ABSTRACT
The use of aspirin in combination with a P2Y12 receptor inhibitor, also known as dual antiplatelet therapy, is at the cornerstone of treatment for patients undergoing coronary stenting. The use of newer generation P2Y12 inhibitors (ie, prasugrel and ticagrelor), characterized by more potent antiplatelet effects and better clinical outcomes compared to clopidogrel, are recommended in high-risk patients, such as those with an acute coronary syndrome. However, this occurs at the expense of increased bleeding that accumulates with the duration of treatment. Given the poor prognostic implication, including an increased mortality rate associated with bleeding, a number of strategies aimed at reducing the risk of this adverse event while preserving efficacy have emerged. Among these, withdrawing aspirin represents an ongoing line of clinical investigation. The pharmacological reason behind such strategy relies on the central role played by the metabolic pathway of P2Y12 receptor inhibitors on platelet activation and its contribution amplifying thrombotic processes. Thus, it has been hypothesized that in the presence of a powerful P2Y12 receptor blockade, aspirin may offer minimal contribution when it comes to reducing thrombotic complications, but rather contribute to increased bleeding complications. A number of ongoing clinical investigations are currently challenging the dogma of aspirin as a mandatory background therapy in patients undergoing coronary stenting.
Keywords: Aspirin. Ticagrelor. Stent. Thrombosis
RESUMEN
El uso del ácido acetilsalicílico en combinación con un inhibidor del receptor P2Y12, es decir, la doble terapia antiplaquetaria, representa la piedra angular del tratamiento para los pacientes en los que se implanta un stent coronario. El uso de inhibidores P2Y12 de nueva generación (prasugrel y ticagrelor), caracterizados por efectos antiplaquetarios más potentes y mejores resultados clínicos en comparación con clopidogrel, se recomienda en pacientes de alto riesgo, como aquellos con un síndrome coronario agudo. Sin embargo, este beneficio es a expensas de un aumento del riesgo de sangrado que se acumula con la duración del tratamiento. Dada la adversa repercusión pronóstica, incluido el aumento de la mortalidad, asociada al sangrado, han surgido una serie de estrategias destinadas a reducir el riesgo de este evento adverso a la vez que se preserva la eficacia. Entre estos, retirar el ácido acetilsalicílico representa una línea de investigación clínica. La justificación farmacológica de dicha estrategia se basa en el papel central de la vía de inhibición de P2Y12 en la activación de las plaquetas y su contribución a la amplificación de los procesos trombóticos. Por lo tanto, se ha planteado la hipótesis de que, en presencia de un potente bloqueo del receptor P2Y12, el ácido acetilsalicílico puede ofrecer una contribución mínima para la reducción de las complicaciones trombóticas, pero de hecho contribuye al aumento de las complicaciones hemorrágicas. Una serie de ensayos clínicos actualmente en curso cuestionan el dogma del ácido acetilsalicílico como una terapia de base obligatoria en pacientes tratados con stents coronarios.
Palabras clave: Ácido acetilsalicílico Ticagrelor Stent Trombosis
Abbreviations: ACS: acute coronary syndrome; CAD: coronary artery disease; DAPT: dual antiplatelet therapy; PCI: percutaneous coronary intervention.
