ABSTRACT
Introduction and objectives: Nonagenarians are a fast-growing age group among cardiovascular patients, especially with aortic stenosis, but data about their prognosis after transcatheter aortic valve implantation (TAVI) is scarce. The objective of our study is to analyze the baseline characteristics of nonagenarians treated with TAVI and determine whether age ≥ 90 years is associated with a worse prognosis compared to non-nonagenarian patients.
Methods: We included all patients ≥75 years enrolled in the multicenter prospective Spanish TAVI registry between 2009 and 2018. Patients < 75 years were excluded.
Results: A total of 8073 elderly patients (≥ 75 years) from 46 Spanish centers were enrolled in the Spanish TAVI registry; 7686 were between ≥ 75 and < 90 years old (95.2%), and 387 were nonagenarian patients (4.79%). A gradual increase of nonagenarians was observed. The transfemoral access was used in 91.6% of the cases, predominantly among the nonagenarian patients (91.4% vs 95.1%, P = .01). Nonagenarians were more likely to die during their hospital stay (4.3% vs 7.0% among nonagenarians, P = .01). However, no difference was seen in the all-cause mortality rates reported at the 1-year follow-up (8.8% vs 11.3%, P =.07). In the multivariate analysis, age ≥ 90 years was not independently associated with a higher adjusted all-cause mortality rate (HR, 1.37, 95%CI, 0.91–1.97, P = .14). The baseline creatinine levels, and the in-hospital bleeding complications were all associated with a worse long-term prognosis in nonagenarians treated with TAVI.
Conclusions: Nonagenarians are a very high-risk and growing population with severe AS in whom TAVI may be a safe and effective strategy. Careful patient selection by the TAVI heart team is mandatory to achieve maximum efficiency in this population where the baseline kidney function and bleeding complications may determine the long-term prognosis after TAVI.
Keywords: TAVI. Aortic stenosis. Nonagenarians. Elderly.
RESUMEN
Introducción y objetivos: Los nonagenarios son un grupo de edad en rápido crecimiento entre los pacientes cardiovasculares, en especial con estenosis aórtica, pero los datos sobre su pronóstico después de la implantación transcatéter de válvula aórtica (TAVI) son escasos. El objetivo de este estudio es analizar las características basales de los nonagenarios tratados con TAVI y determinar si la edad ≥ 90 años está relacionada con un peor pronóstico en comparación con los pacientes no nonagenarios.
Métodos: Se incluyó a todos los pacientes ≥ 75 años inscritos en el registro prospectivo multicéntrico español de TAVI entre 2009 y 2018. Se excluyó a aquellos < 75 años.
Resultados: Se inscribieron en el registro español de TAVI 8.073 pacientes ≥ 75 años de 46 centros de España; 7.686 de > 75 a 90 años (95,2%) y 387 nonagenarios (4,79%). Se observó un aumento progresivo de los nonagenarios. El acceso transfemoral se utilizó en el 91,6% de los casos, predominantemente en los nonagenarios (91,4 frente a 95,1%; p = 0,01). Los nonagenarios tenían más probabilidades de morir durante la hospitalización (4,3 frente a 7,0%; p = 0,01). Sin embargo, no hubo diferencia en la tasa de mortalidad por cualquier causa al año de seguimiento (8,8 frente a 11,3%; p = 0,07). En el análisis multivariable, la edad ≥ 90 años no se asoció de forma independiente con un aumento de la mortalidad por cualquier causa ajustada (HR = 1,37; IC95%, 0,91-1,97; p = 0,14). La creatinina basal y las complicaciones hemorrágicas intrahospitalarias se asociaron a un peor pronóstico a largo plazo en pacientes nonagenarios tratados con TAVI.
