction or suspicion of myocarditis | RV | Undiagnosed | Perforation with cardiac tamponade | Pericardiocentesis |
| 6 | October 2012 | 55 | Female | Study of ventricular dysfunction or suspicion of myocarditis | RV | Undiagnosed | Perforation with cardiac tamponade | Surgery |
| 7 | October 2011 | 82 | Male | Suspicion of RCM or infiltrative cardiomyopathy | RV | Amyloidosis | Severe pericardial effusion with no signs of hemodynamic compromise | Delayed surgery (due to persistent pericardial effusion at follow-up) |
| 8 | July 2011 | 67 | Male | Suspicion of RCM or infiltrative cardiomyopathy | LV | Amyloidosis | TIA | Did not need |
| 9 | June 2008 | 51 | Male | Study of ventricular dysfunction or suspicion of myocarditis | RV | Undiagnosed | Perforation with cardiac tamponade | Pericardiocentesis |
| 10 | May 2007 | 37 | Male | Study of ventricular dysfunction or suspicion of myocarditis | RV | Lymphocytic myocarditis | Perforation with cardiac tamponade and cardiorespiratory arrest | Surgery |
|
EMB, endomyocardial biopsy; IV, intravenous; LV, left ventricle; RCM, restrictive cardiomyopathy; RV, right ventricle; SMVT, sustained monomorphic ventricular tachycardia; TIA, transient ischemic attack. |
||||||||
Table 4. Diagnostic yield and major complications in the main EMB series in the non-transplant setting published to date
| Author (year) | Number of EMBs | Location/vascular access | Average number of specimens/EMBs | Diagnostic yield | Major complications |
|---|---|---|---|---|---|
| Deckers et al.17(1992) | 546 | RV/jugular (96.2%); femoral (1.3%); subclavian (0.5%). | 6 ± 2 | Not indicated | 0.5% perforations 0.4% mortality |
| Felker et al.18 (1999)a | 1278 | RV/jugular | Not published | 16% | 0.9% |
| Bennet et al.19(2013) | 851 | RV/not indicated | 5.6 | 25.5% | 0.9% |
| Hiramitsu et al.20(1998)b | 19 964 | RV (84.3%); LV (56.7%); RA (6.0%) | 2.6 in RV 2.8 in LV 2.2 in RA |
Not indicated | 0.7% perforations 0.05% mortality |
| Holzmann et al.8 (2008)c | 3048 | RV/femoral | 8.2 ± 0.8 (retrospective); 10.1 ± 0.6 (prospective) |
Not indicated | 0.12% in retrospective series 0% in prospective series |
| Yilmaz et al.9 (2010) | 755 | RV (17.1%); LV (35.1%); BiV (47.3%)/femoral | 5.6 ± 1.5 in RV; 5.8 ± 1.5 in LV; 8.4 ± 3.5 BiV |
BiV 79.3% vs UniV 67.3% | 1.1% (BiV 0.56% vs UniV 1.51%) |
| Fiorelli et al.21 (2012) | 1783 | RV/jugular + 5 cases LV | Not indicated | Not indicated | 0.8% 0.2% mortality |
| Jang et al.22 (2013) | 228 | RV/femoral | 5.6 ± 2.3 | Not indicated | 1.3% |
| Chimenti et al.23 (2013) | 4221 | RV (15.9%); LV (27.3%); BiV (56.8%)/femoral | 4.2 ± 1.6 in RV; 4.5 ± 1.2 in LV; 8.7 ± 1.6 BiV |
LV 96.3% vs RV 71.4% in BiV EMBs | 0.39% (LV 0.33% vs RV 0.5%) |
| Isogai et al.24 (2015)d | 9167 | Not indicated | Not indicated | Not indicated | 0.9% |
|
BiV, biventricular; EMB, endomyocardial biopsy; LV, left ventricle; RA, right atrium; RV, right ventricle; UniV, univentricular. |
|||||
In some series, the acquisition of specimens from both ventricles has improved the procedural diagnostic yield without increasing the rate of complications reported.9,23 Our experience on this regard is still limited, but still we could confirm that left-side EMBs were more widely accepted after the implementation of the plan. In Spain this approach has been used until recently for the diagnosis of cardiomyopathies. The difference in the diagnostic criteria used in other left ventricle and biventricular EMB series makes it difficult to draw any comparisons with our own results. We would like to highlight that, in our experience, the left-side EMB is a safe technique (with only one complication reported since the implementation of the protocol) with a diagnostic yield similar to the one of right-side EMBs. This statement is even more valuable if we think that main the indication for left-side EMBs was the suspicion of myocarditis where traditionally the EMB has a limited diagnostic yield.26 In sum, we strongly believe that this is a useful approach that can provide with valuable information in these cases.