Aspirin is at the cornerstone of treatment for patients with clinical manifestations of coronary artery disease (CAD).1 However, the high rate of recurrent ischemic events despite aspirin therapy has inevitably led to explore the effects associated with the use of adjunctive antithrombotic therapies, particularly in high-risk settings. Among these, the adjunctive use of oral P2Y12 inhibitors has proven essential in patients with acute coronary syndromes (ACS) and those undergoing percutaneous coronary interventions (PCI) with stent implantation.2 The combination of aspirin and a P2Y12 inhibitor, also known as dual antiplatelet therapy (DAPT), has been the gold standard treatment for ACS/PCI patients and included in the clinical guidelines for now nearly two decades.3 Clopidogrel is the most commonly used P2Y12 inhibitor. Despite its proven efficacy a number of studies have shown wide variability in individual response profiles to clopidogrel with a considerable number of patients having inadequate platelet inhibitory effects.4 Notably, a number of studies have shown that these individuals also suffer from an increased risk of ischemic events, especially stent thrombosis.5 This has led to the development of P2Y12 inhibitors, such as prasugrel and ticagrelor, characterized by more powerful and reliable antiplatelet effects.2 Indeed, compared to clopidogrel, both agents have proven to reduce significantly ischemic recurrences in ACS patients, including stent thrombosis at the expense of an increased risk of bleeding.2 In the absence of contraindications, practice guidelines advocate for the preferred use of prasugrel or ticagrelor over clopidogrel.3
Although large-scale head-to-head comparisons between prasugrel and ticagrelor are scarce, ticagrelor appears to have a somehow more favorable safety profile in terms of bleeding potential compared to prasugrel.2 These observations may be attributed to the different pharmacological profiles of these agents, being ticagrelor a reversibly-binding P2Y12 inhibitor and