Conclusiones: Los nonagenarios son una población creciente y de muy alto riesgo, con estenosis aórtica grave, para quienes la TAVI podría representar una estrategia segura y efectiva. Una cuidadosa selección de los pacientes por un equipo multidisciplinario de TAVI es obligatoria para lograr la máxima eficiencia en esta población en la que la función renal basal y las complicaciones hemorrágicas pueden determinar el pronóstico a largo plazo tras la TAVI.
Palabras clave: TAVI. Estenosis aórtica. Nonagenarios. Ancianos.
Abbreviations TAVI: transcatheter aortic valve implantation. AS: aortic stenosis. SAVR: surgical aortic valve replacement.
INTRODUCTION
The development of transcatheter aortic valve implantation (TAVI) has marked a milestone in the management of severe aortic stenosis (AS). Thanks to its minimally invasive approach, TAVI allows us to treat patients with severe AS and inoperable or high surgical risk, thus improving their prognosis and quality of life compared to standard therapy and surgical aortic valve replacement (SAVR), respectively.1,2 According to trial results, elderly patients have benefited the most due to their high surgical risk. Given the current demographic trend towards aging3 in developed countries and the increasing prevalence of the corresponding severe AS,4 it seems reasonable to expect that the number of elderly patients with severe AS who will require TAVI within the next few decades will be on the rise. In this sense, nonagenarians are a fast-growing and high-risk segment of the population on whom scarce data on specific outcomes are available. Given their anticipated short life expectancy and comorbidity burden, TAVI may be an excellent option for them. However, we should mention that the absolute cost of TAVI is high.5 Therefore, there is an unmet need for “real-world” data before assessing the impact of this beneficial and expensive technique in a growing and high-risk population like nonagenarians. To this end, the objective of our study was to describe the baseline characteristics, assess the clinical outcomes, and identify the characteristics of futility of this high-risk subgroup based on data from the Spanish TAVI registry.6
METHODS
Patient selection and follow-up
The Spanish TAVI registry is a multicenter prospective registry that enrolled all consecutive patients with severe AS treated with TAVI in 46 Spanish centers (table 1 of the supplementary data). The Spanish TAVI registry has been promoted by the Interventional Cardiology Association of the Spanish Society of Cardiology. For our analysis, we included all patients ≥ 75 years included in the Spanish TAVI registry from 2009 through 2018. Patients < 75 years were excluded. The baseline characteristics, echocardiographic findings, and procedural results were all recorded. The follow-up protocol included a medical consultation 30 days and 1 year after hospital discharge. The registry complies with the Spanish legislation on data protection and has been approved by a central ethics committee. Center participation in this registry was voluntary and all participants gave their informed consent. All data were included in the registry prospectively and systematically reviewed while looking for inconsistencies or lack of data. The data collected included the patients’ demographic characteristics, past medical history, baseline clinical characteristics, echocardiographic findings, procedural characteristics, and in-hospital clinical and follow-up outcomes.
