ABSTRACT
Transcatheter aortic valve implantation (TAVI) is the most commonly used structural technique in the field of interventional cardiology. Initially, this procedure was only used in patients with severe symptomatic aortic stenosis and prohibitive risk for surgical aortic valve replacement. In just 1 decade, TAVI indications have extended to patients at intermediate surgical risk. More recently, the results of the PARTNER 3 and Evolut Low Risk clinical trials has opened that door for patients at low surgical risk. However, there are still some controversial indications that represent the boundaries of TAVI including patients at lower risk with bicuspid aortic valve, valve-in-valve procedures, pure aortic regurgitation or severe valvular heart disease after healed infective endocarditis. Our objective was to summarize the evidence avilable –mostly case series and retrospective registries– that supports the use of TAVI for these new indications.
Keywords: TAVI. Bicuspid aortic valve. Aortic regurgitation. Aortic bioprosthesis.
RESUMEN
El implante percutáneo de válvula aórtica (TAVI) es la técnica de intervencionismo estructural cardiaco más extendida. Inicialmente, el procedimiento se realizaba solo en pacientes con estenosis aórtica grave sintomática, con riesgo prohibitivo para el reemplazo valvular aórtico quirúrgico. En pocos años, esta técnica se extendió a pacientes con riesgo quirúrgico intermedio y, más recientemente, gracias a los resultados de los estudios PARTNER 3 y Evolut Low Risk, se ha abierto esta indicación para pacientes de bajo riesgo. Sin embargo, existen algunas indicaciones controvertidas que marcan la frontera en la evidencia del uso de TAVI, incluyendo pacientes con riesgo quirúrgico intermedio-bajo y válvula aórtica bicúspide, procedimientos válvula en válvula, casos de insuficiencia aórtica pura y pacientes con secuelas valvulares graves tras una endocarditis infecciosa «curada». Nuestro objetivo es resumir la evidencia disponible —fundamentalmente basada en series de casos y registros retrospectivos— referente al empleo de TAVI en estas nuevas indicaciones.
Palabras clave: TAVI. Válvula aórtica bicúspide. Insuficiencia aórtica. Bioprótesis aórtica.
Abbreviations AoR: aortic regurgitation. BAV: bicuspid aortic valve. IE: infective endocarditis. SAVR: surgical aortic valve replacement. SVD: structural valve deterioration. TAVI: transcatheter aortic valve implantation. ViV: valve-in-valve.
INTRODUCTION
The growing incidence of age-related aortic stenosis (AS) has turned the aortic valve into the most commonly treated heart valve, both surgically and percutaneously, in Europe and the United States.1 Since the first off-label transcatheter aortic valve implantation (TAVI) procedure back in 2002,2 the international experience gained with the use of TAVI has grown. Parallel to this there has been an increase of alternative off-label indications for this technology. These indications have encouraged the clinical practice guidelines to gradually include more recommendations. Universally accepted for high and intermediate surgical risk patients,3-6 TAVI has dramatically changed the management of AS over the last decade. However, the recent publication of the PARTNER 37 and Evolut Low Risk8 trials that showed TAVI outcomes at the 1-year follow-up that were similar to those of traditional surgical aortic valve replacement (SAVR) raises the question of whether there are limits to this technology or if it will ever become the gold standard treatment. The doubts on its long-term durability, something essential for its widespread indication in younger patients is increasingly seen as the sword of Damocles rather than the Achilles heel of this technology by surgical defenders blinded to the course of events.
Although it is beyond the scope of this work, it is worth mentioning that another current limitation of TAVI is the challenging trans-vascular approach. Although transfemoral TAVI is the gold standard, this approach is not feasible or too risky in around 15% of the patients.9 The transcarotid TAVI approach has had promising results –better than all the other transthoracic approaches–but are still far from the outcomes obtained with transfermoral cases.10 Importantly, a recent meta-analysis showed that the trans-subclavian approach may not only be an alternative route to transfemoral access but also a competitive one in certain patients with higher risk of femoral artery injury.11
New indications and alternative approaches for TAVI have increased gradually preceded by its use as a compassionate alternative. In this study we describe the current boundaries of these indications by reviewing the main off-label uses of TAVI and the reported outcomes in such challenging scenarios.
SPECIFIC INDICATIONS FOR TAVI
There are several controversial TAVI indications today; however, we have decided to exclude certain uncommon indications and focus on the following ones: a) TAVI for bicuspid AS; b) TAVI for valve-in-valve (ViV) procedures; c) TAVI for pure aortic regurgitation (AoR); and d) TAVI for valvular severe dysfunction following healed infective endocarditis (IE).
TAVI for the management of biscuspid AS
Incidence and specific challenges of bicuspid AS
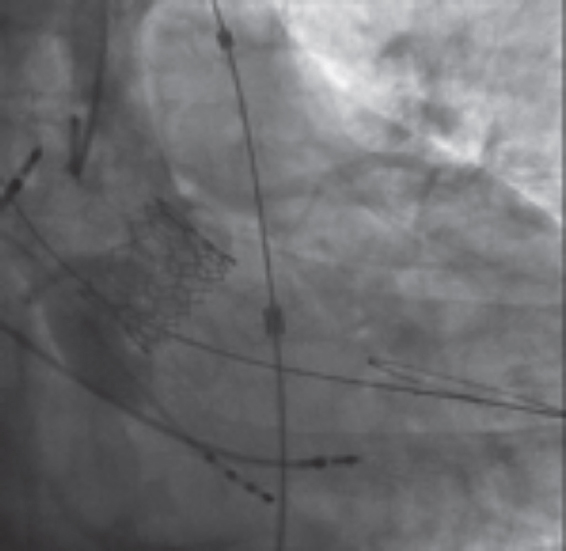
Bicuspid aortic valve (BAV) is the most common congenital valvular defect. It has been reported in up to 1% to 2% of the general population.12 It is more common in younger patients with severe AS, but it is present in elderly patients as well. BAV is associated with increased mechanical stress, which predisposes to calcification and the development of AS.13 BAV stenosis has been considered an anatomical challenge for TAVI for the following reasons: a) the shape of the annulus is often extremely elliptical and tends to aortic dilation compared to the characteristic annular oval shape of calcified tricuspid aortic valve (TAV) that may be associated with greater leakage; b) BAVs usually have a higher cusp coaptation point that can be a confounding factor during the procedure and increase the risk of valve embolization (figure 1); and, c) the asymmetric distribution of calcium with a tendency to bulky formations increases the risk of paravalvular leak and annular rupture.14 All these elements should be taken into account when considering TAVI for patients with BAV since stent malapposition is more common in patients with these abnormalities and may be associated with higher rates of paravalvular regurgitation, valvular dysfunction or early degeneration of the implanted valve.15
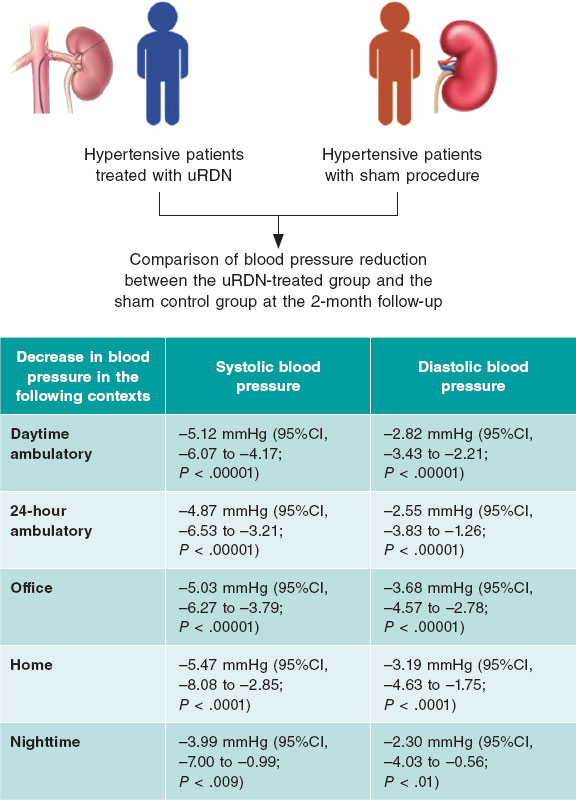
Figure 1. Example of balloon-expandable TAVI (Myval, Meril Lifesciences, India) in a biscuspid aortic stenosis. The relatively high position is due to the high coaptation level of the leaflets that is common of bicuspid aortic valves.