prasugrel an irreversible binding agent.2 Moreover, in ACS patients, ticagrelor associates lower cardiovascular mortality compared to clopidogrel, a finding not seen with prasugrel compared to clopidogrel.2 These findings have been reported, although a causal relationship has never been attributed to ticagrelor,6 as off-target effects (ie, inhibition of ENT-1 transporter leading to increased adenosine levels). Overall, these observations as well as the expanded ACS clinical scenarios in which ticagrelor has proven beneficial, have led ticagrelor being more widely used than prasugrel. Nevertheless, concerns surrounding the risk for bleeding associated with longer DAPT courses of aspirin and ticagrelor persist. 7 It should be noted here that the occurrence of a bleeding complication even during the DAPT maintenance phase, has importance prognostic implications including increased mortality. 8 These observations have led to a series of investigations aimed at identifying strategies associated with reduced bleeding without an efficacy trade-off. These include shortening DAPT duration, de-escalating the antiplatelet therapy, and withdrawing aspirin. 9–12 Indeed, evolution in stent design has led to safer (ie, less thrombogenic) platforms that have facilitated investigations in this field. 10
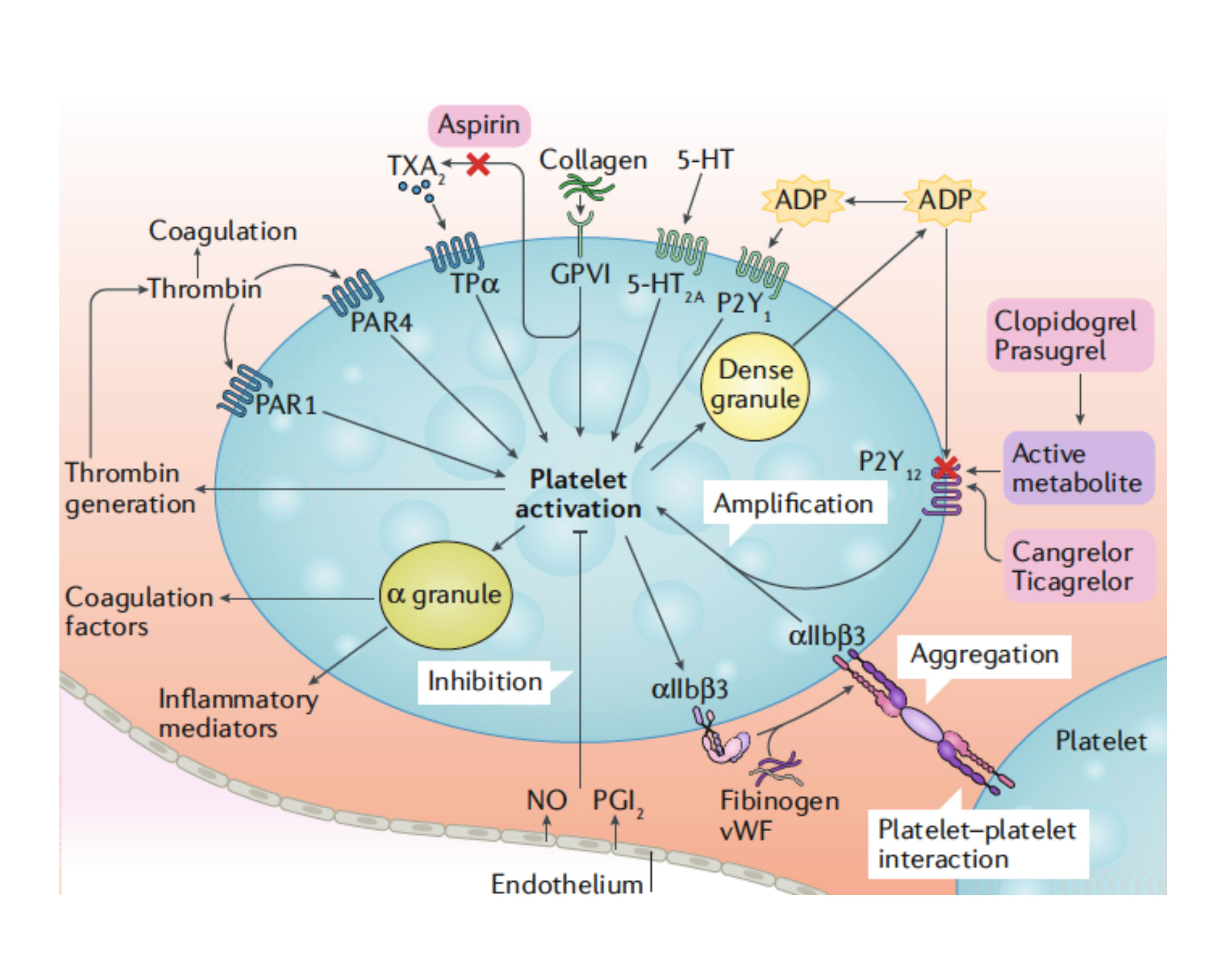
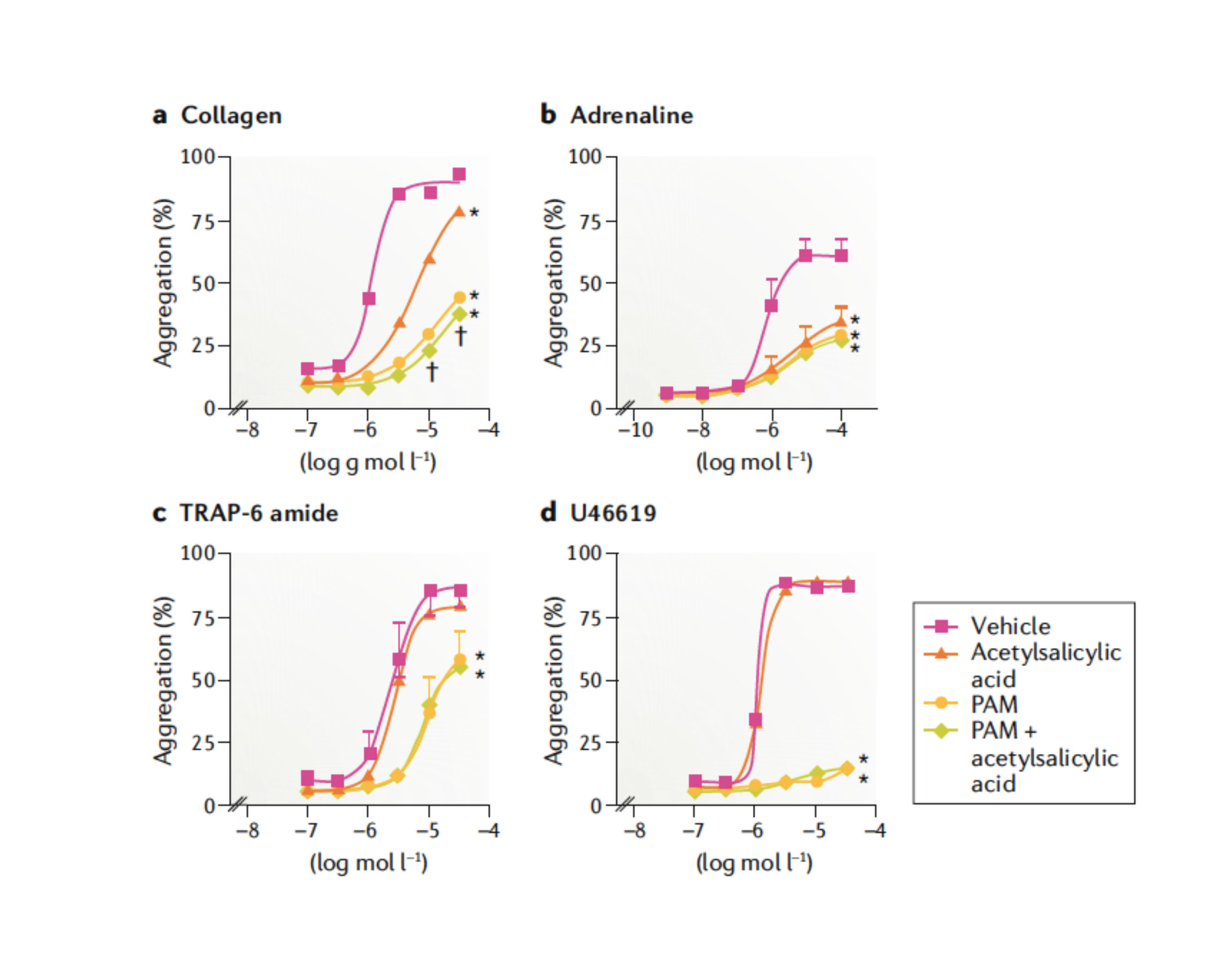
The use of aspirin-free strategies following PCI has been prospectively tested in randomized trials of patients with atrial fibrillation undergoing PCI and requiring oral anticoagulant therapy. 13 These studies have consistently shown that withdrawing aspirin as early as possible and favoring a double antithrombotic therapy approach (mostly clopidogrel combined with an oral anticoagulant) significantly reduced bleeding without an efficacy trade-off. Consequently, a double antithrombotic therapy strategy is now immediately recommended after PCI.13,14 The reason behind considering an aspirin-free strategy among PCI patients not requiring oral