Table 1. Clinical characteristics
| Variable | All patients (N = 8073) | Elderly < 90 y (N = 7686) | Nonagenarians (N = 387) | P |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 82.9 ± 4.01 | 82.6 ± 3.66 | 91.1 ± 1.29 | < .001 |
| Women | 4476 (55.5%) | 4250 (55.3%) | 226 (58.4%) | .23 |
| Body mass index (kg/m2) | 27.8 ± 4.60 | 27.9 ± 4.70 | 26.3 ± 4.20 | < .001 |
| Medical history | ||||
| Hypertension | 6572 (82.9%) | 6259 (83.0%) | 313 (81.9%) | .59 |
| Hyperlipidemia | 4502 (59.7%) | 4313 (59.2%) | 189 (49.7%) | < .001 |
| Diabetes mellitus | 2660 (34.5%) | 2586 (35.3%) | 74 (19.3%) | < .001 |
| Smoker | 1254 (20.4%) | 1207 (20.6%) | 47 (15.9%) | .05 |
| Peripheral arterial disease | 972 (12.9%) | 943 (13.2%) | 29 (8.4%) | .01 |
| Chronic kidney disease | ||||
| Hemodialysis | 98 (1.4) | 98 (1.5) | 0 | .02 |
| Baseline estimated glomerular filtration rate (mL/min) | 54.0 ± 27 | 54.5 ± 27 | 44.5 ± 21 | < .001 |
| Previous stroke | 847 (11.1%) | 805 (11.1%) | 42 (11.1%) | .99 |
| Heart disease | ||||
| Previous ischemic heart disease | 4553 (57.9%) | 4346 (59.9%) | 207 (55.3%) | .08 |
| Previous myocardial infarction | 935 (12.6%) | 904 (12.8%) | 31 (8.8%) | .03 |
| Previous percutaneous coronary intervention | 1563 (20.6%) | 1508 (20.9%) | 55 (14.9%) | .006 |
| Percutaneous coronary intervention 1-month before | 1888 (24.8%) | 1816 (25.0%) | 72 (19.5%) | .02 |
| Previous coronary artery bypass grafting | 539 (7.30%) | 536 (7.6%) | 3 (0.9%) | < .001 |
| Previous heart valve surgery | ||||
| Aortic | 227 (2.9%) | 223 (3.0%) | 4 (1.1%) | .03 |
| Mitral | 115 (1.5%) | 113 (1.5%) | 2 (0.5%) | .12 |
| Atrial fibrillation | 2169 (27.9%) | 2075 (28.1%) | 94 (24.9%) | .19 |
| Previous pacemaker or ICD | 582 (7.50%) | 552 (7.50%) | 30 (8.00%) | .73 |
| Risk scores | ||||
| Logistic EuroSCORE, % | 16.72 ± 11.6 | 16.54 ± 11.6 | 20.31 ± 11.9 | < .001 |
| Clinical presentation | ||||
| Angina | 578 (7.30%) | 559 (7.40%) | 19 (4.90%) | .06 |
| Dyspnea | 5000 (67.0%) | 4730 (66.7%) | 270 (72.4%) | .02 |
| Echocardiographic characteristics | ||||
| LVEF, % | 57.4 ± 15.4 | 57.4 ± 15.5 | 58.8 ± 11.8 | .47 |
| Mean gradient, mmHg | 48.2 ± 15.0 | 48.1 ± 14.9 | 51.0 ± 15.1 | < .001 |
| Peak gradient, mmHg | 78.9 ± 22.9 | 78.7 ± 22.8 | 83.8 ± 23.0 | < .001 |
| Aortic valve area, cm2 | 0.66 ± 0.2 | 0.66 ± 0.2 | 0.62 ± 0.2 | .06 |
| Moderate or severe mitral regurgitation | 469 (6.9%) | 444 (6.9%) | 25 (7.8%) | .57 |
| Moderate or severe aortic regurgitation | 151 (3.1%) | 143 (3.0%) | 8 (3.3%) | .79 |
| Pulmonary artery systolic pressure, mmHg | 46.55 ± 18.0 | 46.24 ± 13.6 | 46.56 ± 18.2 | .83 |
| Diameter of the aortic annulus, mm | 23.90 ± 2.8 | 23.07 ± 2.8 | 22.70± 2.7 | .08 |
| TAVI indication | .10 | |||
| Contraindication | 1656 (29.3%) | 1584 (29.5%) | 72 (25.3%) | |
| High risk | 2379 (42.0%) | 2242 (41.7%) | 137 (48.1%) | |
| Intermediate risk | 1626 (28.7%) | 1550 (28.8%) | 76 (26.7%) | |
ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; TAVI, transcatheter aortic valve implantation. Data are expressed as no. (%) or mean ± standard deviation. | ||||
Study endpoints and definitions
Standardized definitions of all patient-related variables, clinical diagnoses, and in-hospital complications and outcomes were used according to the Valve Academic Research Consortium (VARC) definitions.7,8 The primary endpoint was all-cause mortality occurring within the first year after TAVI between nonagenarians and elderly non-nonagenarian patients. Also, the rates of in-hospital mortality, stroke, myocardial infarction, major or life-threatening bleeding events as defined by the VARC criteria,8,9 and permanent pacemaker implantation were compared too. High surgical risk was defined as logistic EuroSCORE values > 20% or Society of Thoracic Surgeons (STS) risk model values > 8%. The unadjusted and adjusted short- (in-hospital) and long-term (within the first year after TAVI) mortality rates were assessed in the general cohort.
