INTRODUCTION
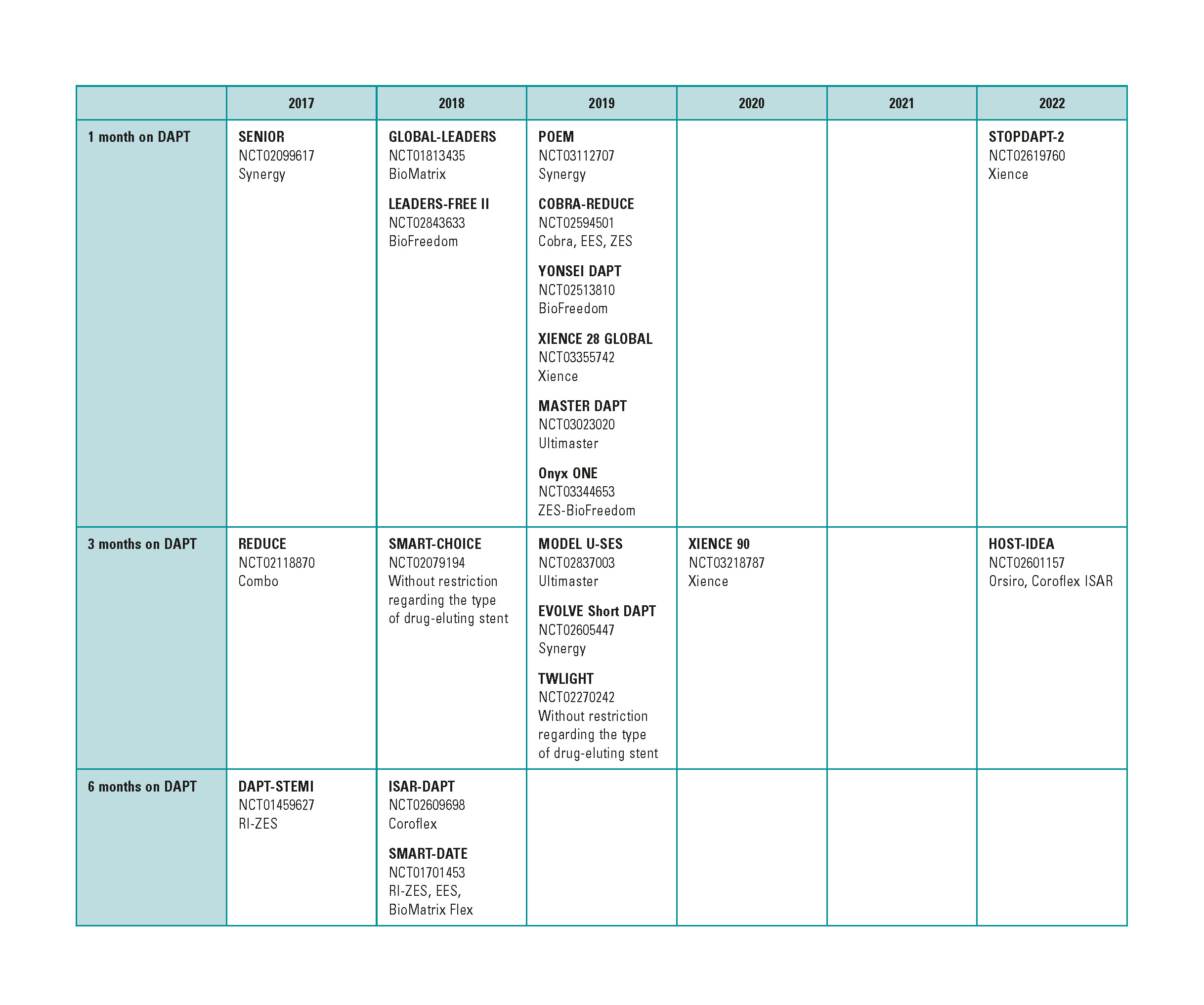
Due to the commotion caused by the growing number of late thrombosis after drug-eluting stents implantation back in 2005,1 1 year courses of dual antiplatelet therapy (DAPT) were recommended after the implants. The lower rate of this complication thanks to improved designs of the device has made it possible to shorten these courses. The shortest courses of DAPT are still under discussion2-4 (figure 1), yet 1-month courses of DAPT with further withdrawal of 1 of the 2 drugs has been agreed on. However, most 1-month DAPT studies have been conducted with low-risk patients5,6 or have focused on patients with stable coronary artery disease.7
Figure 1. Studies with short courses of dual antiplatelet therapy. DAPT, dual antiplatelet therapy.