Over the last few years, the use of radial access to acquire left-side EMBs has been gradually replaced by the femoral access in our series. There is evidence on the medical literature of its feasibility and safety27-31 with a growing interest in its implementation in the clinical practice since this technique has been perfected with thinner catheters and bioptomes, and sheathless catheters specially designed for this access. The risk of complications is potentially lower. Also, same as it happens with coronary interventions through radial access,32 this technique allows to reduce hospital stays and discharge patients just after a few hours under hospital observation.
Performing electroanatomic mapping-guided EMBs is a promising strategy to improve the diagnostic yield of this procedure. Ever since Corrado et al.33 described for the first time the correlation between areas of low voltage and fibrofatty replacement in patients with arrhythmogenic right ventricular dysplasia, several studies have validated and confirmed the safety of this combined approach for the diagnosis of several cardiomyopathies.34 Our own experience with these cases is still limited. Nevertheless, we believe this is a promising technique for the diagnosis of cardiomyopathies with patchy distribution such as myocarditis or cardiac sarcoidosis. Also, it allows us to optimize the acquisition of specimens, reduce its number, and direct the bioptome towards transition areas instead of areas of greater necrosis where the risk of perforation is higher.
Limitations
Our study has several limitations. In the first place, this was a retrospective study with all the biases associated with a study of this nature when it comes to obtaining relevant data. Secondly, this study included the experience of a single center, which is why results are not easy to generalize. On the other hand, and since this is a reference center on the management of cardiomyopathies in advanced functional class and amyloidosis, it is possible that patients were overrepresented in our series.
CONCLUSIONS
In our own experience, the EMB is a technique with an attractive diagnostic potential in patients with suspected cardiomyopathy. However, we should not forget that this procedure can also lead to potentially serious complications. This study shows that the implementation of a plan of action and safety allows to minimize the appearance of complications and improve the diagnostic yield of EMBs.
CONFLICTS OF INTEREST
None declared.
WHAT IS KNOWN ABOUT THE TOPIC?
- The EMB is a key tool for the diagnosis of several cardiomyopathies.
- Yet despite its huge diagnostic potential, this procedure can lead to serious complications.
- The large series of EMBs published so far show rates of complications that are usually low (< 1%) and variable data of diagnostic yield.
WHAT DOES THIS STUDY ADD?
- The results of safety and diagnostic yield of an EMB series in the non-transplant setting performed at our center in a great variety of clinical contexts were presented here. This is the largest Spanish series ever published to this date.
- We describe the technical characteristics of the EMB technique, and the details of the plan of action and safety approved by our center to perform EMBs.
- We proved, for the very first time, that the implementation of a plan of action and safety generates lower rates of major complications and improves the diagnostic yield of EMBs.
SUPPLEMENTARY DATA
REFERENCES
1. Costanzo MR, Dipchand A, Starling R, et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant Off Publ Int Soc Heart Transplant. 2010;29:914-956.
2. Cooper LT, Baughman KL, Feldman AM, et al. The role of endomyocardial biopsy in the management of cardiovascular disease:a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J Am Coll Cardiol. 20076;50:1914-1931.
3. Caforio ALP, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis:a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636-2648.
4. Caforio ALP, Adler Y, Agostini C, et al. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases:a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur Heart J. 2017;38:2649-2662.
5. Kent G, Sutton DC, Sutton GC. Needle biopsy of the human ventricular myocardium. Q Bull Northwest Univ Evanst Ill Med Sch. 1956;30:213-214.
6. Sakakibara S, Konno S. Endomyocardial biopsy. Jpn Heart J. 1962;3:537-543.
7. Francis R, Lewis C. Myocardial biopsy:techniques and indications. Heart. 2018;104:950-958.
8. Holzmann M, Nicko A, Kühl U, et al. Complication rate of right ventricular endomyocardial biopsy via the femoral approach:a retrospective and prospective study analyzing 3048 diagnostic procedures over an 11-year period. Circulation. 2008;118:1722-1728.
9. Yilmaz A, Kindermann I, Kindermann M, et al. Comparative evaluation of left and right ventricular endomyocardial biopsy:differences in complication rate and diagnostic performance. Circulation. 2010;122:900-909.
10. Sławek S, Araszkiewicz A, Gaczkowska A, et al. Endomyocardial biopsy via the femoral access —still safe and valuable diagnostic tool. BMC Cardiovasc Disord. 2016;16:222.
11. Miller CA, Fildes JE, Ray SG, et al. Non-invasive approaches for the diagnosis of acute cardiac allograft rejection. Heart Br Card Soc. 2013;99:445-453.
12. Mingo-Santos S, Moñivas-Palomero V, Garcia-Lunar I, et al. Usefulness of Two-Dimensional Strain Parameters to Diagnose Acute Rejection after Heart Transplantation. J Am Soc Echocardiogr. 2015;28:1149-1156.