Statistical analysis
Categorical variables were expressed as frequencies (percentages), and the differences were assessed using the chi-square test (or Fisher’s exact test, when appropriate). Continuous variables were expressed as mean ± standard deviation or as median [interquartile range]. The Kolmogorov-Smirnov test was used to guarantee a normal distribution. Continuous variables were compared using the analysis of variance (ANOVA) test or the Kruskal-Wallis test, when appropriate. Using all follow-up data available survival curves were built for the time-to-event variables using the Kaplan-Meier method. To identify the independent predictors of first year all-cause mortality in the general cohort, the multivariate Cox proportional hazard regression model was used. The proportionality assumption was assessed graphically using log-minus-log plots. Also, the Cox proportional hazard models for the primary endpoint satisfied the proportional hazards assumption. In all analyses, 2-tailed P values < .05 were considered statistically significant. Follow-up was scheduled to end on the date of the last follow-up or at the 1-year mark, whichever came first. The analyses were performed using IBM SPSS statistical software (V 19.0, IBM, United States).
RESULTS
Baseline and echocardiographic characteristics
From January 2012 through December 2018, a total of 8073 elderly patients (≥ 75 years) from 46 Spanish centers were enrolled in the Spanish TAVI registry; 7686 (95.2%) were elderly non-nonagenarian patients (≥ 75-< 90 years) while 387 (4.79%) were nonagenarians (≥ 90 years). The patients’ baseline characteristics in both groups are shown on table 1. The mean age ± standard deviation of the non-nonagenarian group was 82.6 ± 3.66 years (91.06 ± 1.29 years in nonagenarians). Women were predominant in both groups (55.3% vs 58.4% in nonagenarians; P = .23). Nonagenarians had a lower prevalence of diabetes mellitus, peripheral arterial disease or previous myocardial infarction. On the other hand, they showed a lower estimated glomerular filtration rate at baseline, and a higher EuroSCORE model I. There were no differences in the TAVI indications between both groups. Figure 1 shows the number and percentage of nonagenarians treated with TAVI over the years. In absolute terms, there is a gradual increase of nonagenarians from the 11 patients reported in 2011 to the 78 patients reported in 2018.
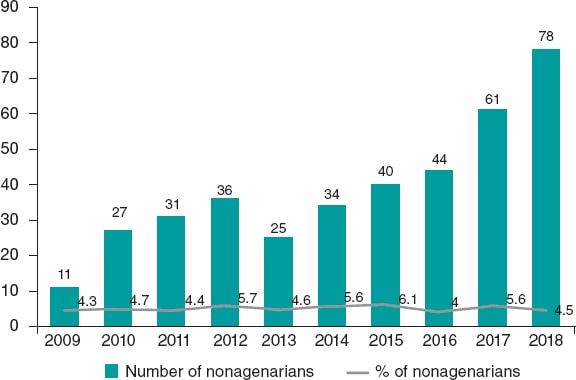
Figure 1. Absolute number and rate of nonagenarians treated with TAVI by year. The bar chart and line graph respectively express the number and rate of nonagenarian patients with severe aortic stenosis treated with TAVI from 2009 to 2018. TAVI, transcatheter aortic valve implantation.