With the progressive aging of the population, comorbidity, and the higher rate of atrial fibrillation, it has become more and more common to find patients at high risk of bleeding and rates of 15%.8 In the LEADERS FREE clinical trial9, the BioFreedom biolimus A9-eluting stent (Biosensors, Switzerland) without polymer and a stainless steel stent platform proved superior to conventional stents in patients at high risk of bleeding and on a 1-month course of DAPT with clopidogrel. Ever since, it has become the reference device in this type of patients.
To this day, the Resolute Onyx chromium-cobalt stent with zotarolimus and permanent polymer (Medtronic, United States) showed safety data with the 1 month course of DAPT,10 but no specific randomized clinical trials had been conducted on this issue. However, there were data available on a previous model with zotarolimus in this context, the Endeavor Sprint stent (Medtronic, United States), but with a different drug-release kinetics and off the market for quite a few years now.11 In the Onyx ONE clinical trial (NCT03344653), the Resolute Onyx stent was compared to the BioFreedom stent in patients at high risk of bleeding. The objective of this review was to analyze such study and put it into context with recent studies published on 1-month courses of DAPT.
DESIGN
The Onyx ONE is a prospective, randomized, multicenter study that compared the safety and efficacy profiles of the chromium-cobalt Resolute Onyx stent with permanent polymer and zotarolimus to the BioFredom biolimus A9-eluting bare-metal stent without polymer in patients at high risk of bleeding and on a 1-month course of DAPT.
The primary endpoint was a composite of cardiac death, infarction, and definitive or probable stent thrombosis at 1 year. The secondary endpoint for which the statistical power of the study was designed, was target lesion failure defined as the factors already mentioned plus ischemia-related lesion revascularization.
Other secondary endpoints were the success of the target vessel, device, procedure, BARC (Bleeding Academic Research Consortium Definition of Bleeding) bleeding score, and each particular component of the primary endpoint. Inclusion criteria are shown on table 1, they are exactly the same ones as those used in the LEADERS FREE study,9 and focus on patients at high risk of bleeding.
Table 1. Inclusion criteria of the Onyx ONE clinical trial
| Indication for percutaneous coronary intervention and, at least, one of the following criteria: |
|---|
| Age ≥ 75 years-old |
| Oral anticoagulation after stent implantation |
| Hemoglobin 11 g/L or transfusion during the previous 4 weeks |
| Platelets < 100 000/mm3 |
| Hospital admission due to bleeding during the previous 12 months |
| Stroke during the previous 12 months |
| Past medical history of intracranial bleeding |
| Acute liver failure |
| Creatinine clearance < 40 mL/min |
| Cancer within the previous 3 years |
| Scheduled surgery during the 12 following months |
| Corticosteroids or non-steroidal anti-inflammatory drugs during the first month following stent implantation |
| Suspicious compliance to dual antiplatelet therapy from the first month |
A crucial aspect of this study is antithrombotic regimens. During the first month, all patients should receive 75-100 mg/day of acetylsalicylic acid (ASA) plus a P2Y12 receptor inhibitor, preferably clopidogrel. In patients on oral anticoagulation, during this first month, single antiplatelet therapy or DAPT were allowed. After the first month, 1 of the 2 antiplatelet drugs was withdrawn, one or the other.
Its non-inferiority design included 2000 patients randomized on a 1:1 basis with an estimated event rate in each arm of 9.4 for the primary endpoint and a non-inferiority margin of 4.1%. In record time, the study was completed with 1996 patients and a final follow-up period of 98% in both study groups between November 2017 and September 2018.
RESULTS
The mean age of both groups was > 74 years-old and the percentage of diabetics was > 38%. A third of these patients showed atrial fibrillation and the indication was distributed equally between the group of stable patients and those with acute coronary syndrome (ACS), yet only 5% showed ST-segment elevation. The 4 most common inclusion criteria were age ≥ 75 years-old (61% of the patients); oral anticoagulation (38%); anemia or transfusion during the last year (15%) and creatinine clearance < 40 mL/min (15%).