Current evidences of TAVI for the management of BAV
Patients with BAV have not been included in landmark trials of TAVI devices. Patients who need aortic valve replacement due to AS at a younger age (< 60 years) often have congenital BAV. For this reason, patients with BAV often have less comorbidities and the heart team usually decides to perform SAVR. When TAVI is the preferred option, meticulous valve sizing and procedural planning are important to achieve good results.16 To this day, all the specific studies dedicated to analyze the different outcomes of TAVI in the management of patients with BAV and TAV have been retrospective studies. We identified 13 studies that proved the feasibility and safety of TAVI in BAV stenosis. The main baseline characteristics and procedural outcomes are shown on table 1 and table 2.16-28 In their meta-analysis Quintana et al.29 reviewed the results of studies that focused on early-generation devices mainly.16-20 This analysis showed that the TAVI therapy was feasible and safe in BAV disease. The primary endpoint of the 1-year all-cause mortality revealed an 11.8% mortality rate in patients with BAV compared to 15.06% in patients with TAV. No differences were seen between the 2 groups (relative risk [RR], 1.03; 95% confidence interval [95%CI], 0.70-1.51). However, the BAV group was associated with less procedural success with the device and more significant valve regurgitation after TAVI compared to patients with TAV. Yoon et al.22 compared the procedural and clinical outcomes of patients with BAV versus TAV including new-generation devices. In the group that received early-generation devices, the BAV more commonly presented with aortic root injury (4.5% vs 0.0%; P = .015) when the balloon-expandable device was used and moderate-to-severe paravalvular leak (19.4% vs 10.5%; P = .02) when self-expanding devices were used. However, in patients with new-generation devices procedural results were similar with different valves. The 2-year cumulative all-cause mortality rates were similar between bicuspid and tricuspid AS (17.2% vs 19.4%; P = .28). Takagi et al.30 conducted the last meta-analysis available to this day and showed no statistical differences in the rates of pacemaker implantation and early- and mid-term mortality (RR, 1.35; 95%CI, 0.94-1.93 and RR, 1.00; 95CI, 0.77-1.31, respectively). However, the BAV group showed significantly more aortic valve regurgitation compared to the TAV group (RR, 1.42; 95%CI, 1.11-1.82). This setback was less common when using balloon-expandable devices compared to self-expandable ones. Maybe because of this, as shown on table 1, balloon-expandable devices have been the preferred options in most recent studies.
Table 1. Comparison between bicuspid and tricuspid aortic stenosis. Baseline data and procedural characteristics
| Reference, year | N | Age (years) | STS score (%) | Logistic EuroSCORE (%) | TF approach (%) | Balloon-expandable (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BAV | TAV | BAV | TAV | BAV | TAV | BAV | TAV | BAV | TAV | BAV | TAV | |
| Hayashida et al.,17 2013 | 21 | 208 | 82.0 ± 7.0 | 83.2 ± 6.5 | N/A | N/A | 19.9 ± 11.9 | 20.1 ± 11.4 | 61.9 | 50.5 | 52.4 | 83.7 |
| Bauer et al.,18 2014 | 38 | 1357 | 80.7 ± 6.6 | 81.8 ± 6.2 | N/A | N/A | 18.0 ± 10.0 | 20.0 ± 13.0 | 81.6 | 88.0 | 31.6 | 18.0 |
| Costopoulos et al.,16 2014 | 21 | 447 | 76.7 ± 7.1 | 79.8 ± 7.4 | 7.6 ± 4.2 | 7.8 ± 7.3 | 23.9 ± 12.0 | 24.4 ± 17.3 | 71.4 | 83.9 | 38.1 | 58.6 |
| Kochman et al.,19 2014 | 28 | 84 | 77.6 ± 5.5 | 79.1 ± 6.8 | N/A | N/A | 19.2 ± 9.0 | 18.8 ± 8.7 | 78.6 | 77.4 | 17.9 | 17.9 |
| Liu et al.,20 2015 | 15 | 25 | 75.4 ± 5.7 | 75.8 ± 5.5 | 5.6 ± 4.1 | 7.5 ± 5.9 | 16.1 ± 11.1 | 21.8 ± 14.7 | 86.7 | 92.0 | 0.0 | 0.0 |
| Sannino et al.,21 2017 | 88 | 735 | 80.2 ± 8.4 | 81.8 ± 7.9 | 7.4 ± 3.9 | 7.6 ± 3.9 | N/A | N/A | 88.6 | 87.1 | 52.3 | 59.7 |
| Yoon et al.,22 2017 | 546 | 546 | 77.2 ± 8.2 | 77.2 ± 8.8 | 4.6 ± 4.6 | 4.3 ± 3.0 | 16.1 ± 12.0 | 16.9 ± 13.9 | 79.1 | 78.8 | 57.7 | 57.1 |
| Arai et al.,23 2017 | 10 | 143 | 81.3 ± 5.1 | 82.6 ± 6.2 | N/A | N/A | 19.0 ± 12.5 | 18.1 ± 11.0 | 70.0 | 87.4 | 100.0 | 100.0 |
| Liao et al.,24 2018 | 87 | 70 | 80.2 ± 8.4 | 81.8 ± 7.9 | 7.9 ± 4.0 | 8.6 ± 4.4 | N/A | N/A | 100.0 | 100.0 | 0.0 | 0.0 |
| De Biase et al.,25 2018 | 83 | 166 | 81.4 ± 7.6 | 82.9 ± 5.7 | 5.1 ± 3.3 | 5.1 ± 2.9 | N/A | N/A | 98.8 | 98.8 | 60.2 | 36.7 |
| Xiong et al.,26 2018 | 67 | 49 | 74.0 (68.0–77.0) | 75.0 (68.0-79.0) | 6.5 (4.4–9.3) | 8.3 (5.2–9.5) | N/A | N/A | 98.5 | 100.0 | 0.0 | 0.0 |
| Kawamori et al.,27 2018 | 41 | 239 | 80 (70.5–83.0) | 83 (78.0–87.0) | N/A | N/A | N/A | N/A | 97.6 | 98.7 | 100.0 | 100.0 |
| Makkar et al.,28 2019 | 2691 | 2691 | 74.0 (66.0–81.0) | 74.0 (66.0–81.0) | 4.9 ± 4.0 | 5.1 ± 4.2 | N/A | N/A | 93.6 | 93.9 | 100.0 | 100.0 |
|
Data are expressed as mean ± standard deviation or median (interquartile range) or n (%). BAV, bicuspid aortic valve; N/A, not available; STS score, Society of Thoracic Surgeons predicted risk of mortality; TAV, tricuspid aortic valve; TF, transfemoral. |
||||||||||||
Table 2. Comparison between bicuspid and tricuspid aortic stenosis. Main outcomes
| Reference, year | Mean valve gradient (mmHg) | Valvular AoR > 2 (%) | Permanent pacemaker (%) | 30-day mortality (%) | ||||
|---|---|---|---|---|---|---|---|---|
