HOW WOULD I APPROACH IT?
The authors hereby present the clinical case of an 80-year old male with symptomatic severe aortic stenosis, preserved ventricular function, mild mitral regurgitation and no coronary heart disease. He had low surgical risk (3.1% score in the Society of Thoracic Surgeons risk scale) and, in medical-surgical session, it was decided to proceed with a surgical aortic valvular replacement using the Perceval Sutureless Aortic Heart Valve, Sorin – size L. The follow-up echocardiogram prior to the patient’s discharge showed lack of sig- nificant aortic transvalvular gradient and periprosthetic regurgitation jets indicative of mild-to-moderate aortic regurgitation. Five months after the implantation of the valve, the patient was admitted to the hospital due to acute pulmonary edema. The echocardiogram showed severe aortic regurgitation due to the lack of stent coverage of the aortic bioprosthesis at the level of the aortic annulus and in the area of the non-coronary sinus and most of the right coronary sinus with two regurgitation jets towards the left dilated ventricle. The left ventricular ejection fraction was slightly depressed.
Before considering any therapeutic approaches in a patient with aortic regurgitation following a valvular implantation there are 3 fundamental issues we should all be aware of: 1) the exact degree of regurgitation severity; 2) the location (transvalvular or paravalvular); and 3) the underlying mechanism according to the appropriate imaging modalities.
The information we got told us that the aortic regurgitation was serious. When it comes to the location of this regurgitation, if we take a look at the results obtained following the implantation and the description of the echocardiogram at admission, the location was periprosthetic. The third main aspect to decide the future therapeutic approach is to find out what mechanism is causing the periprosthetic severe aortic regurgitation in a patient carrying a Perceval device. Here wer are dealing with a bovine pericardial prosthesis mounted on a nitinol stent frame (nickel and titanium). The frame structure has two cylindrical rings (figura 1): one proximal (outflow ring) and one distal (inflow ring). 1 Once mounted, it is placed inside the valve and deployed in the aortic root. Three different suture sites are used inside the native aortic annulus as a guide to find the exact spot for the implant. It is deployed by turning a release screw and is fully expanded with the use of a balloon catheter. Once deployed, the sutures are removed. As far as we know, there are four different sizes available in the market today: size S (19 mm to 21 mm), size M (21 mm to 23 mm), size L (23 mm to 25 mm), and size XL (25 mm to 27 mm). Every size is the right size for different aortic annuli and sinotubular diameters, which is why it is essential to know the patient’s exact anatomical measures. Its use is not recommended in patients with a dilated ascending aorta and with a sinotubular junction/aortic annulus diameter ratio ≥ 1.3, or in patients with bicuspid aortic valve.
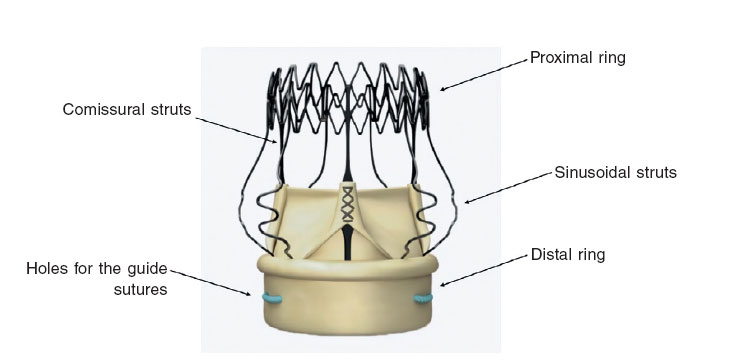
Figure 1. Components of the Perceval Sutureless Aortic Heart Valve, Sorin.
As it is the case with transcatheter prostheses, after implanting the Perceval device, it is not rare to find some sort of perivalvular leakage. These are some of the mechanisms we already know:
Valvular malapposition: the valve is deployed correctly, but the distal ring is placed above or below the aortic annulus causing the leakage.
Inadequate size of the prosthesis: small (undersizing) and big devices (oversizing) with respect to the ring can lead to perivalvular leakages.
Taking into consideration the description of the echocardiogram and the fact that the most common mistakes are due to oversizing, and assuming the correct placement of the device, I think that the mechanism that made the prosthesis fail was the recoil or collapse of the metallic structure that harbors the bioprosthetic leaflets and that usually affects the area of the non-coronary sinus – a complication that surgeons know so well and that has been described extensive in the medical literature. 2 We should bear in mind that this deformity of the stent that causes the lack of contact between the device and the aortic root anywhere from the aortic annulus to the sino- junction can occur intraoperatively or days after the intervention and it is more common with small rings; that is why various authors recommend using the smallest prosthesis available when the dimension of the aortic annulus is somehow between two different device sizes.
According to the medical literature, when the metallic frame collapses, these are the therapeutic options available:3
Reintervention or valvular replacement for a smaller device, Perceval M, or a valve with sutures.
Percutaneous treatment with a 22 mm to 23 mm balloon valvuloplasty, since the true internal diameter of the Perceval device size L goes from 21.5 mm to 23 mm according to the manufacturer.
Percutaneous treatment through the percutaneous implantation of the aortic valve, the so-called valve-in-valve procedure.
Personally, I would do a valvuloplasty as my first option, but since the underlying problem is still unsolved (too big of a device for the aortic annulus), such a maneuver would probably be ineffective. In that case, I would implant a transcatheter valve with the strongest possible radial strength (valve-in-valve procedure).
ACKNOWLEDGEMENTS
I wish to thank the critical review of this paper conducted by C. Moris de la Tassa, (interventional cardiologist) and D. Hernández- Vaquero, (heart surgeon).
REFERENCES
1. Baert J, Astarci P, Noirhomme P, de Kerchove L. The risk of oversizing with sutureless bioprosthesis in small aortic annulus. J Thorac Cardiovasc Surg. 2017;153:270-272.
2. Chandola R, Teoh K, Elhenawy A, Christakis G. Perceval Sutureless Valve —are Sutureless Valves Here?Curr Cardiol Rev. 2015;11:220-228.
3. Powell R, Pelletier MP, Chu MWA, Bouchard D, Melvin KN, Adams C. The Perceval Sutureless Aortic Valve:Review of Outcomes, Complications, and Future Direction. Innovations (Phila). 2017;12:155-173.
Corresponding author: Servicio de Cardiología, Hospital Universitario Central de Asturias, Avda. Roma s/n, 33011, Oviedo, Asturias, Spain.
E-mail address: avanzas@secardiologia.es (P. Avanzas).