Lesions were type B2 or C in 80% of the cases, and the length of the vessel covered by the stent was 37 mm. No differences were seen in the target lesion and procedural success, yet the Resolute Onyx stent group had better results in success with the device. Crossing from this group to the BioFreedom group occurred in 2 cases, and from this group to the Resolute Onyx group in 40 cases. On the other hand, the zotarolimus-eluting stent also showed significant differences with less residual stenosis and more initial angiographic gain.
Two months after the procedure, 92% of the patients remained on single antiplatelet therapy, 56% on ASA and 44% on clopidogrel. These same percentages remained for a whole year, when 88% of the patients were still on single antiplatelet therapy.
The primary endpoint of non-inferiority was met with an event rate of 17.1% in the Resolute Onyx group and 16.9% in the BioFreedom group (difference, 0.2%; upper limit of the confidence interval, 3.0%; P value for non-inferiority = .011). No significant differences were found on the event rate for each particular component of the primary endpoint or on the secondary endpoint of target lesion failure. No differences were reported either in the rates of BARC bleeding (table 2).
Table 2. 12-month results of the Onyx ONE clinical trial
| Resolute Onyx | BioFreedom | P | |
|---|---|---|---|
| Cardiac death, infarction, stent thrombosis | 17.1 | 16.9 | .84 |
| Cardiac death | 4.6 | 3.9 | .40 |
| Infarction | 13.5 | 15 | .50 |
| Periprocedural | 9.4 | 7.9 | .26 |
| Spontaneous at follow-up | 4.6 | 7.1 | .02 |
| Probable or definitive stent thrombosis | 1.3 | 2.1 | .22 |
| Early (first month) | 0.6 | 1.3 | |
| Late (between the first month and 1 year) | 0.7 | 0.7 | |
| Target lesion failure | 18 | 17.9 | .84 |
| Cardiac death | 4.5 | 3.7 | .43 |
| Target lesion-related infarction | 12.8 | 14.0 | .43 |
| Ischemia-guided revascularization | 2.8 | 4.0 | .17 |
| Successful device | 92.8 | 89.7 | .007 |
| BARC bleeding | |||
| 1-5 | 17.7 | 16.3 | .43 |
| 2-5 | 15.1 | 13.7 | .40 |
| 3-5 | 4.5 | 4.9 | .67 |
|
BARC, Bleeding Academic Research Consortium Definition of Bleeding. |
|||
DISCUSSION
Who is the winner in this study?
In conclusion, it can be said that the results obtained have been similar with both stents, except for the greater success achieved with the Resolute Onyx stent due to a lower crossover rate. This is not unexpected given the different platform designs. The Resolute Onyx is a single sinusoidal strut with an external cobalt alloy frame and an internal core of 90% platinum and 10% iridium alloy with an 81 µm-thick mesh. The BioFreedom has an older 316-L and 120 µm stainless steel design. However, to this day, both stents are the only devices we have evidence of in patients at high risk of bleeding, and both with positives results. On the one hand, the Onyx ONE study confirms the good results obtained by the BioFreedom stent in the LEADERS FREE trial and comes as a response to the criticism on the quality of the conventional stent used whose strut thickness was far beyond that of other available stents. On the other hand, the Resolute Onyx study showed promising data when if DAPT should be withdrawn after the first month,10 but these data came from studies that we not designed for this analysis and, therefore, with limited reliability. This study confirms the safety profile of this device in patients at high risk of bleeding.
How should we interpret the study results?
Added to the strut thickness of the conventional stent used in the LEADERS FREE, the rate of events was controversial too. The Onyx ONE study used similar inclusion criteria and, as a consequence, patients were very similar. In this case there is a cardiovascular mortality rate of 4% and an overall stent thrombosis rate slightly < 2%. However, the myocardial infarction rate doubles that of the LEADERS FREE study, which is certainly surprising since the criterion used was similar in both studies: the third universal definition of myocardial infarction.12 It seems obvious that we have to wait for the publication of the study to know if this is due to differences in the patients’ baseline risk or to other reasons. In any case, there is no doubt that the higher event rate seen at the primary endpoint and in its particular components compared to most studies on new generations of stents is explained by the higher risk of the patients included; it is clearly a higher risk profile because of age; percentage of diabetes; prior history of bleeding; and oral anticoagulation.