Procedural characteristics
The post-implantation TAVI echocardiographic findings and procedural characteristics are shown on table 2. Transfemoral access was used in 91.6% of the cases, predominantly among nonagenarians (91.4% vs 95.1%; P = .01). No differences were seen in the type or size of valve used. The device implantation success achieved was high in both groups (94.9% vs 95.6%; P = .55).
Table 2. Procedural characteristics
| Variable | All patients (N = 8073) | Elderly < 90 y (N = 7686) | Nonagenarians (N = 387) | P |
|---|---|---|---|---|
| Procedural characteristics | ||||
| Balloon-expandable valve | 3663 (45.7%) | 3477 (45.6%) | 186 (48.1%) | .34 |
| Self-expanding valve | 4356 (54.3%) | 4155 (54.4%) | 201 (51.9%) | |
| Valve size | .82 | |||
| < 23 | 2126 (28.3%) | 2017 (28.3%) | 109 (29.1%) | |
| 24-28 | 3146 (41.9%) | 2987 (41.9%) | 159 (42.5%) | |
| < 29 | 2236 (29.8%) | 2130 (29.9%) | 106 (28.3%) | |
| Transfemoral access | 7360 (91.6%) | 6993 (91.4%) | 367 (95.1%) | .01 |
| Predilatation | 2617 (49.9%) | 2508 (50.2%) | 109 (44.0%) | .06 |
| Postdilatation | 1671 (22.4%) | 1593 (22.3%) | 78 (22.7%) | .86 |
| Device implantation success | 7666 (95.0%) | 7296 (94.9%) | 370 (95.6%) | .55 |
| Procedural duration, min | 104.2 ± 48 | 103.9 ± 48 | 109.7 ± 51 | .05 |
| Post-TAVI echocardiographic characteristics | ||||
| Mean gradient, mmHg | 10.1 ± 5.5 | 10.1 ± 5.5 | 9.5 ± 4.3 | .18 |
| Peak gradient, mmHg | 18.9 ± 9.9 | 18.9 ± 10.0 | 18.0 ± 8.3 | .14 |
LVEF, left ventricular ejection fraction; TAVI, transcatheter aortic valve implantation. Data are expressed as no. (%) or mean ± standard deviation. | ||||
Clinical outcomes
A comparison of clinical outcomes between the non-nonagenarian group and the nonagenarian one is shown on table 3. Compared with the non-nonagenarian group, nonagenarians were more likely to die during their hospital stay (4.3% vs 7.0% among nonagenarians; P = .01) and have major or life-threatening bleeding events (1.2% vs 3.1%; P < .05). No differences were found in stroke, myocardial infarction, vascular complications, permanent pacemaker implantation or acute kidney injury. The median follow-up was 308 days (31-365). At the 1-year follow-up, a total of 719 patients had died (8.9%). The unadjusted risk of all-cause mortality at the 1-year follow-up was similar among nonagenarians compared to the non-nonagenarian group (8.8% vs 11.6%; P = .07) (figure 2).
