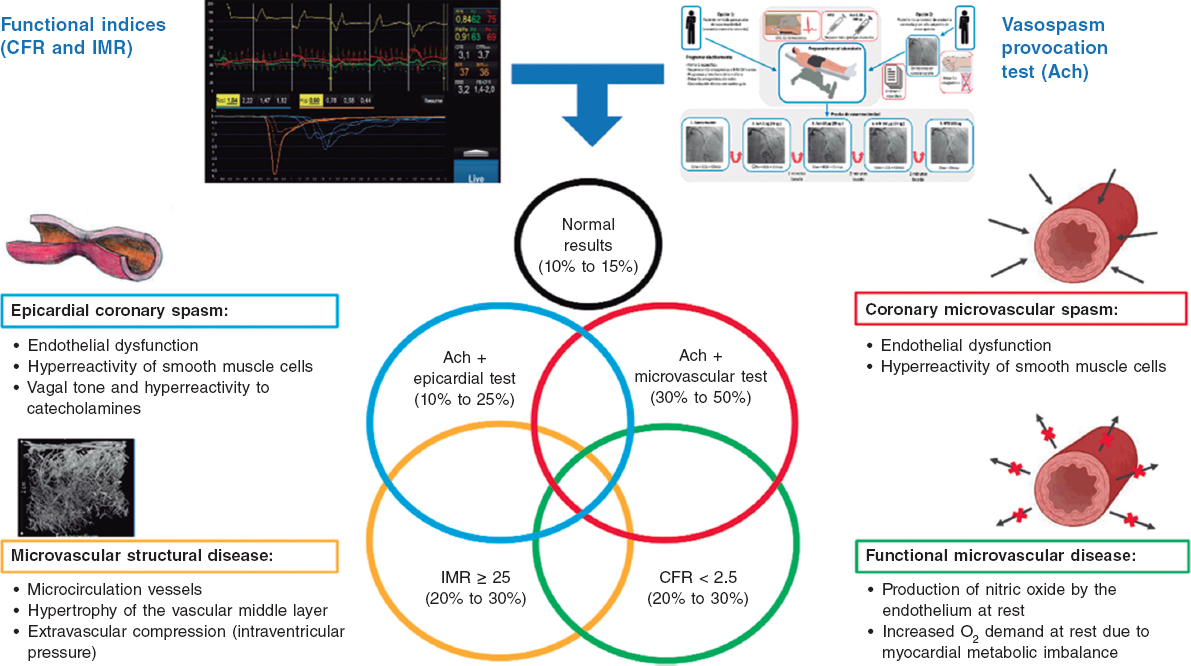
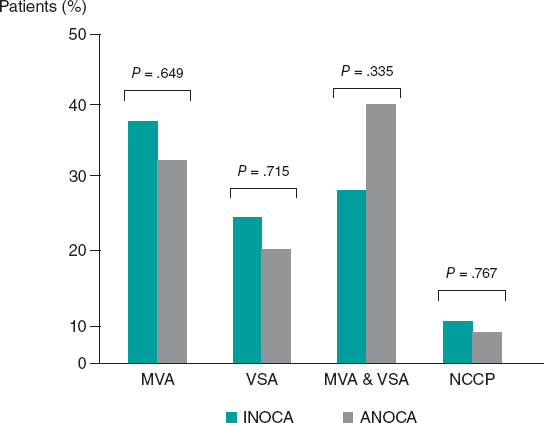
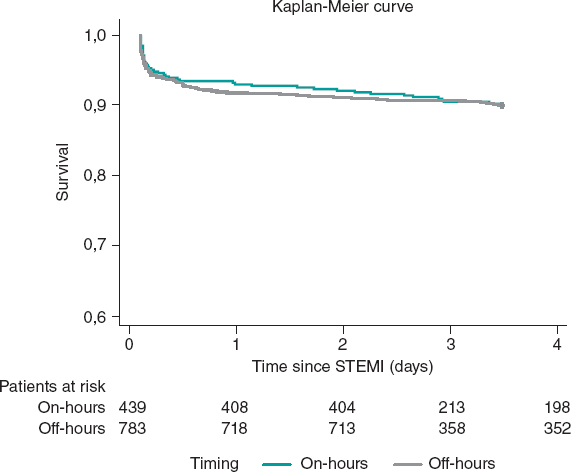
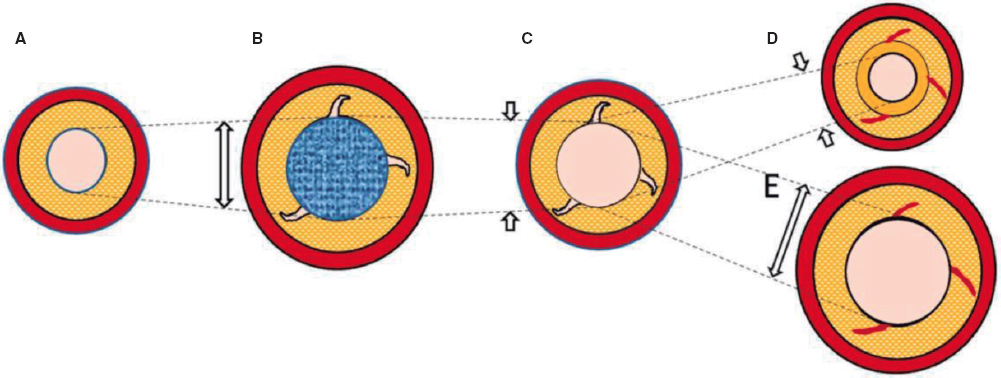
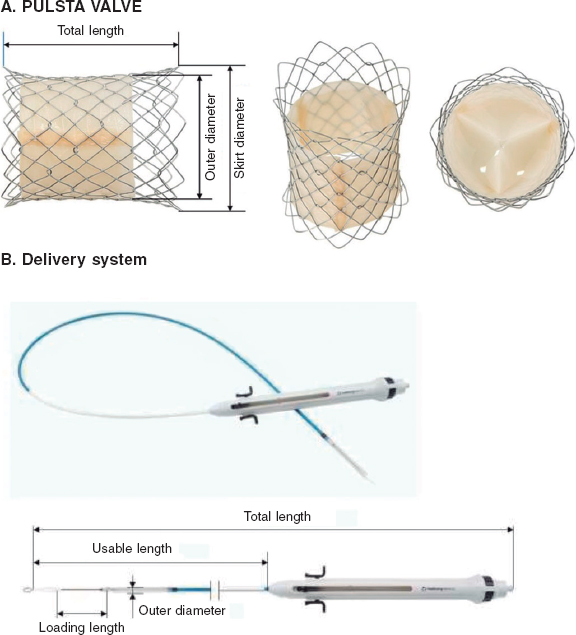
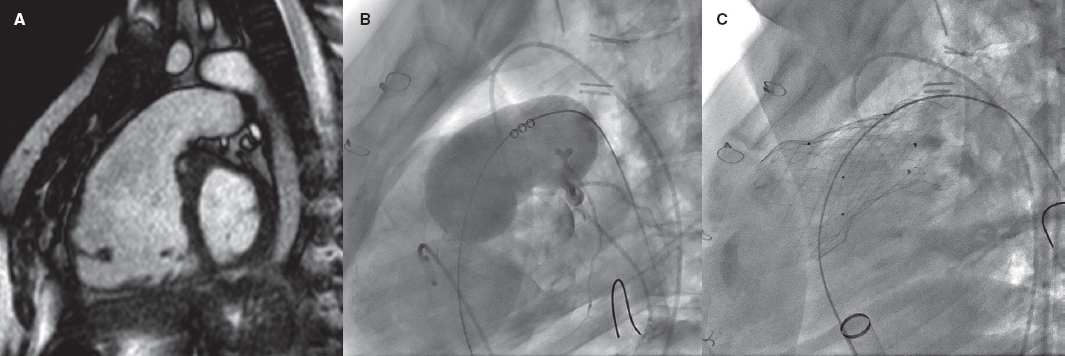
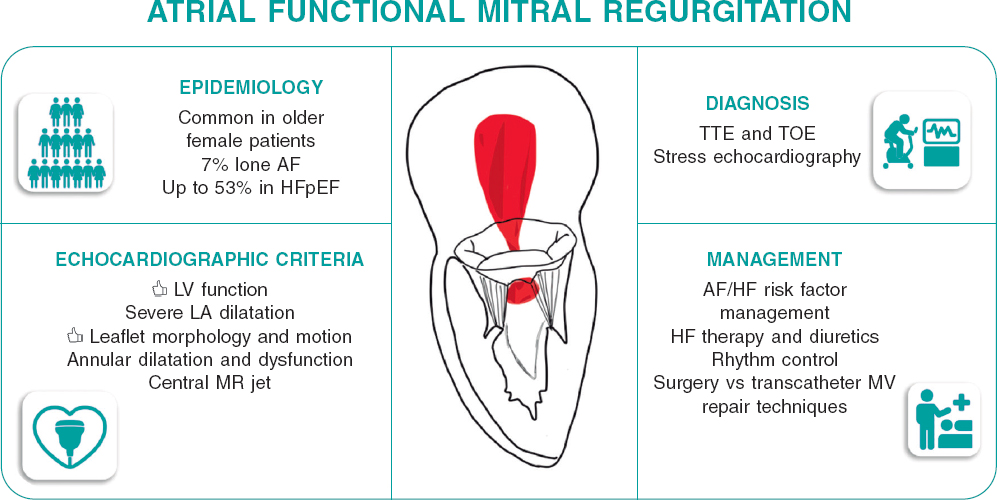
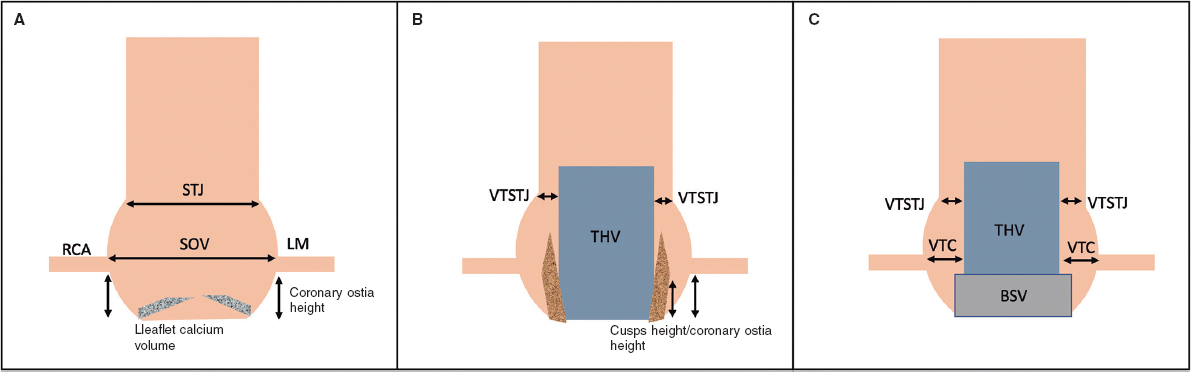
Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 was published to regulate medical devices with the aim of bolstering the safety, quality, and efficacy of medical products in Europe.1
The regulation covers medical products intended for the diagnosis and treatment of numerous cardiovascular conditions, including high-risk devices such as pacemakers, defibrillators, artificial hearts, stents, cardiovascular sutures, heart valves, catheters, cardiovascular wires, and cardiac ablation instruments. According to the classification rules outlined in the regulation, all these high-risk medical products are classified as Class III.
The process for obtaining the CE mark for a medical device requires the manufacturer to demonstrate that the product meets the established safety and performance requirements and to conduct a clinical evaluation to validate the intended indication and purpose of use. For Class III product certification, a notified body designated by a member state authority must verify that the manufacturer has the objective technical and clinical documentation required to demonstrate that the product meets all the claims made by the manufacturer on the product. The authorized body issues a “CE Declaration of Conformity” including the manufacturer’s information, the product’s unique