| BAV | TAV | BAV | TAV | BAV | TAV | BAV | TAV | |
| Hayashida et al.,17 2013 | 10.0 ± 3.4 | 9.7 ± 4.1 | 19.0 | 14.9 | 14.3 | 7.2 | 1 (4.8) | 17 (8.2) |
| Bauer et al.,18 2014 | 5.5 ± 7.1 | 5.9 ± 6.8 | 23.7 | 15.0 | 15.8 | 35.0 | 4 (10.5) | 5 (11.0) |
| Costopoulos et al.,16 2014 | 10.3 ± 5.7 | 10.5 ± 4.7 | 23.8 | 21.7 | 14.3 | 15.0 | 3 (14.3) | 3.6 (3.6) |
| Kochman et al.,19 2014 | 11.5 ± 6.4 | 10.4 ± 4.5 | 32.1 | 22.6 | 28.6 | 33.3 | 1 (3.6) | 6 (7.1) |
| Liu et al.,20 2015 | 9.6 ± 3.1 | 11.0 ± 4.2 | 0.0 | 4.0 | 13.3 | 12.0 | 1 (6.7) | 2 (8.0) |
| Sannino et al.,21 2017 | 7.96 ± 4.15 | 8.5 ± 4.2 | 5.3 | 5.0 | 22.7 | 18.1 | 3 (3.4) | 23 (3.1) |
| Yoon et al.,22 2017 | 10.8 ± 6.7 | 10.2 ± 4.4 | 10.4 | 6.8 | 15.4 | 15.4 | 20 (3.7) | 18 (3.3) |
| Arai et al.,23 2017 | N/A | N/A | 0.0 | 6.0 | 0.0 | 8.4 | 0 (0.0) | 1 (0.7) |
| Liao et al.,24 2018 | 13.7 ± 8.4 | 13.0 ± 7.5 | 1.2 | 0.0 | 24.1 | 28.6 | 8 (9.2) | 3 (4.3) |
| De Biase et al.,25 2018 | 10.0 ± 4.0 | 9.8 ± 4.5 | 3.6 | 2.4 | 14.5 | 10.2 | 4 (4.8) | 5 (3.0) |
| Xiong et al.,26 2018 | 13.5 (10.0 - 17.0) | 13.0 (10.0 - 18.0) | N/A | N/A | 25.4 | 22.4 | 6 (9.0) | 2 (4.1) |
| Kawamori et al.,27 2018 | 11.9 ± 4.2 | 10.8 ± 4.0 | 2.4 | 1.3 | 22.0 | 9.6 | 0 (0.0) | 1 (0.4) |
| Makkar et al.,28 2019 | N/A | N/A | N/A | N/A | 9.1 | 7.5 | 66 (2.6) | 63 (2.5) |
|
Data are expressed as mean ± standard deviation or median (interquartile range) or n (%). AoR, aortic regurgitation; BAV, bicuspid aortic valve; N/A, not available; TAV, tricuspid aortic valve. |
||||||||
Will TAVI be the future gold standard treatment for the management of BAV?
TAVI has proven to be an excellent option for selected BAV cases, which is consistent with the data collected so far. In order to extend its indications, Elbadawi et al.31 compared TAVI to SAVR and showed similar in-hospital mortality rates (3.1% vs. 3.1%; odds ratio [OR], 1.00; 95%CI, 0.60-1.67). No differences between TAVI and SAVR were reported in the rates of procedural complications and early outcomes such as cardiac arrest, cardiogenic shock, acute kidney injury, cardiac tamponade or acute stroke. TAVI was associated with lower rates of acute myocardial infarction, postoperative bleeding complications, and shorter hospital stays. However, TAVI was associated with higher rates of complete heart block and permanent pacemaker implantation (13.8% vs 4.6%; OR, 3.32; 95%CI, 2.34-4.71; P < .001).
Keypoints: use of TAVI in the management of BAV
In conclusion, the use of TAVI in BAV seems like a good alternative regarding mortality and major complications. However, balloon-expandable devices may have a slightly higher rate of annular rupture and self-expandable devices a higher rate of pacemaker and paravalvular leak as in alternative scenarios. Dedicated randomized trials that compare TAVI versus SAVR are justified in the future and may open the way to a new gold standard treatment in younger patients with BAV.
TAVI for ViV procedures
Bioprosthetic structural valve deterioration
Bioprosthetic valves have limited durability compared to mechanical valves and eventually fail between 5 and 20 years after the intervention; however, when this happens they can be treated using ViV procedures instead of mechanical valves. Also, bioprosthetic valves do not require anticoagulation, which minimizes the risks associated with this procedure.32,33 These factors have led to a significant increase in the use of these procedures over the last 2 decades.
Structural valve deterioration (SVD) is an acquired intrinsic bioprosthetic valve abnormality defined as the deterioration of the leaflets or supporting structures that results in thickening, calcification, tearing or disruption of the prosthetic valve materials eventually leading to prosthetic valve hemodynamic dysfunction. SVD may present as stenosis, regurgitation or both. Mechanical stress, collagen fiber disruption, and tissue calcification are the main contributors to this process.32 Although there is not a standard definition of SVD,33-35 the growing use of TAVI for ViV procedures with certain cases wrongly indicated to treat pre-existing severe mismatch makes it necessary to establish clear diagnostic criteria on the indication for ViV34. Dvir et al.32 proposed a practical definition of SVD in the valve-in-valve international data (VIVID) registry and gave recommendations on the timing of clinical and imaging assessment at the follow-up. This definition is built on different stages and each stage is associated with a specific recommendation to show SVD as a continuum instead of a binary categorical variable. Therefore, stage 1 is associated with early morphological changes in the leaflet without hemodynamic effects. Stage 2 SVD refers to the valve leaflets morphological abnormalities associated with hemodynamic dysfunction. Depending on the type of dysfunction this stage is divided into: stenosis (stage 2S) or regurgitation (stage 2R) since the clinical implications and progression of deterioration are different between these 2 failure modes. Investigators categorized a mixed moderate stenosis/regurgitation condition as Stage 2RS. In this stage 2 SVD there are symptomatic patients who may be eligible for reintervention. The most severe stage of SVD (stage 3) is the development of severe stenosis and/or regurgitation.