Table 3. Outcomes
| Variable | All patients (N = 8073) | Elderly < 90 y (N = 7686) | Nonagenarians (N = 387) | P |
|---|---|---|---|---|
| Procedural | ||||
| Conversion to open-heart surgery | 54 (0.7%) | 51 (0.7%) | 3 (0.8%) | .74 |
| In-hospital | ||||
| Death | 357 (4.4%) | 330 (4.3%) | 27 (7.0%) | .01 |
| Stroke | 148 (1.8%) | 144 (1.9%) | 4 (1.0%) | .23 |
| Myocardial infarction | 99 (1.2%) | 94 (1.2%) | 5 (1.3%) | .81 |
| Vascular complication | 976 (12.1%) | 919 (12.0%) | 57 (14.7%) | .10 |
| Major/life- threatening bleeding | 108 (1.3%) | 96 (1.2%) | 12 (3.1%) | .002 |
| Permanent | ||||
| pacemaker implantation | 1213 (15.0%) | 1155 (15.0%) | 58 (15.0%) | .98 |
| AKI > 1 | 466 (5.8%) | 440 (5.5%) | 26 (6.7%) | .41 |
| Follow-up | ||||
| Median 1-year follow-up | 308 (31-365) | 306 (31-365) | 362 (19-365) | .60 |
| 1-year all-cause mortality rate | 719 (8.9%) | 674 (8.8%) | 45 (11.6%) | .072 |
AKI, acute kidney injury. Data are expressed as no. (%) or mean ± standard deviation. | ||||
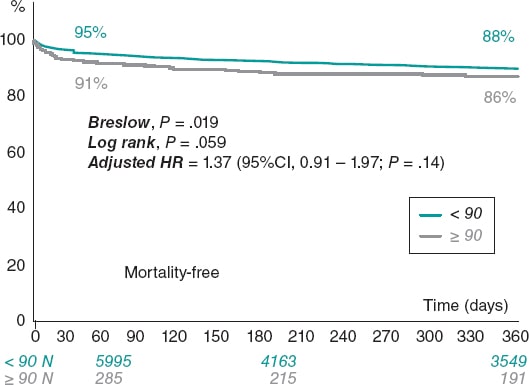
Figure 2. Kaplan-Meier survival estimates for the 1-year all-cause mortality and survival rates. 95%CI, 95% confidence interval; HR, hazard ratio.
The multivariate Cox proportional hazard models identified independent predictors of all-cause mortality in the cohort (table 4). Age ≥ 90 years was not independently associated with a higher adjusted all-cause mortality rate (hazard ratio [HR], 1.37; 95% confidence interval [95%CI], 0.91–1.97; P = .14). The baseline creatinine levels prior to the procedure (HR, 1.28; 95%CI, 1.15–1.44; P < .001), dyspnea as the predominant symptom of severe AS (HR, 1.49, 95% CI 1.14–1.3, P < .01), surgical risk assessment as high-risk or inoperable (HR, 1.34; 95%CI, 1.01–1.79; P = .04), and atrial fibrillation (HR, 1.37; 95% CI, 1.09–1.73; P = .008) were independently associated with a higher adjusted all-cause mortality rate. On the other hand, the body mass index (HR, 0.97; 95%CI, 0.95-0.99; P = .02), the use of femoral access (HR, 0.68; 95%CI, 0.51–0.92; P = .02), and the device implantation success rate (HR, 0.18; 95%CI, 0.13–0.23; P < .001) were associated with a lower adjusted all-cause mortality rate at the follow-up.
Table 4. Independent predictors of all-cause mortality
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Age ≥ 90 years | 1.59 (1.16-2.18) | .007 | 1.37 (0.91-1.97) | .14 |
| Women | 0.94 (0.80-1.11) | .49 | – | – |
| Body mass index (kg/m2) | 0.97 (0.95-0.98) | < .001 | 0.97 (0.95-0.99) | .02 |
| Peripheral vascular disease | 1.34 (1.07-1.68) | .06 | – | – |
| Baseline creatinine levels (mL/min) | 1.22 (1.11-1.34) | < .001 | 1.28 (1.15-1.44) | < .001 |
| Atrial fibrillation | 1.28 (1.07-1.52) | .008 | 1.37 (1.09-1.73) | .008 |
| Previous pacemaker or ICD | 0.70 (0.49-1.01) | .05 | – | – |
| LVEF, % | 0.99 (0.98-0.99) | .007 | – | – |
| Moderate or severe mitral regurgitation | 1.39 (1.02-1.89) | .05 | – | – |
| Moderate or severe aortic regurgitation | 1.49 (0.88-2.56) | .16 | ||
| Dyspnea | 1.26 (1.04-1.52) | .02 | 1.49 (1.14-1.93) | .002 |
| High-risk/inoperable | 1.57 (1.23-2.00) | < .001 | 1.34 (1.01-1.79) | .04 |
| Transfemoral access | 0.61 (0.48-0.78) | < .001 | 0.68 (0.51-0.92) | .02 |
| Device implantation success | 0.19 (0.15-0.23) | < .001 | 0.18 (0.13-0.23) | < .001 |
95%CI, 95% confidence interval; HR, hazard ratio; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction. Hazard ratios and their 95% confidence intervals were calculated using the multivariate Cox regression analysis. | ||||
Table 2 of the supplementary data shows differences in the baseline characteristics, echocardiographic findings, and procedural characteristics between the nonagenarian patients who died at the follow-up and those who did not. The nonagenarians who died after TAVI had higher baseline creatinine levels (1.16 ± 0.42 vs 1.34 ± 0.56; P = .02), and more in-hospital complications: vascular complications (12.2% vs 35.7%; P = .001), major or life-threatening bleeding events (0.9% vs 21.4%; P < .001), and acute kidney injury (5.2% vs 19.0%; P = .004).