Is there a class effect for all the drug-eluting stents in these patients?
There are several stents available with CE marking for short DAPT regimens, 1 month included. It should be reminded that CE marking is not an indication but an on-label use under certain circumstance and that this recommendation is always accompanied by the message that the courses recommended by the guidelines should be followed. Also, that early interruptions are the responsibility of the treating physician and that the individual condition of every patient needs to be taken into consideration.
This study shows the results of these 2 stents in patients at high risk of bleeding. Another 3 studies with 1-month DAPT regimens focused on only one model of stent have been conducted: the SENIOR7, theSTOPDAPT-2,6 and the GLOBAL LEADERS.5 Other studies like the SMART-CHOICE13 and the recently published TWILIGHT4 assessed 3-month courses of DAPT and included patients with different stents, which is why they design is different from the Onyx ONE.
The SENIOR study7 randomized 1200 patients ≥ 75 year old to receive a conventional stent or the SYNERGY stent (Boston Scientific, United States) plus a 1-month course of DAPT in stable patients, and a 6-month course in patients with ACS. In 88% of the cases, clopidogrel was used during DAPT. The results were favorable to the drug-eluting stent with a 12% vs 16% event rate in the primary endpoint of all-cause mortality, infarction, stroke or target lesion revascularization, and a similar rate of bleeding (5%), and stent thrombosis (1%). This study provided data on the safety and efficacy profiles of the SYNERGY stent in old patients and followed a 6-month course for the management of ACS, which corresponds to 45% of the patients included. On the other hand, and although they were older patients compared to the Onyx ONE, the percentage of diabetics and patients with atrial fibrillation was significantly lower. For all this, although this study shows favorable data on how the SYNERGY behaved in old patients, we should bear in mind that they were different patients on a different course.
The STOPDAPT-2 study6 analyzed 3045 patients treated with the chromium-cobalt Xience stent (Abbott Vascular, United States) and compared a standard 12-month course of DAPT plus ASA and clopidogrel (with clopidogrel withdrawal after this time) to a 1-month course of DAPT (with ASA withdrawal after this time and continuation with clopidogrel for another 5 years). During the first month, prasugrel was allowed, but from that moment on the P2Y12 receptor inhibitor was always clopidogrel. The primary endpoint of non-inferiority was reached, but again the population risk was lower compared to the Onyx ONE. Mean age was 68.6 years-old, only 38% showed ACS, less than 1% received oral anticoagulation and, above all, 90% had low or intermediate thrombotic and bleeding risk according to the CREDO-Kyoto and PARIS risk scores. Once again, although it is a very important study with favorable data for the Xience stent, they were different patients on a different course.
Finally, the GLOBAL LEADERS study5 analyzed 15 968 patients and compared a 1-month course of DAPT plus ticagrelor and ASA followed by a 24-month course of ticagrelor to a 12-month standard therapy of DAPT plus ASA an clopidogrel in patients with stable angina or ticagrelor in patients with ACS followed by another 12 months with ASA only. All patients received the biolimus A9 stent. In this study, the course of the intervention was not superior to the standard one since, although results were favorable during the first year, the heavy bleeding seen during the second year led to a negative study primary endpoint at 2 years. On the other hand, once again the risk profile of patients was lower compared to the Onyx ONE: age was much younger; there were fewer diabetic patients; bleeding rate was < 1%; no patients on oral anticoagulation were included.