Indication of ViV for the management of bioprosthetic SVD
Until the past decade, when SVD would reach stage 3, the standard of care for bioprosthetic valve deterioration was to replace the valve again. The ViV proof-of-concept was described by Walther et al. back in 2007.36 Since then and due to its less invasive and more appealing nature for both patients and operators compared to having to perform open-heart surgery again, the rates of ViV procedures have grown rapidly32 even without the CE mark approval for some of the current devices. Relatively small series and some long registries on the devices used have been published since then. The results of the studies include over 20 cases of ViV procedures and are shown on table 3.37-49
Table 3. Cases series (> 20 patients) and registries of aortic valve-in-valve procedures
| Reference, year | N | THV | Age (years) | STS score (%) | Logistic EuroSCORE (%) | Procedural success (%) | Mean gradient after ViV (mmHg) | AoR > 2 (%) | PPI (%) | THV malap- position (%) | 30-day mortality (%) | 1-year mortality (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eggebrecht et al.,37 2011 | 47 | ES | 79.8 ± 7.1 | 11.6 ± 8.5 | 35.0 ± 18.5 | 100 | 17.0 ± 10 | 2 | N/A | 8 | 17 | N/A |
| Bedogni et al.,38 2011 | 25 | CV | 82.4 ± 3.2 | 8.2 ± 4.2 | 31.5 ± 14.8 | 100 | 13.8 | 0 | 12 | N/A | 12 | 16 |
| Bapat et al.,39 2012 | 23 | ES | 76.9 (43-92) | 7.6 ± 3.8 | 31.8 ± 15.3 | 100 | 9.1 | 0 | 0 | 4.3 | 0 | 12.5 |
| Linke et al.,40 2012 | 27 | CV | 74.8 ± 8 | N/A | 31.3 ± 17 | 100 | 18 ± 8 | 7.4 | 3.7 | 3.7 | 7.4 | 12.5 |
| Dvir et al.,41 2012 | 202 | CV/ES | 77.7 ± 10.4 | 11.8 ± 9.9 | 31.1 ± 16.4 | 93.1 | 15.9 ± 8.6 | 5.0 | 7.4 | 15.3 | 8.4 | 14.2 |
| Dvir et al.,42 2014 | 459 | CV/ES | 77.6 ± 9.8 | 9.8 (6.2-16.1) | 29 (19.1-42.3) | 93.1 | 15.8 ± 8.9 | 5.4 | 8.3 | 15.3 | 7.6 | 16.8 |
| Ihlberg et al.,43 2013 | 45 | CV/ES | 80.6 (61-91) | 15.0 ± 10.8 | 35.4 ± 16.1 | 95.6 | 16.4 ± 8.7 | 2 | 7 | 2.2 | 4.4 | 11.9 |
| Camboni et al.,44 2015 | 31 | CV/ES/ME/SA | 77.8 ± 6.3 | 20.9 ± 8.8% | N/A | 88 | 16.1 ± 7.2 | N/A | 6 | N/A | 22.5 | N/A |
| Webb et al.,45 2017 | 365 | ES | 78.9 ± 10.2 | 9.1 ± 4.7 | 12.3 ± 9.8 | 97.5* | 17.6 (16.2 – 19.1) | 1.9 | 1.9 | 2.7 | 12.4 | |
| Zenses et al.,46 2018 | 79 | CV/ES/P | 74.5 ± 11.0 | N/A | 10.2 ± 2.7 | 78.5 | 22.2 ± 9.3 | 3.9 | 3.8 | N/A | N/A | N/A |
| de Freitas Campos Guimaraes et al.,47 2018 | 116 | CV/ES | 76 ± 11 | 8.0±5.1% | N/A | 94.8 | 18.5±10.5 | 4.3 | 5.2 | N/A | 6.9 | 25.9 (3-years) |
| Tuzcu et al.,48 2018 | 1150 | CV/ES | 79 (74–85) | 6.9 (4.5-10.8) | N/A | 96.9* | 16.0 (10.0-22.0) | 3.5 | 3.0 | < 1% | 2.9 | 11.7 |
| Holzamer et al.,49 2019 | 85 | AN | 77 ± 8 | 6.8 ± 6.0 | 11.4 ± 7.9 | 99 | 16 ± 8 | 10 | 1 | N/A | 5 | 8 |
|
Data are expressed as mean ± standard deviation or median (interquartile range) or n (%). *Not explicit in the text. Procedural success according to the Valve Academic Research Consortium (VARC) criteria. AoR, aortic regurgitation; AN, ACURATE neo; CV, CoreValve; ES, Edwars Sapien; ME, Medtronic Engager; N/A, not available; P, Portico; PPI, permanent pacemaker implantation; SA, Symetis ACURATE; STS score, Society of Thoracic Surgeons predicted risk of mortality; THV, transcatheter heart valve; ViV, valve-in-valve. |
||||||||||||
Although in 2012 Dvir et al.41 suggested that the ViV procedure was technically demanding and should be spared for highly experienced centers, nowadays this procedure is performed in all TAVI-capable centers and –unlike Dvir et al. predicted–is probably not considered as one of the most complex scenarios anymore. However, operators need to be skilled on valve malapposition, retrieval techniques, implantation of a second TAVI device, and management of the feared coronary occlusion. During the screening stage, the heart team should take all these factors into consideration. Also, the mechanism of SVD should be assessed by cardiac imaging experts familiar with structural procedures and taken into consideration when having to choose the TAVI model and the right size.
New techniques and challenges for ViV procedures
Positioning during ViV procedures can be very challenging as it is predictive of the risk of coronary obstruction, which is more likely when the leaflets are sutured outside the sewing ring or in stentless valves.49 Better devices and dedicated techniques are being rapidly developed to help operators achieve better outcomes including fracturing the ring during postdilatation to improve the transvalvular gradients of patients with prior small bioprosthetic valves and certain degree of mismatch50 or the BASILICA technique (bioprosthetic or native aortic scallop intentional laceration to prevent coronary artery obstruction).51 These procedures are based on short series of cases but are growing rapidly given their promising results.
A relatively new problem which will become eventually bigger is the TAVI-in-TAVI procedure. Little is known about the mid- and long-term durability of transcatheter aortic valves beyond the first decade of implantation.52 Although the transcatheter ViV procedure is now accepted as a good alternative to having to perform surgery again in high-risk patients with failed surgical bioprosthetic valves, the TAVI-in-TAVI procedure is associated with specific risks depending on the type of device used. On the one hand, supra-annular self-expandable valves may present a higher risk of coronary occlusion if treated with the current devices, which makes access to the coronary ostia even more challenging. On the other hand, intra-annular devices may have worse residual gradients after the ViV or a higher risk of annular rupture when postdilatation is performed. Overall, the scarce data available today regarding this new scenario seem favorable.52,53
Keypoints: ViV
Despite the tendency to underestimate the risks of ViV or the long-term impact of poor acute hemodynamic results, the truth is that the ViV procedure is far from being a well-established technique despite the large number of cases performed to this day. To have optimal outcomes technical improvements and new devices are needed in both the transcatheter and surgical fields.
TAVI for the management of pure AoR
Mechanisms of AoR and current management
AoR is characterized by its prolonged silent clinical course. When patients with severe AoR become symptomatic, they present with volume overload related congestive heart failure, increased wall stress, and left ventricular dysfunction.1 There are other differences compared to AS. On the one hand, the anatomy of patients with native aortic valve regurgitation is often challenging with dilated aortic root, dilated ascending aorta, and often an elliptical annulus.54 On the other hand, patients with AoR are usually referred for valve replacement at a younger age due to the different mechanisms involved in the AoR like degenerative, congenital, rheumatic and, less commonly, infectious disease or radiotherapy.55 For these reasons, SAVR is the standard therapy.1
The role of TAVI in the management of patients with AoR
However, the advances made in the technology of the valves and the accumulated experience have led to the off-label use of TAVI for the management of inoperable or high-risk patients with AoR.56 As a matter of fact, TAVI has been contraindicated for the management of pure AoR due to absent or scarce valve calcification, which makes fixing the device even more challenging.57 Since Roy et al.55 published the first case series of TAVI for the management of pure native AoR other retrospective studies have been published trying to generate evidence and show the feasibility and safety of TAVI for this indication. As it occurs with other TAVI indications, performing a preoperative echocardiography and a three-dimensional multislice computed tomography should be mandatory. Careful assessment of the diameters of the annulus and sinus of Valsalva followed by the measurement of the ascending aortic diameter become essential. Valve sizing should match the perimeter and area too. However, proper annular contrast enhancement is often challenging during the computed tomography scan and the dimensions of the annulus can quickly change if the procedure is not performed shortly after the assessment of the images.
Table 4 and table 5 show the main registries on this topic.55,57-65 Studies have been arranged chronologically and, as Takagi et al.66 did in their recent meta-analysis, the information has been classified into early- and new-generation devices with educational purposes.