identifier, class, intended purpose, test reports and documentation, date and issue of validity, and details of the notified body involved in the process of granting the CE mark. The manufacturer’s company must also implement a quality management system to ensure that the manufactured products meet specified standards. After auditing the manufacturer’s facilities, the body issues an “EU quality management system certificate” detailing the scope of the quality system and type of manufactured products.
In other words, for the marketing of Class III medical products, the manufacturer must hold 2 different EU certificates issued by a notified body: one for the product and one for the quality management system.
Health care workers or users of a medical product can easily identify which notified body participated in its assessment by checking the product label, which is identified by a 4-digit number appearing alongside the CE mark. The name of the organization behind that number can be found on the European Commission’s website.2 For example, if the digits 0318 appear next to the CE mark on the label or the instructions for use of a medical product, it indicates that the evaluation was conducted by the National Certification Center for Medical Product, the sole notified body designated by the Ministry of Health.
The main change introduced in the regulation on product requirements involves the clinical evaluation. The assessment is especially strict for Class III products, which, as previously mentioned, are high risk. The first requirement is that the clinical evaluation validating the indication for use must be based on clinical data obtained from clinical investigations conducted with the product itself or a product that is technically, biologically, and clinically equivalent. The second requirement is that manufacturers must have access to the primary clinical data supporting the clinical evaluation of the medical product in question, either because they own them, or because the data have been published, or because they have a contractual agreement with the owner allowing permanent access and availability.
Although it may seem trivial, since the publication of the regulation, the availability of a compliant clinical evaluation has been the Achilles’ heel for manufacturers of medical products intending to market their products in Europe in the coming years.
During the 3 decades since the implementation of the directives, special emphasis has been placed on ensuring the safety and quality of medical products, while the available objective evidence supporting their clinical benefit has been relegated to a secondary role. Consequently, manufacturers of medical products that have been on the market for years have had to make considerable efforts and investments to obtain sufficient clinical data with the necessary level of evidence to support the clinical risk-benefit ratio esta- blished by the new legislation. Many have had to devise new clinical evaluation plans or review existing ones, including conducting specific postmarket clinical follow-up studies to provide clinical data with an adequate level of evidence. Therefore, we could say that a culture of the need for clinical research and publication of the obtained data is emerging in the medical products sector.