anticoagulant therapy largely comes from the overall very effective degree of P2Y12 inhibition achieved with ticagrelor.15 Notably, the P2Y12 signaling pathway plays a key role in platelet activation and amplification of thrombotic processes (figure 1).16 In vitro investigations have also shown that the use of aspirin offers limited pharmacodynamic effects in the presence of effective P2Y12 receptor blockade (figure 2). 17 In light of the well-established association between aspirin and bleeding, especially GI bleeding, it has been hypothesized that withdrawing aspirin therapy after the highest thrombotic risk phase (eg, 1-3 months post-PCI) has passed, can reduce the risk of bleeding complications without any efficacy trade-off. 9 It has also been suggested that in light of the detrimental impact of bleeding on clinical outcomes, an aspirin-free strategy can actually improve efficacy. 18
Figure 1. Platelet activation mechanisms. Platelet activation is initiated by soluble agonists, such as thrombin, thromboxane A2 (TXA2), 5-hydroxytryptamine (5-HT), adenosine diphosphate (ADP [via P2Y purinoceptor 1, P2Y1]), and adenosine triphosphate (ATP), and by adhesion ligands, such as collagen and the von Villebrand factor (vWF). Consequently, the dense granule secretion of platelet agonists and the secretion of TXA2, as a result of phospholipase A2 activation, lead to amplification of platelet activation and the associated responses. The P2Y purinoceptor 12 (P2Y12) receptor plays a major role in the amplification of platelet activation, which is also supported by outside-in signaling via integrin αIIbβ3 (the glycoprotein IIb/IIIa receptor). Thus, the combined blockade of P2Y12 and integrin αIIbβ3 has additive effects on platelet activation and the associated platelet responses. 5-HT2A, 5-HT receptor 2A; GPVI, platelet glycoprotein VI; NO, nitric oxide; PAR, proteinase-activated receptor; PGI2, prostacyclin receptor; TPα, TXA2 receptor isoform α. Adapted from Capodanno et al. 9 with permission of Springer Nature Ltd.