Table 4. Cases series (> 20 patients) and registries of TAVI on the management of pure aortic regurgitation. Baseline clinical and echocardiographic data
| Reference, year | N | THV | Age (years) | STS score (%) | Logistic EuroSCORE (%) | Mean LVEF (%) | Mean LVEDD (mm) | Significant MR* (%) |
|---|---|---|---|---|---|---|---|---|
| Roy et al.,55 2013 | 43 | CV | 75.3 ± 8.8 | 10.2 ± 5.3 | 26.9 ± 17.9 | 45.5 ± 12.9 | 59.4 ± 13.7 | 32.6 |
| Seiffert et al.,58 2014 | 31 | JE | 73.8 ± 9.1 | 5.4 ± 3.6 | 23.6 ± 14.5 | 46.8 ± 16.1 | N/A | 20.0 |
| Testa et al.,59 2014 | 26 | CV | 73 ± 10 | 13.1 ± 2 | 24 ± 8 | 45 ± 14 | N/A | 50.0 |
| Frerker et al.,60 2015 | 22 | CV/ES | 80 ± 7.6 | N/A | 25 ± 18 | N/A | N/A | N/A |
| Zhu et al.,61 2016 | 33 | JV | 74.2 ± 5.2 | N/A | 24.4 ± 5.1 | N/A | N/A | N/A |
| Yoon et al.,57 EGD 2017 | 119 | CV/ES | 74.2 ± 13.1 | 7.6 ± 6.7 | N/A | 44.5 ± 14.3 | 62 ± 11 | 35.1 |
| Yoon et al.,57 NGD 2017 | 212 | CV/ES/JE/JV/SA/DF/ME/LO/P | 74.2 ± 11.6 | 6.2 ± 6.7 | N/A | 46.3 ± 14.8 | 60 ± 11 | 35.6 |
| Sawaya et al.,62 2017 | 78 | CV/ES/JE/DF/LO | 74 ± 10 | 6.7 ± 4.8 | 23.6 ± 14.5 | 42.7 ± 13.8 | 58.5 ± 10.2 | 43.3 |
| Liu et al.,63 2018 | 43 | JV | 73.9 ± 5.7 | N/A | 25.5 ± 5.3 | 55.9 ± 10.8 | 60.5 ± 8.4 | 9.3 |
| De Backer et al.,64 EGD 2018 | 109 | CV/ES | 74 ± 13 | 6.9 ± 7.5 | N/A | 44 ± 15 | N/A | 42.9 |
| De Backer et al.,64 NGD 2018 | 145 | CV/ES/JE/SA/DF/ME/LO/P | 75 ± 10 | 6.2 ± 4.9 | N/A | 45 ± 15 | N/A | 43.5 |
| Silaschi et al.,65 2018 | 30 | JE | 74.4 ± 9.3 | 17.7 ± 14.8 | 4.9 ± 3.5 | 49.6 ± 13.3 | N/A | 50.0 |
|
Data are expressed as mean ± standard deviation. *Significant MR, mitral regurgitation (at least grade). CV, CoreValve; DF, Direct Flow; EDG, early-generation devices; ES, Edwars Sapien; JV, J Valve; JE, JenaValve; LO, Lotus; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic dimension; ME, Medtronic Engager; MR, mitral regurgitation; N/A, not available; NGD, new-generation devices; P, Portico; SA, Symetis ACURATE; STS score, Society of Thoracic Surgeons predicted risk of mortality; TAVI, transcatheter aortic valve implantation; THV, transcatheter heart valve. |
||||||||
Table 5. Cases series (> 20 patients) and registries of TAVI on the management of pure aortic regurgitation. Procedural and follow-up outcomes
| Reference, year | Procedural success (%) | Conversion to open surgery | THV-in-THV (%) | Annulus rupture | Reintervention | PPI (%) | ≥ moderate PVR | 30-day mortality (%) |
|---|---|---|---|---|---|---|---|---|
| Roy et al.,55 2013 | 74.4 | 2.3 | 18.6 | N/A | N/A | 16.3 | 4.7 | 9.3 |
| Seiffert et al.,58 2014 | 96.8 | 0.0 | 0.0 | 0.0 | N/A | 6.5 | 0.0 | 12.9 |
| Testa et al.,59 2014 | 76.9 | 0.0 | 19.2 | N/A | N/A | 7.7 | 23.1 | 23.1 |
| Frerker et al.,60 2015 | 81.8 | N/A | N/A | N/A | N/A | 27.3 | N/A | 22.7 |
| Zhu et al.,61 2016 | 93.9 | 3.0 | N/A | N/A | N/A | 6.1 | 3.0 | 3.0 |
| Yoon et al.,57 EGD 2017 | 61.3 | 3.4 | 24.4 | 1.7 | 5.0 | 17.5 | 18.8 | 13.4 |
| Yoon et al.,57 NGD 2017 | 81.1 | 3.8 | 12.7 | 1.4 | 3.8 | 18.6 | 4.2 | 9.4 |
| Sawaya et al.,62 2017 | 70.5 | N/A | 16.7 | N/A | 2.6 | 18.5 | 13.4 | 14.3 |
| Liu et al.,63 2018 | 97.7 | 2.3 | 0.0 | 0.0 | 4.7 | 2.3 | 2.4 | 2.3 |
| De Backer et al.,64 EGD 2018 | 46.5 | N/A | N/A | N/A | 3.7 | N/A | 25.5 | 17.1 |
| De Backer et al.,64 NGD 2018 | 82.5 | N/A | N/A | N/A | 4.4 | N/A | 4.7 | 7.7 |
| Silaschi et al.,65 2018 | 88.9 | 3.7 | 0.0 | 0.0 | 3.3 | 3.8 | 0.0 | 10.0 |
|
EGD, early-generation devices; N/A, not available; NGD, new-generation devices; PPI, permanent pacemaker implantation; PVR, paravalvavular regurgitation; TAVI, transcatheter aortic valve implantation; THV, transcatheter heart valve. |
||||||||
Alternative TAVI devices for the management of AoR
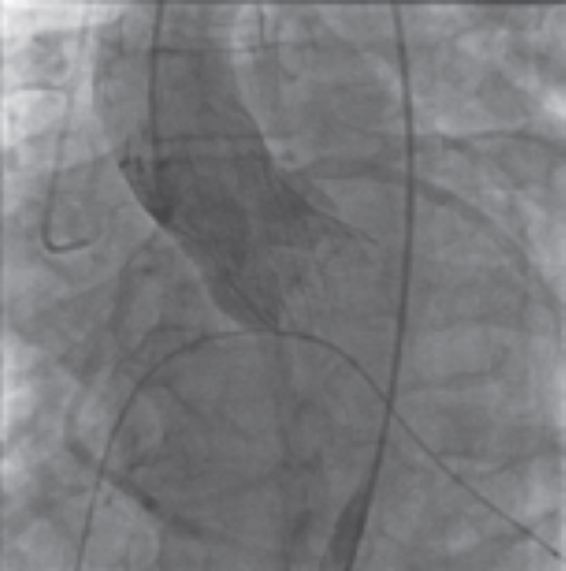
The Medtronic CoreValve (Medtronic, United States) was the preferred option in most of the early reports of TAVR in patients with pure native AoR.60,67-74 Its self-expandable properties were thought to offer stability during implantation and guarantee valve fixation even in the absence of heavy calcification (figure 2). However, the regular need for ViV implantation and the moderate-to-high rates of postoperative AoR grade III-IV (table 5, results from Roy et al.55 and Testa et al.59) resulted in a modest change in the definition of device success according to the Valve Academic Research Consortium.35 This was a heads-up on the limitations of this device for its use in the setting of this specific off-label indication.54 Other self-expandable transcatheter valves such as the ACURATE neo (Boston Scientific, United States), Lotus (Boston Scientific, United States), Portico (Abbott, United States), and the balloon-expandable Edwards SAPIEN XT/S3 (Edwards Lifesciences, United States) have been used for the management of AoR (table 4) with variable outcomes but poorer results compared to the management of patients with AS.
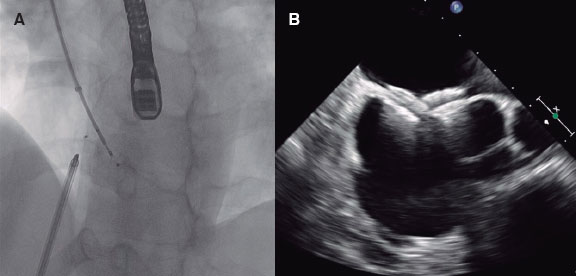
Figure 2. Example of a self-expandable TAVI (Evolut R, Medtronic, United States) in pure aortic regurgitation. Note that in this case there is no residual aortic regurgitation in the angiographic assessment.