13. Yoshida A, Ishibashi-Ueda H, Yamada N, et al. Direct comparison of the diagnostic capability of cardiac magnetic resonance and endomyocardial biopsy in patients with heart failure. Eur J Heart Fail. 2013;15:166-175.
14. Zhao L, Fang Q. Recent advances in the noninvasive strategies of cardiac amyloidosis. Heart Fail Rev. 2016;21:703-721.
15. Bami K, Haddad T, Dick A, Dennie C, Dwivedi G. Noninvasive imaging in acute myocarditis. Curr Opin Cardiol. 2016;31:217.
16. Spieker M, Katsianos E, Gastl M, et al. T2 mapping cardiovascular magnetic resonance identifies the presence of myocardial inflammation in patients with dilated cardiomyopathy as compared to endomyocardial biopsy. Eur Heart J Cardiovasc Imaging. 2018;19:574-582.
17. Deckers JW, Hare JM, Baughman KL. Complications of transvenous right ventricular endomyocardial biopsy in adult patients with cardiomyopathy:a seven-year survey of 546 consecutive diagnostic procedures in a tertiary referral center. J Am Coll Cardiol. 1992;19:43-47.
18. Felker GM, Hu W, Hare JM, Hruban RH, Baughman KL, Kasper EK. The spectrum of dilated cardiomyopathy. The Johns Hopkins experience with 1,278 patients. Medicine (Baltimore). 1999;78:270-283.
19. Bennett MK, Gilotra NA, Harrington C, et al. Evaluation of the role of endomyocardial biopsy in 851 patients with unexplained heart failure from 2000-2009. Circ Heart Fail. 2013;6:676-684.
20. Hiramitsu S, Hiroe M, Uemura A, Kimura K, Hishida H, Morimoto S. National survey of the use of endomyocardial biopsy in Japan. Jpn Circ J. 1998;62:909-912.
21. Fiorelli AI, Benvenuti L, Aielo V, et al. Comparative analysis of the complications of 5347 endomyocardial biopsies applied to patients after heart transplantation and with cardiomyopathies:a single-center study. Transplant Proc. 2012;44:2473-2478.
22. Jang SY, Cho Y, Song JH, et al. Complication Rate of Transfemoral Endomyocardial Biopsy with Fluoroscopic and Two-dimensional Echocardiographic Guidance:A 10-Year Experience of 228 Consecutive Procedures. J Korean Med Sci. 2013;28:1323-1328.
23. Chimenti C, Frustaci A. Contribution and risks of left ventricular endomyocardial biopsy in patients with cardiomyopathies:a retrospective study over a 28-year period. Circulation. 2013;128:1531-1541.
24. Isogai T, Yasunaga H, Matsui H, et al. Hospital volume and cardiac complications of endomyocardial biopsy:a retrospective cohort study of 9508 adult patients using a nationwide inpatient database in Japan. Clin Cardiol. 2015;38:164-170.
25. Elbadawi A, Elgendy IY, Ha LD, et al. National Trends and Outcomes of Endomyocardial Biopsy for Patients With Myocarditis:From the National Inpatient Sample Database. J Card Fail. 2018;24:337-341.
26. Kindermann I, Kindermann M, Kandolf R, et al. Predictors of outcome in patients with suspected myocarditis. Circulation. 2008;118:639-648.
27. Schulz E, Jabs A, Gori T, et al. Feasibility and safety of left ventricular endomyocardial biopsy via transradial access:Technique and initial experience. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. 2015;86:761-765.
28. Bagur R, Bertrand OF, Béliveau P, et al. Feasibility of using a sheathless guiding catheter for left ventricular endomyocardial biopsy performed by transradial approach. J Invasive Cardiol. 2014;26:E161-163.
29. Schäufele TG, Spittler R, Karagianni A, et al. Transradial left ventricular endomyocardial biopsy:assessment of safety and efficacy. Clin Res Cardiol Off J Ger Card Soc. 2015;104:773-781.
30. Bagur R, Gilchrist IC. Transradial approach to take a little piece of heart. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. 2015;86:766-767.
31. Choudhury T, Schäufele TG, Lavi S, et al. Transradial Approach for Left Ventricular Endomyocardial Biopsy. Can J Cardiol. 2018;34:1283-1288.
32. Córdoba-Soriano JG, Jiménez-Mazuecos J, Rivera Juárez A, et al. Safety and Feasibility of Outpatient Percutaneous Coronary Intervention in Selected Patients:A Spanish Multicenter Registry. Rev Esp Cardiol. 2017;70:535-542.
33. Corrado D, Basso C, Leoni L, et al. Three-dimensional electroanato-mic voltage mapping increases accuracy of diagnosing arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2005;111:3042-3050.
34. Vaidya VR, Abudan AA, Vasudevan K, et al. The efficacy and safety of electroanatomic mapping-guided endomyocardial biopsy:a systematic review. J Interv Card Electrophysiol. 2018;53:63-71.