Figure 2. In the presence of strong P2Y12 receptor blockade, acetylsalicylic acid provides little additional inhibition of platelet aggregation. In these studies, platelet aggregation was induced by four different platelet agonists: collagen 0.1–30.0 µg/mL (part a), adrenaline 0.001–100.0 µmol/L (part b), the synthetic protease-activated receptor 1 (PAR1) antagonist TRAP-6 amide (H-Ser–Phe–Leu–Leu–Arg–Asn–NH2) 0.1–30.0 µmol/L (part c), and the thromboxane A2 mimetic U46619 0.1–30.0 µmol/L in the presence of acetylsalicylic acid 30.0 µmol/L and/or prasugrel active metabolite (PAM) 3.0 µmol/L (part d). Data are expressed as mean ± standard deviation of the mean responses measured by 96-well plate aggregometry in citrated platelet-rich plasma prepared from four different individuals. *P < .05 for difference from vehicle by two-way analysis of variance (ANOVA) plus a Bonferroni post hoc test. †P < .05 for difference between PAM and PAM plus acetylsalicylic acid. Symbols at the end of lines mean difference in sets; symbols at individual points mean particular differences. Adapted from Capodanno et al. 9 with permission of Springer Nature Ltd.
GLOBAL LEADERS (Comparative Effectiveness of 1 Month of Ticagrelor Plus Aspirin Followed by Ticagrelor Monotherapy Versus a Current-day Intensive Dual Antiplatelet Therapy in All-comers Patients Undergoing Percutaneous Coronary Intervention With Bivalirudin and BioMatrix Family Drug-eluting Stent Use) was a superiority trial conducted in 15 968 patients undergoing PCI with Biolimus A9-eluting stents, designed to assess whether a 24-month antithrombotic regimen with ticagrelor and one month with aspirin improves the composite of all-cause mortality or new Q-wave myocardial infarction compared to conventional DAPT for 12 months followed by aspirin monotherapy. 19 However, despite a directional trend towards the benefit of P2Y12 monotherapy, the trial failed to meet its primary endpoint (experimental strategy 3.81% vs reference strategy 4.37%; rate ratio, 0.87; 95%CI, 0.75-1.01; P = .073). Moreover, there were no differences in the key safety endpoint of class 3 or 5 bleeding according to the Bleeding Academic Research Consortium (BARC) criteria. A series of considerations need to be made when interpreting the GLOBAL LEADERS trial. First, the study is one of the largest PCI studies ever conducted with a new generation drug-eluting stent platform and even though it did not meet its primary endpoint, there were no safety signals associated with early (ie, one-month post-PCI) aspirin withdrawal. These observations provide reassurance for other ongoing studies evaluating aspirin-free strategies post-PCI and support the findings from pharmacodynamic investigations on the maintained efficacy associated with powerful P2Y12 inhibitor monotherapy. It is worth mentioning that, at 12 months, a statistically significant difference between groups was observed, but this was not maintained after a 2-year follow-up. It is also worth taking into consideration that during the first year of the trial a comparison was made between 2 DAPT regimens the first month, followed by a comparison of ticagrelor monotherapy versus DAPT the following 11 months. Conversely, between 12 and 24 months, ticagrelor was compared to aspirin, showing no difference and ultimately diluting the overall treatment effect of the experimental strategy. Reduced adherence to randomized therapy has also been suggested as a contributing factor. Indeed, a study with a larger sample would have likely been statistically significant enough and would have favored P2Y12 inhibitor mejor mon-therapy at 2 years. Second, the inclusion of patients