DISCUSSION
There are 3 main findings in our study. First, the absolute number of nonagenarians treated with TAVI has been growing gradually over time. Secondly, age ≥ 90 years was not independently associated with a higher adjusted all-cause mortality rate. Thirdly, both the baseline kidney function and in-hospital complications have been associated with the prognosis of nonagenarians treated with TAVI.
The number of nonagenarians treated with TAVI has been growing gradually over time
Aortic stenosis is a slowly progressive heart disease associated with dismal outcomes within a few years after symptom onset if it goes untreated. Surgical aortic valve replacement (SAVR) has been the only effective treatment of severe AS for many years. This has resulted in a high percentage of patients with severe AS going untreated because the risks of this surgical procedure outweigh its possible benefits. Over the last few years, the development of minimally invasive TAVI has changed the decision-making process regarding valvular procedures.10 Initially introduced as a ‘bailout’ therapy for inoperable patients with severe AS, TAVI is currently a feasible option for high- and intermediate- risk patients,11 which widens the spectrum of patients with severe AS who get treated and moves away from the poor outcomes associated with the standard treatment (an all-cause mortality risk at 5 years of nearly 93%).1 The great beneficiaries are elderly patients who used to be considered noneligible for SAVR as confirmed by the CURRENT AS (Contemporary outcomes after surgery and medical treatment in patients with severe aortic stenosis) registry.12
In our study, we saw an increase in the absolute number of nonagenarians treated with TAVI from only 11 cases reported before 2009 to 78 cases reported back in 2018 (figure 1). The gradual increase in the number of nonagenarians referred for TAVI can be explained by the greater knowledge gained on this technique, the excellent early results reported,6 the higher number of TAVIs performed, and the gradual aging of the population, which is a global phenomenon. The aging of the population is a global phenomenon. There were 703 million people > 65 years in the world in 2019. The number of elderly people is projected to double to 1.5 billion by 2050.3 It has been predicted that, in Spain, by 2040, life expectancy will exceed 85 years of age in both sexes.13 Other study claims that chances are over 50% that by 2030, female life expectancy will break the 90-year barrier, a level deemed unattainable by some at the turn of the 21st century. South Korea would have the highest projected female life expectancy followed by France, Spain, and Japan.14 Taking these data into consideration, the natural evolution of valvular heart disease, the advances made with TAVI devices, and the results associated with this procedure,15 a higher number of nonagenarians with severe AS should be expected in the coming future. This should lead to more routine assessments of these patients by the heart team on the possibility to perform TAVI.