New devices have been developed for the management of patients with pure severe AoR. The JenaValve (JenaValve Technology, Germany) was the only new-generation repositionable valve with self-positioning geometry and specific fixation mechanisms with Conformité Européenne mark for the management of AoR. A nitinol stent frame that houses a trileaflet porcine pericardial valve with 3 feelers that allow the correct anatomical orientation of the valve plus a special clipping mechanism that fixes the device onto the native leaflets. The first report of a TAVR with the JenaValve included 5 patients with moderate or severe AoR and high surgical risk; procedural success was achieved in all cases and no death or stroke was reported at the 30-day follow-up.75 A similar performance was observed in longer registries as shown on table 4 and table 5. However, this device is no longer in the market. The J-Valve system61,76 (JieCheng Medical Technology, China), not available in Europe yet, has 3 U-shaped and anatomically oriented device “graspers” for “self-positioning” purposes during valve prosthesis implantation and provides radial fixation by embracing the native valve leaflets in a “clip mechanism”. The Direct Flow Medical Transcatheter Aortic Valve System (Direct Flow Medical, California) consists of 2 systems of rings (ventricular and aortic) that are independently inflated with a contrast-saline mixture during the deployment phase plus a polymer that solidifies and brings support while the device is being fixated in its final position. Schofer et al.77 reported their experience with this device in 11 high-risk patients with 100% device success and an early safety rate of 91% according to VARC-2 criteria.35
Keypoints: TAVI for the management of pure AoR
TAVR for the management of pure native AoR with early-generation devices has been associated with relatively high rates of procedural complications. The development of new-generation devices improved procedural outcomes with lower rates of second valve implantation need57 or significant postoperative AoR (≥ grade 2).57,64 However, recent studies57 prove that a significant reduction of the degree of AoR is not enough since postoperative AoR ≥ 2 is associated with higher rates of re-hospitalization and all-cause mortality. This is indicative that there is still a long way to go before reaching the level of evidence currently available for TAVI for the management of patients with AS. New devices on the pathophysiology of AoR are needed but for the time being TAVI should be only considered in selected cases of non-calcified AoR after clinical and imaging assessment.
TAVI for the management of severe valvular dysfunction after healed IE
Current relevance of IE
IE affects between 1 and 10 cases per 100 000 individuals each year.78,79 The detection, management, and treatment have slightly improved in recent years, although a concomitant rise in its incidence has been reported.80,81 Also, the rates of mortality and complication remain stable.81 The life-threatening aspect of this entity is evident in its mortality rates (between 15% and 30%) depending on the patients’ baseline conditions, the causative organism, and the presence of other complications like cerebrovascular events.78 Approximately, half of the patients affected by IE require cardiac surgery to treat the infection or the associated complications. However, many of the patients with an indication for surgery due to residual valvular lesion are not eligible for surgery due to high surgical risk. When the aortic valve is damaged, TAVI may be a potential therapeutic option despite its current contraindication established by the guidelines due to risk of reinfection concerns. However, the damaged valve can be treated with TAVI once the infection has been resolved.78
Experience with TAVI in the setting of healed IE with residual valvular damage
There are very few cases in the medical literature on the use of TAVI following IE. Back in 2013, Albu et al.82 described the first case of a healed IE related severe aortic homograft stenosis successfully treated with a self-expandable TAVI. In 2015, Nguyen et al.83 described the first case of valve-in-valve procedure to treat a healed IE in a patient treated with TAVI inside a surgical bioprosthetic valve. Both cases had good clinical outcomes at the mid-term follow-up (6 and 12 months, respectively). There are no larger series that confirm the good results of TAVI in healed IE leaving dysfunctional valves. However, in a subanalysis of their long-term registry of surgical treatment in patients with AS, Pechlivanidis et al.84 suggested the possibility of using transcatheter valves to treat patients who overcame an IE and were at very high risk for conventional surgery.
Evidences supporting the use of TAVI after healed IE
To our knowledge, the most complete review of potential candidates for TAVI following IE was the study conducted by Garcia-Granja et al.85 They analyzed 182 patients treated with aortic valve surgery due to IE and looked for predictors of active local infection at the time of the intervention through explant tissue cultures. The main independent predictors of active local infection were diabetes mellitus, Staphylococcus aureus, and concomitant compromised mitral valve. In contrast, an interval between the diagnosis and the intervention of over 9 days was predictive of healed infection. Without predisposing criteria for active infection, the risk of positive cultures in the explanted tissue was ~3%. This hypothesis-generating research supports the use of TAVI in selected cases with healed infections but residual valve damage, high surgical risk, and no predisposing criteria for active local infection.
Keypoints: use of TAVI after healed IE
In patients who are not eligible for surgery but have a low risk of local infection according to the «IE team», TAVI may be an option to treat residual aortic valve damage. More evidence is still needed before knocking down the current contraindication of TAVI in this setting.
CONCLUSIONS
Although it may be controversial there is a growing interest in the off-label uses of TAVI devices to solve several uncovered clinical scenarios. However, the level of evidence is variable across these indications and several technological advances and controlled clinical trials are still needed. Although there is a large number of studies that support the use of TAVI in patients with bicuspid AS or SVD of a prior surgical bioprosthetic valve, the indication for the management of pure native AoR or healed IE with residual aortic valve dysfunction is, at least for now, under discussion as a last-resort procedure.
CONFLICTS OF INTEREST
None declared.
REFERENCES
1. Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739-2791.
2. Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis:first human case description. Circulation. 2002;106:3006-3008
3. Leon MB, Smith CR, Mack M, et al.;PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597-1607.
4. Arnold SV, Reynolds MR, Wang K, et al. Health status after transcatheter or surgical aortic valve replacement in patients with severe aortic stenosis at increased surgical risk:results from the CoreValve US pivotal trial. JACC Cardiovasc Interv. 2015;8:1207-1217. ?
5. Reardon MJ, Van Mieghem NM, Popma JJ, et al., for the SURTAVI Investigators. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321-1231.
6. Leon MB, Smith CR, Mack MJ, et al., for the PARTNER 2 Investigators. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609-1220.
7. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Eng J Med. 2019;380:1695-1705.
8. Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706-1715.
9. Guedeney P, Mehran R. Non-femoral TAVR:Time to stratify alternative vascular approaches. Catheter Cardiovasc Interv. 2018;92:1194-1195.
10. Overtchouk P, Folliguet T, Pinaud F, et al. Transcarotid Approach for Transcatheter Aortic Valve Replacement With the SAPIEN 3 Prosthesis:A Multicenter French Registry. JACC Cardiovasc Interv. 2019;12:413-419.
11. Amat-Santos IJ, Rojas P, Gutiérrez H, et al. Transubclavian approach:A competitive access for transcatheter aortic valve implantation as compared to transfemoral. Catheter Cardiovasc Interv. 2018;92:935-944.
12. Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation. 2008;117:2776-2784.
13. Roberts WC, Janning KG, Ko JM, Filardo G, Matter GJ. Frequency of congenitally bicuspid aortic valves in patients ≥80 years of age undergoing aortic valve replacement for aortic stenosis (with or without aortic regurgitation) and implications for transcatheter aortic valve implantation. Am J Cardiol. 2012;109:1632e1636.
14. Watkins C, Gupta A, Griffith BP. Preoperative imaging. In:Transcatheter Aortic Valve Replacement A How-to Guide for Cardiologists and Cardiac Surgeons. Watkins C, Gupta A, Griffith BP, eds. 1st ed. Springer;2018. 40-41.
15. Buellesfeld L, Stortecky S, Kalesan B, et al. Aortic root dimensions among patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2013;1:72-83.
16. Costopoulos C, Latib A, Maisano F, et al. Comparison of results of transcatheter aortic valve implantation in patients with severely stenotic bicuspid versus tricuspid or nonbicuspid valves. Am J Cardiol. 2014;113:1390-1393.
17. Hayashida K, Bouvier E, Lefèvre T, et al. Transcatheter aortic valve implantation for patients with severe bicuspid aortic valve stenosis. Circ Cardiovasc Interv. 2013;6:284-291.
18. Bauer T, Linke A, Sievert H, et al. Comparison of the effectiveness of transcatheter aortic valve implantation in patients with stenotic bicuspid versus tricuspid aortic valves (from the German TAVI Registry). Am J Cardiol. 2014;113:518-521.
19. Kochman J, Huczek Z, Scisło P, et al. Comparison of one- and 12-month outcomes of transcatheter aortic valve replacement in patients with severely stenotic bicuspid versus tricuspid aortic valves (results from a multicenter registry). Am J Cardiol. 2014;114:757-762.