with stable CAD (53% of the overall study population) may have diluted the potential benefit of the study. In fact, a significant interaction for BARC 3 or 5 bleeding (P = .007) was observed in favor of patients with ACS. Indeed, extending the study up to 24-months and including stable CAD patients with no established benefit of ticagrelor may also be reasons for the lack of differences seen in the primary safety endpoint of bleeding. Third, it may be argued that the primary endpoint (all-cause mortality and Q-wave myocardial infarction) chosen for this study was overly ambitious. While the selection of these endpoints was specifically chosen to facilitate the assessment of events, including other traditional endpoints would have facilitated even more the detection of differences between the treatment arms. Although in this trial nonfatal ischemic recurrences or bleeding events were not adjudicated, the GLASSY study will assess the superiority of the experimental treatment strategy over standard of care in more than 7000 patients on a composite endpoint of fatal and non-fatal ischemic and bleeding events. 20
The TWILIGHT (Ticagrelor With Aspirin or Alone in High-Risk Patients After Coronary Intervention) trial is a double-blind, superiority study that is analyzing the comparative efficacy and safety of antiplatelet therapy with ticagrelor plus placebo versus continued DAPT with aspirin and ticagrelor in up to 9000 high-risk patients on DAPT who are event-free at 3 months from PCI treated with commercially available drug-eluting stents. 21 There are key differences between the TWILIGHT and the GLOBAL LEADERS. First, the double-blind design (aspirin vs placebo), which is one of the strengths of the study, aimed at eliminating the chance of reporting bias. Second, the primary endpoint is focused on safety (BARC class 2, 3 or 5 bleeding at 12 months), which is a more plausible endpoint to achieve following withdrawal of aspirin therapy. The non-inferiority of DAPT for ischemic events is also being studied. Third, the study population is enriched with clinical and angiographic risk factors which increases the anticipated event and, in turn, the probability to detect treatment effect. This trial has recently completed recruitment, and the primary results are expected for Q2 2019. Other trials are being conducted to provide new insights on the potential role of P2Y12 monotherapy as an alternative to long-term platelet inhibition in patients undergoing PCI (table 1).
Table 1. Ongoing trials of aspirin-free strategies in patients undergoing percutaneous coronary interventions
| Study | n | Population | Treatment arms | Primary outcome measure |
|---|---|---|---|---|
| TWILIGHT (NCT02270242) | 9000 | High risk PCI on ticagrelor, event-free at 3 months | Placebo for 12 months versus ASA for 12 months | Bleeding at 12 months |
| TICO (NCT02494895) | 3056 | ACS-PCI | DAPT for 3 months followed by ticagrelor for 9 months versus DAPT for 12 months | MACCE at 12 months, major bleeding at 12 months |
| SMART CHOICE (NCT02079194) | 3000 | PCI | DAPT for 3 months followed by clopidogrel for 9 months versus DAPT for 12 months | Death, MI or stroke at 12 months, major bleeding at 12 months |
| SHORT-DAPT 2 (NCT02619760) | 3045 | PCI | DAPT for 1 month followed by clopidogrel for 59 months versus DAPT for 12 months followed by ASA for 48 months | NACE at 12 months |
| ASET (NCT03469856) | 200 | PCI | Prasugrel monotherapy | Cardiac death, target-vessel MI (spontaneous > 48 h) or definite stent thrombosis BARC 3 or 5 bleeding |
| AUGUSTUS (NCT02415400) | 4600 | Atrial fibrillation on oral anticoagulation with ACS and/or undergoing PCI | ASA for 6 months versus placebo for 6 months | Major or clinically relevant bleeding at 6 months |