FINAL CONSIDERATIONS
Although we still have to wait for its publication, we can say that the Onyx ONE is a landmark study for 2 reasons. First, because it shows that, to this day, the Resolute Onyx stent has the same clinical results, even superior in terms of device success, compared to the reference BioFreedom stent in patients at high risk of bleeding. This means that now we have 2 highly valid options in this context. Second, because of the high prevalence of ACS patients in the trial. Half of the patients included had stable coronary artery disease and the guidelines recommend the administration of 1-month course of DAPT.14 It is precisely in patients with ACS that the study is more important because it changes completely the course recommended by clinical practice guidelines that indicate DAPT between 6 and 12 months according to the PRECISE-DAPT score.14 The study is important because it includes patients at high risk of bleeding in whom this complication can be more important than ischemic risk. We should mention that the 12-month recommendation in patients with ACS comes from the CURE study15 that, although included 12 562 patients, did not show statistically significant differences regarding death and stroke (2 of the 3 major adverse events that were part of the primary endpoint). The study was positive after confirmation of a reduced non-fatal infarction rate. We should also mention that the information provided by the Onyx ONE study on patients with ACS is still limited. This is so because we only have data of the overall study and, although in the LEADERS FREE this subgroup benefited from the use of drug-eluting stents vs conventional stent,16 we still don’t have comparative data of both stents in patients with ACS.
Lastly, in this study, the short course of DAPT includes 2 strategies. One strategy recommends shortening the therapy compared to the guidelines recommendations, 6 to 12 months for the management of ACS. The other strategy is withdrawing ASA in half of the patients as the only antiplatelet agent from the second to the twelfth month. Although the strategy of withdrawing ASA from the first or third months has become a matter of study,4-6,13 we will still have to wait for the publication of the study to know if there is a really significant interaction here.
CONFLICTS OF INTEREST
None declared.
REFERENCES
1. Camenzind E, Steg PG, Wijns W. Stent thrombosis late after implantation of first-generation drug-eluting stents:a cause for concern. Circulation. 2007;115:1440-1455;discussion 1455.
2. Feres F, Costa RA, Abizaid A, et al. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents:the OPTIMIZE randomized trial. JAMA. 2013;310:2510-2522.
3. Kim BK, Hong MK, Shin DH, et al. A new strategy for discontinuation of dual antiplatelet therapy:the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol. 2012;60:1340-1348.
4. Mehran R, Baber U, Sharma SK, et al. Ticagrelor with or without Aspirin in High-Risk Patients after PCI. N Engl J Med. 2019;381:2032-2042.
5. Vranckx P, Valgimigli M, Juni P, et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent:a multicentre, open-label, randomised superiority trial. Lancet. 2018;392:940-949.
6. Watanabe H, Domei T, Morimoto T, et al. Effect of 1-Month Dual Antiplatelet Therapy Followed by Clopidogrel vs 12-Month Dual Antiplatelet Therapy on Cardiovascular and Bleeding Events in Patients Receiving PCI:The STOPDAPT-2 Randomized Clinical Trial. JAMA. 2019;321:2414-2427.
7. Varenne O, Cook S, Sideris G, et al. Drug-eluting stents in elderly patients with coronary artery disease (SENIOR):a randomised single-blind trial. Lancet. 2018;391:41-50.
8. Morice MC, Urban P, Greene S, Schuler G, Chevalier B. Why are we still using coronary bare-metal stents?J Am Coll Cardiol. 2013;61:1122-1123.
9. Urban P, Meredith IT, Abizaid A, et al. Polymer-free Drug-Coated Coronary Stents in Patients at High Bleeding Risk. N Engl J Med. 2015;373:2038-2047.
10. Silber S, Kirtane AJ, Belardi JA, et al. Lack of association between dual antiplatelet therapy use and stent thrombosis between 1 and 12 months following resolute zotarolimus-eluting stent implantation. Eur Heart J. 2014;35:1949-1956.
11. Valgimigli M, Patialiakas A, Thury A, et al. Zotarolimus-eluting versus bare-metal stents in uncertain drug-eluting stent candidates. J Am Coll Cardiol. 2015;65:805-815.
12. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581-1598.
13. Hahn JY, Song YB, Oh JH, et al. Effect of P2Y12 Inhibitor Monotherapy vs Dual Antiplatelet Therapy on Cardiovascular Events in Patients Undergoing Percutaneous Coronary Intervention:The SMART-CHOICE Randomized Clinical Trial. JAMA. 2019;321:2428-2437.
14. Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS:The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39:213-260.
15. Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494-502.
16. Naber CK, Urban P, Ong PJ, et al. Biolimus-A9 polymer-free coated stent in high bleeding risk patients with acute coronary syndrome:a LEADERS FREE ACS sub-study. Eur Heart J. 2017;38:961-969.