On the other hand, to minimize potential discrepancies between notified bodies in the assessment of the clinical evaluation of Class III implantable medical products (such as pacemakers), the regulation has established a centralized supervision procedure by a panel of experts in medical products from the European Medicines Agency (EMA). The role of this panel is to review and confirm the adequacy of both the clinical evaluation conducted by the manufacturer and the assessment made by the notified body, and provide any recommendations deemed appropriate regarding the decision on certifying the medical product. These recommendations may include proposing to certify or not certify the product, or to limit or restrict indications, among others.
An interesting point is that 18 out of the 43 applications received by the panel so far correspond to medical products within the “circulatory system” clinical area. In particular, the clinical evaluations of some stents, implantable defibrillators, and various types of heart valves have already undergone this procedure, and the resulting public opinions can be consulted in a list within the framework of the European Commission’s clinical evaluation consultation procedure.3
In addition, manufacturers of these types of products can seek guidance from the panel of experts before starting clinical development to confirm that the strategy designed for clinical development is appropriate and ensure that the resulting clinical evaluation will fully comply with the current legislation. If manufacturers decide to submit this voluntary query, the response issued by the panel will be binding. In other words, manufacturers will not be able to implement a different clinical evaluation plan from that recommended by the panel if they want to obtain the CE mark for the product.
The cornerstone of the CE certification model described is the competence of the personnel conducting the evaluation tasks. The personnel involved in the process of conducting or assessing the clinical evaluation of a medical product must have adequate knowledge. At the forefront of this chain are the manufacturers because they have had to review the competence of their staff to ensure that clinical evaluations are conducted by personnel experienced in clinical evaluation, competent in bibliographic searches, and with sufficient clinical knowledge and use of the products. Next are the notified bodies, which have to ensure that they have sufficient personnel with relevant clinical knowledge to issue a clinical judgment on the product’s risk-benefit ratio after analyzing and scientifically testing the clinical data collected in the clinical evaluation provided by the manufacturers. Furthermore, clinicians internal to the notified bodies must verify that the personnel conducting the clinical evaluations provided by the manufacturers are qualified to perform the task. Further along the review chain, notified bodies are audited by European teams of qualified professionals, who, in turn, must verify that the competencies of the personnel conducting the assessments of the clinical evaluations in the notified bodies meet the criteria of experience and training established in the regulation.
This regulation also encourages the manufacturers of medical products to hire health care workers with clinical experience, who have their own opinions on the products they use in their routine clinical practice. These professionals can participate in the early stages of product design, engage in usability testing, and, naturally, as occurs with drugs, promote clinical research both before and after product marketing. This helps to confirm the clinical benefit of medical products throughout their life cycle.
Health care workers must be aware of the value of their experience and clinical knowledge in ensuring that the medical products entering the market are truly innovative and meet the needs of patients.
The responsible and committed contribution made by each of the parties involved in conducting and reviewing the clinical evaluation of medical products will, on the one hand, provide greater assurance of the rigor, robustness, and sufficiency of the clinical data supporting a product’s indication. On the other hand, it will serve to standardize the criteria applied in the evaluation and ensure that the level of evidence required for all medical products bearing the CE mark under the new regulation is the same. These measures will restore confidence in the legislative model of medical products, ensuring that all manufacturers marketing their medical products in the European common market play by the same rules. Therefore, that the CE certification under which products are marketed will provide identical safeguards to patients, regardless of the country of origin, manufacturer, or issuing body.
FUNDING
None declared.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence has been used in the preparation of this article.
CONFLICTS OF INTEREST
None declared.
REFERENCES
1. Parlamento Europeo. Reglamento (UE) 2017/745 del Parlamento Europeo y del Consejo, de 5 de abril de 2017, sobre los productos sanitarios, por el que se modifican la Directiva 2001/83/CE, el Reglamento (CE) n.º178/2002 y el Reglamento (CE) n.º1223/2009, y por el que se derogan las Directivas 90/385/CEE y 93/42/CEE del Consejo. DOUE. 2017;117:1-175.
2. European Commission. New Approach Notified and Designated Organisations -NANDO. Available online:EUROPA –European Commission –Growth–Regulatory policy SMCS. Accessed 18 Dec 2023.
3. European Commission. List of opinions provided under the CECP. Available online:List of opinions provided under the CECP European Commission (europa.eu). Accessed 18 Dec 2023.
* Corresponding author.
E-mail address: ghernandez@certificaps.gob.es (G. Hernández Hernández).