| ENTRUST-AF PCI (NCT02866175) | 1500 | Atrial fibrillation on oral anticoagulation undergoing PCI | Edoxaban and clopidogrel or ticagrelor for 12 months versus vitamin K antagonist for 12 months plus DAPT for 1–12 months | Major or clinically relevant bleeding at 12 months |
ACS, acute coronary syndrome; ASA, acetylsalicylic acid; DAPT, dual antiplatelet therapy; MACCE, major adverse cardiac and cerebrovascular events; MI, myocardial infarction; n, number of patients; NACE, net adverse cardiac events; PCI, percutaneous coronary intervention. | ||||
In sum, the advances made in interventional pharmacotherapy with the introduction of antithrombotic agents with more effective pharmacodynamics effects have called into question the standard approach that consisted on piling up on aspirin as a background therapy. The growing recognition of the importance of reducing bleeding complications has called into question whether in a more modern arsenal of antithrombotic therapies aspirin is still irreplaceable. The long-term use of aspirin is not indispensable as already proven in certain settings such as patients treated with oral anticoagulants and current evidence shows that in the presence of effective blockade of other pivotal platelet signaling pathways withdrawing aspirin is harmless. Thus, what would have been a myth a few years ago, the possibility of doing without long-term aspirin following coronary stenting is not that far from reality anymore. Whether we will be able to overcome the dogma of mandatory long-term use of aspirin will indeed depend on the findings from the ongoing clinical trials that are being conducted in this field.
CONFLICTS OF INTEREST
D. Caponanno declared receiving consulting fees/honoraria from Bayer and AstraZeneca. R. Mehran declares that she has received consulting fees from Abbott Vascular, Abiomed, Boston Scientific, Bristol-Myers Squibb, Cardiovascular Systems, Elixir, Medscape, Shanghai BraccoSine Pharmaceutical, The Medicines Company, and executive committee fees from Janssen Pharmaceuticals, Osprey Medical. She also declares that her institution receives funding from AstraZeneca, Bayer, Beth Israel Deaconess, Bristol-Myers Squibb, Cardiokinetix, Claret Medical, CSL Behring, Eli Lilly/DSI, Medtronic, Novartis Pharmaceuticals, OrbusNeich, Spectranetics and Watermark Research Partners. D.J. Angiolillo declared receiving: a) consulting fees/honoraria from Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PLx Pharma, Pfizer, Sanofi, and The Medicines Company; b) consulting fees/honoraria for his participation in review activities from CeloNova and St Jude Medical. Institutional grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli-Lilly, Gilead, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, and Renal Guard Solutions; Also, D.J. Angiolillo received funding from the Scott R. MacKenzie Foundation and the NIH/NCATS Clinical and Translational Science Award to the University of Florida UL1 TR000064 and NIH/NHGRI U01 HG007269, outside the submitted work.
REFERENCES
1. Angiolillo DJ. The Evolution of Antiplatelet Therapy in the Treatment of Acute Coronary Syndromes. Drugs. 2012;72:2087-2116.
2. Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol. 2015;12:30-47.
3. Capodanno D, Alfonso F, Levine GN, Valgimigli M., Angiolillo DJ. Dual Antiplatelet Therapy:Appraisal of the ACC/AHA and ESC Focused Updates. J Am Coll Cardiol. 2018;72:103-19.a
4. Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Variability in Individual Responsiveness to Clopidogrel. J Am Coll Cardiol. 2007;49:1505-1516.