20. Liu XB, Jiang JB, Zhou QJ, et al. Evaluation of the safety and efficacy of transcatheter aortic valve implantation in patients with a severe stenotic bicuspid aortic valve in a Chinese population. J Zhejiang Univ Sci B. 2015;16:208-214.
21. Sannino A, Cedars A, Stoler RC, Szerlip M, Mack MJ, Grayburn PA. Comparison of efficacy and safety of transcatheter aortic valve implantation in patients with bicuspid versus tricuspid aortic valves. Am J Cardiol. 2017;120:1601-1606.
22. Yoon SH, Bleiziffer S, De Backer, O et al. Outcomes in transcatheter aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J Am Coll Cardiol. 2017;69:2579-2589.
23. Arai T, Lefèvre T, Hovasse T, et al. The feasibility of transcatheter aortic valve implantation using the Edwards SAPIEN 3 for patients with severe bicuspid aortic stenosis. J Cardiol. 2017;70:220-224.
24. Liao YB, Li YJ, Xiong TY, et al. Comparison of procedural, clinical and valve performance results of transcatheter aortic valve replacement in patients with bicuspid versus tricuspid aortic stenosis. Int J Cardiol. 2018;254:69-74.
25. De Biase C, Mastrokostopoulos A, Philippart R, et al. Aortic valve anatomy and outcomes after transcatheter aortic valve implantation in bicuspid aortic valves. Int J Cardiol. 2018;266:56-60.
26. Xiong TY, Wang X, Li YJ, et al. Less pronounced reverse left ventricular remodeling in patients with bicuspid aortic stenosis treated with transcatheter aortic valve replacement compared to tricuspid aortic stenosis. Int J Cardiovasc Imaging. 2018;34:1761-1767.
27. Kawamori H, Yoon SH, Chakravarty T, et al. Computed tomography characteristics of the aortic valve and the geometry of SAPIEN 3 transcatheter heart valve in patients with bicuspid aortic valve disease. Eur Heart J Cardiovasc Imaging. 2018;19:1408-1418.
28. Makkar RR, Yoon SH, Leon MB, et al. Association Between Transcatheter Aortic Valve Replacement for Bicuspid vs Tricuspid Aortic Stenosis and Mortality or Stroke. JAMA. 2019;321:2193-2202.
29. Quintana RA, Monlezun DJ, DaSilva-DeAbreu A, et al. One- Year Mortality in Patients Undergoing Transcatheter Aortic valve Replacement for Stenotic Bicuspid versus Tricuspid Aortic Valves:A Meta-Analysis and Meta- Regression. J Interv Cardiol. 2019;2019:8947204.
30. Takagi H, Hari Y, Kawai N, et al. Meta-analysis of transcatheter aortic valve implantation for bicuspid versus tricuspid aortic valves. J Cardiovasc Med. 2019;20:237-244.
31. Elbadawi A, Saad M, Elgendy IY, et al. Temporal Trends and Outcomes of Transcatheter Versus Surgical Aortic Valve Replacement for Bicuspid Aortic Valve Stenosis. JACC Cardiovasc Interv. 2019;12:1811-1822.
32. Dvir D, Bourguignon T, Otto CM, et al. Standardized definition of structural valve degeneration for surgical and transcatheter bioprosthetic aortic valves. Circulation. 2018;137:388-399.
33. Paradis JM, Del Trigo M, Puri R, Rodés-Cabau J. Transcatheter valve-in-valve and valve-in-ring for treating aortic and mitral surgical prosthetic dysfunction. J Am Coll Cardiol. 2015;66:2019-2037.
34. Rodriguez-Gabella T, Voisine P, Puri R, et al. Aortic bioprosthetic valve durability. J Am Coll Cardiol. 2017;70:1013-1028.
35. Kappetein AJ, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation:The Valve Academic Research Consortium-2 consensus document. J Thorac Cardiovasc Surg. 2013;145:6-23.
36. Walther T, Falk V, Dewey T, et al. Valve-in-a- valve concept for transcatheter minimally invasive repeat xenograft implantation. J Am Coll Cardiol. 2007;50:56-60.
37. Eggebrecht H, Schäfer U, Treede H, et al. Valve-in-valve transcatheter aortic valve implantation for degenerated bioprosthetic heart valves. JACC Cardiovasc Interv. 2011;4:1218-1227.
38. Bedogni F, Laudisa ML, Pizzocri S, et al. Transcatheter valve-in-valve implantation using Corevalve Revalving System for failed surgical aortic bioprostheses. JACC Cardiovasc Interv. 2011;4:1228-1234.
39. Bapat V, Attia R, Redwood S, et al. Use of transcatheter heart valves for a valve-in- valve implantation in patients with degenerated aortic bioprosthesis:technical considerations and results. J Thorac Cardiovasc Surg. 2012;144:1372-1379.
40. Linke A, Woitek F, Merx MW, et al. Valve- in-valve implantation of Medtronic CoreValve prosthesis in patients with failing bioprosthetic aortic valves. Circ Cardiovasc Interv. 2012;5:689-697.
41. Dvir D, Webb JG, Brecker, et al. Transcatheter Aortic Valve Replacement for Degenerative Bioprosthetic Surgical Valves:Results From the Global Valve-in-Valve Registry. Circulation. 2012;126:2335-2344.
42. Dvir D, Webb JG, Bleiziffer S, et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA. 2014;312:162-170.
43. Ihlberg L, Nissen H, Nielsen NE, et al. Early clinical outcome of aortic transcatheter valve- in-valve implantation in the Nordic countries. J Thorac Cardiovasc Surg. 2013;146:1047-1054.
44. Camboni D, Holzamer A, Flörchinger B, et al. Single institution experience with transcatheter valve-in-valve implantation emphasizing strategies for coronary protection. Ann Thorac Surg. 2015;99:1532-1538.
45. Webb JG, Mack MJ, White JM, et al. Transcatheter Aortic Valve Implantation Within Degenerated Aortic Surgical Bioprostheses:PARTNER 2 Valve-in-Valve Registry. J Am Coll Cardiol. 2017;69:2253-2262.
46. Zenses AS, Dahou A, Salaun E, et al. Haemodynamic outcomes following aortic valve- in-valve procedure. Open Heart. 2018;5:e000854.
47. de Freitas Campos Guimaraes, Urena M, Wijeysundera HC, et al. Long-Term Outcomes After Transcatheter Aortic Valve-in-Valve Replacement. Circ Cardiovasc Interv. 2018;11:e007038.
48. Tuzcu EM, Kapadia SR, Vemulapalli S, et al. Transcatheter Aortic Valve Replacement of Failed Surgically Implanted Bioprostheses:The STS/ACC Registry. J Am Coll Cardiol. 2018;72:370-382.
49. Holzamer A, Kim WK, Andreas Rück, et al. Valve-in-Valve Implantation Using the ACURATE Neo in Degenerated Aortic Bioprostheses. An International Multicenter Analysis. JACC Cardiovasc Interv. 2019;12:2309-2316.
50. Amat-Santos IJ, Gutiérrez H, Sathananthan J, Webb JG. Fracture of small Mitroflow aortic bioprosthesis following valve-in-valve transcatheter aortic valve replacement with ACURATE neo valve valve –From bench testing to clinical practice. Catheter Cardiovasc Interv. 2019;10:e005216.
51. Khan JM, Dvir D, Greenbaum AB, et al. Transcatheter laceration of aortic leaflets to prevent coronary obstruction during transcatheter aortic valve replacement:concept to first-in-human. JACC Cardiovasc Interv. 2018;11:677-89.
52. Salaun E, Zenses AS, Clavel MA, et al. Valve-in-Valve Procedure in Failed Transcatheter Aortic Valves. JACC Cardiovasc Imaging. 2019;12:198-202.
53. Barbanti M, Webb JG, Tamburino C, et al. Outcomes of redo transcatheter aortic valve replacement for the treatment of postprocedural and late occurrence of paravalvular regurgitation and transcatheter valve failure. Circ Cardiovasc Interv. 2016;9:e003930.
54. Franzone A, Piccolo R, Siontis GCM, et al. Transcatheter aortic valve replacement for the treatment of pure native aortic valve regurgitation:a systematic review. JACC Cardiovasc Interv. 2016;9:2308-2317.
55. Roy DA, Schaefer U, Guetta V, et al. Transcatheter aortic valve implantation for pure severe native aortic valve regurgitation. J Am Coll Cardiol. 2013;61:1577-1584.
56. Hira RS, Vemulapalli S, Li Z, et al. Trends and outcomes of off-label use of transcatheter aortic valve replacement:insights from the NCDR STS/ACC TVT registry. JAMA Cardiol. 2017;2:846-854.
57. Yoon SH, Schmidt T, Bleiziffer S, et al. Transcatheter aortic valve replacement in pure native aortic valve regurgitation. J Am Coll Cardiol. 2017;70:2752-2763.
58. Seiffert M, Bader R, Kappert U, et al. Initial German experience with transapical implantation of a second-generation transcatheter heart valve for the treatment of aortic regurgitation. JACC Cardiovasc Interv. 2014;7:1168-1174.
59. Testa L, Latib A, Rossi ML, et al. CoreValve implantation for severe aortic regurgitation:a multicentre registry. EuroIntervention. 2014;10:739-745.
60. Frerker C, Schewe lJ, Schewel D, et al. Expansion of the indication of transcatheter aortic valve implantation —feasibility and outcome in “off-label“patients compared with “on-label“patients. J Invasive Cardiol. 2015;27:229-236.
61. Zhu D, Wei L, Cheung A, et al. Treatment of pure aortic regurgitation using a second-generation transcatheter aortic valve implantation system. J Am Coll Cardiol. 2016;67:2803-2805.
62. Sawaya FJ, Deutsch MA, Seiffert M, et al. Safety and efficacy of transcatheter aortic valve replacement in the treatment of pure aortic regurgitation in native valves and failing surgical bioprostheses:results from an international registry study. JACC Cardiovasc Interv. 2017;10:1048-1056.
63. Liu H, Yang Y, Wang W, et al. Transapical transcatheter aortic valve replacement for aortic regurgitation with a second-generation heart valve. J Thorac Cardiovasc Surg. 2018;156:106-116.
64. De Backer O, Pilgrim T, Simonato M, et al. Usefulness of transcatheter aortic valve implantation for treatment of pure native aortic valve regurgitation. Am J Cardiol. 2018;122:1028-1035.
65. Silaschi M, Conradi L, Wendler O, et al. The JUPITER registry:one-year outcomes of transapical aortic valve implantation using a second generation transcatheter heart valve for aortic regurgitation. Catheter Cardiovasc Interv. 2018;91:1345-1351.
66. Takagi H, Hari Y, Kawai N, Ando T;ALICE (All-Literature Investigation of Cardiovascular Evidence) Group. Meta-Analysis and Meta-Regression of Transcatheter Aortic Valve Implantation for Pure Native Aortic Regurgitation. Heart Lung Circ. 2019. https://doi.org/10.1016/j.hlc.2019.04.012.
67. Rossi ML, Barbaro C, Pagnotta P, Lucarelli C, Mennuni M, Presbitero P. Transcatheter aortic valve implantation in patients with pure severe native aortic regurgitation:results after 3 year of follow-up [abstract]. J Am Coll Cardiol. 2014;64(Suppl B):B220-B221.
68. Munoz-Garcia E, Munoz-Garcia M, Munoz- Garcia AJ, et al. Safety of transcatheter aortic valve implantation in patients with pure native aortic valve regurgitation [abstract]. Eur Heart J. 2015;36(Supl 1):793.
69. Seiffert M, Diemert P, Koschyk D, et al. Transapical implantation of a second-generation transcatheter heart valve in patients with noncalcified aortic regurgitation. JACC Cardiovasc Interv. 2013;6:590-597.
70. Cabasa AS, Eleid MF, Suri RM. Transcatheter aortic valve replacement for native aortic valve regurgitation as bridge to liver transplantation. Catheter Cardiovasc Interv. 2016;88:665-670.
71. Nakamura Y, Teefy P, Kiaii B, et al. Transcatheter aortic valve implantation in a patient with severe aortic insufficiency and minimal aortic annular calcification. Can J Cardiol. 2013;29:1138.e9-11.
72. Hildebrandt HA, Erbel R, Kahlert P. Compas- sionate use of the self-expandable Medtronic CoreValve prosthesis for the treatment of pure aortic regurgitation in a patient at prohibitive risk for surgical valve replacement. Catheter Cardiovasc Interv. 2013;82:E939-943.
73. Stachon P, Kaier K, Heidt T, et al. Nationwide outcomes of aortic valve replacement for pure aortic regurgitation in Germany 2008-2015. Catheter Cardiovasc Interv. 2019. https://doi.org/10.1002/ccd.28361.
74. Dumonteil N, Marcheix B, Lairez O, Laborde JC. Transcatheter aortic valve implantation for severe, non-calcified aortic regurgitation and narrow aortic root:description from a case report of a new approach to potentially avoid coronary artery obstruction. Catheter Cardiovasc Interv. 2013;82:E124-127.
75. Seiffert M, Diemert P, Koschyk D, et al. Transapical implantation of a second-generation transcatheter heart valve in patients with non- calcified aortic regurgitation. JACC Cardiovasc Interv. 2013;6:590-597.
76. Zhu D, Hu J, Meng W, Guo Y. Successful transcatheter aortic valve implantation for pure aortic regurgitation using a new second generation self-expanding J-Valve™system –the first-in-man implantation. Heart Lung Circ. 2015;24:411-414.
77. Schofer J, Nietlispach F, Bijuklic K, et al. Transfemoral implantation of a fully reposition- able and retrievable transcatheter valve for non- calcified pure aortic regurgitation. JACC Cardiovasc Interv. 2015;8:1842-1849.
78. Habib G, Lancellotti P, Antunes MJ, et al. ESC Guidelines for the management of infective endocarditis:The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Eur Heart J. 2015;36:3075-3128.
79. Fernández-Hidalgo N, Tornos Mas P. Epidemiology of Infective Endocarditis in Spain in the Last 20 Years. Rev Esp Cardiol. 2013;66:728-733.
80. Pant S, Patel NJ, Deshmukh A, et al. Trends in Infective Endocarditis Incidence, Microbiology, and Valve Replacement in the United States From 2000 to 2011. J Am Coll Cardiol. 2015;65:2070-2076.
81. Dayer MJ, Jones S, Prendergast B, Baddour LM, Lockhart PB, Thornhill MH. Incidence of infective endocarditis in England, 2000-13:a secular trend, interrupted time-series analysis. Lancet. 2015;385:1219-1228.
82. Albu C, Swaans MJ, ten Berg JM. With the back against the wall:TAVI in a patient with endocarditis. Catheter Cardiovasc Interv. 2013;82:E595-E597.
83. Nguyen C, Cheong AP, Himbert D. Valve-in-valve-in-valve:Treating endocarditis of a transcatheter heart valve. Catheter Cardiovasc Interv. 2015;86:E200-E204.
84. Pechlivanidis K, Onorati F, Petrilli G, et al. In which patients is transcatheter aortic valve replacement potentially better indicated than surgery for redo aortic valve disease?Long-term results of a 10-year surgical experience. J Thorac Cardiovasc Surg. 2014;148:500-508.
85. García-Granja PE, Amat-Santos IJ, Vilacosta I, Olmos C, Gómez I, San Román Calvar JA. Predictors of Sterile Aortic Valve Following Aortic Infective Endocarditis. Preliminary Analysis of Potential Candidates for TAVI. Rev Esp Cardiol. 2019;72:428-430.
Corresponding author: Instituto de Ciencias del Corazón (ICICOR), Hospital Clínico Universitario de Valladolid, Ramón y Cajal 3, 47005 Valladolid, Spain.
E-mail address: ijamat@gmail.com (I.J. Amat Santos).