5. Tantry US, Bonello L, Aradi D, et al. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. 2013;62:2261-2273.
6. Cattaneo M, Schulz R, Nylander S. Adenosine-Mediated Effects of Ticagrelor. J Am Coll Cardiol. 2014;63:2503-2509.
7. Becker RC, Bassand JP, Budaj A, et al. Bleeding complications with the P2Y12 receptor antagonists clopidogrel and ticagrelor in the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J. 2011;32:2933-2944.
8. Généreux P, Giustino G, Witzenbichler B, et al. Incidence, Predictors, and Impact of Post-Discharge Bleeding After Percutaneous Coronary Intervention. J Am Coll Cardiol. 2015;66:1036-1045.
9. Capodanno D, Mehran R, Valgimigli M, et al. Aspirin-free strategies in cardiovascular disease and cardioembolic stroke prevention. Nat Rev Cardiol. 2018;15:480-496.
10. Moon JY, Franchi F, Rollini F, Angiolillo DJ. The quest for safer antithrombotic treatment regimens in patients with coronary artery disease:new strategies and paradigm shifts. Expert Rev Hematol. 2018;11:5-12.
11. Angiolillo DJ, Rollini F, Storey RF, et al. International Expert Consensus on Switching Platelet P2Y12 Receptor-Inhibiting Therapies. Circulation. 2017;136:1955-1975.
12. Moon JY, Franchi F, Rollini F, Angiolillo DJ. Evolution of Coronary Stent Technology and Implications for Duration of Dual Antiplatelet Therapy. Prog Cardiovasc Dis. 2018;60:478-490.
13. Angiolillo DJ, Goodman SG, Bhatt DL, et al. Antithrombotic Therapy in Patients with Atrial Fibrillation Treated with Oral Anticoagulation Undergoing Percutaneous Coronary Intervention:A North American Perspective - 2018 Update. Circulation. 2018;138:527-536.
14. Lip GYH, Collet JP, Haude M, et al. 2018 Joint European consensus document on the management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous cardiovascular interventions:a joint consensus document of the European Heart Rhythm Association (EHRA), European Society of Cardiology Working Group on Thrombosis, European Association of Percutaneous Cardiovascular Interventions (EAPCI), and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA). Europace. 2018. http://doi.org/10.1093/europace/euy174.
15. Rollini F, Franchi F, Cho JR, et al. A head-to-head pharmacodynamic comparison of prasugrel vs ticagrelor after switching from clopidogrel in patients with coronary artery disease:results of a prospective randomized study. Eur Heart J. 2016;37:2722-2730.
16. Storey RF. Biology and pharmacology of the platelet P2Y12 receptor. Curr Pharm Des. 2006;12:1255-1259.
17. Armstrong PCJ, Leadbeater PD, Chan MV, et al. In the presence of strong P2Y12 receptor blockade, aspirin provides little additional inhibition of platelet aggregation. J Thromb Haemost. 2011;9:552-561.
18. Vranckx P, Valgimigli M, Windecker S, et al. Long-term ticagrelor monotherapy versus standard dual antiplatelet therapy followed by aspirin monotherapy in patients undergoing biolimus-eluting stent implantation:rationale and design of the GLOBAL LEADERS trial. EuroIntervention. 2016;12:1239-1245.
19. Vranckx P, Valgimigli M, Jüni P, et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent:a multicentre, open-label, randomised superiority trial. Lancet. 2018;392:940-949.
20. GLOBAL LEADERS Adjudication Sub-Study (GLASSY). https://clinicaltrials.gov/ct2/show/NCT03231059 . Accessed 15 Nov 2018.
21. Baber U, Dangas G, Cohen DJ, et al. Ticagrelor with aspirin or alone in high-risk patients after coronary intervention:Rationale and design of the TWILIGHT study. Am Heart J. 2016;182:125-134.
Corresponding author: University of Florida College of Medicine-Jacksonville, 655 West 8th Street, Jacksonville, FL 32009, United States.
E-mail address: dominick.angiolillo@jax.ufl.edu (D.J. Angiolillo).