Being a nonagenarian is not associated with a higher adjusted all-cause mortality rate in TAVI
Over the last decade, healthcare spending has been gradually rising at a higher speed than the gross domestic product, which challenges the sustainability of healthcare systems.16 A number of factors have been identified as contributors to increasing spending, among them, the aging of the population and the development of new medical technologies.17 Both are implicated in our study. Given the economic implications of TAVI,18 identifying the patients in whom TAVI would likely be futile, as defined by the composite endpoint of death and/or absence of functional improvement at the postoperative short-term follow-up (6 months to 1 year),19 must be a priority. In our study, age ≥ 90 years was not independently associated with a higher adjusted all-cause mortality rate. This may be due to the fact that nonagenarians treated with TAVI are a highly selected population with a healthier clinical profile compared to younger patients. This is a selection bias introduced by the heart team that selects the healthiest nonagenarians to perform TAVIs while looking for the highest benefit possible, which is seen in the preferential use of femoral access. Our results are consistent with other cohorts of nonagenarians treated with TAVI.20,21 In addition, several studies have shown that the benefit of the procedure goes beyond a higher survival rate, thus improving the quality of life.22,23 This is a remarkable aspect in the elderly setting: the so-called idea of “adding life to years” rather the “adding years to life”. Nevertheless, we should be cautious when interpreting the results reported: we found that 1 in 10 nonagenarians treated with TAVI died within the first 30 days.
Prognosis in this high-risk population can improve if TAVI is performed in advance in asymptomatic patients with severe AS. Detecting the early signs of symptoms can be challenging in many sedentary and deconditioned elderly patients in whom irreversible left ventricular decompensation may have appeared when detected. This strategy is supported by evidence from the recently published RECOVERY trial (The randomized comparison of early surgery versus conventional treatment in very severe aortic stenosis).24 It was designed to compare the long-term clinical outcomes of early surgical aortic valve replacement and the outcomes of a conservative strategy in asymptomatic patients with fairly severe aortic stenosis (transvalvular velocity ≥ 4.5 m per second) based on the current clinical practice guidelines. It was found that the rate of the composite endpoint of procedural mortality or cardiovascular death at the follow-up was significantly lower in those treated with early aortic valve replacement surgery compared to those treated conservatively [1% vs 15%, HR 95%CI, 0.009 (0.001–0.67)]. The performance of TAVI as a less invasive procedure compared to surgery can be easily justified as a preventive measure in nonagenarians instead of having to wait for the development of early symptom to trigger this valve procedure. Two randomized controlled trials, the EARLY-TAVR (Evaluation of transcatheter aortic valve replacement compared to surveillance for patients with asymptomatic severe aortic stenosis; NCT03042104) and the EVoLVeD (Early valve replacement guided by biomarkers of left ventricular decompensation in asymptomatic patients with severe aortic stenosis) trials25 are currently recruiting asymptomatic patients with AS to study if an early therapeutic approach may actually improve the outcomes compared to the current standard of care. These trials have the potential to change clinical practice and reduce the threshold for the procedure.26
The finding on mid-term futility characteristics in nonagenarians
This is the third and last point we would like to underline. In our cohort, we identified several characteristics associated with a worse prognosis (table 2 of the supplementary data), and their recognition may help clinicians select the eligible nonagenarians for TAVI. Among them, 2 deserve special attention: the presence of chronic kidney disease and a bleeding event as in-hospital complications. Chronic kidney disease is widely known as one of the worst prognostic factors among patients treated with SAVR,27 and similar results have been reported in TAVI.28 In our study, higher baseline creatinine levels at admission were associated with a worse long-term prognosis. Our results are consistent with those from different registries that found that the presence of chronic kidney disease has been consistently associated with poorer outcomes after TAVI.7 Yamamoto et al29 studied the prognostic value of an impaired renal function based on a chronic kidney disease classification in very elderly patients treated with TAVI. They found that, in stage 4 patients (eGFR < 30 mL/min/1.73 m2), the 30-day, 1-year, and cumulative 2-year mortality rates were 26.2%, 47.8%, and 68.2%, respectively. On the other hand, something that would explain the higher in-hospital mortality rate of nonagenarians is the presence of major or life-threatening bleeding compared to younger patients. Patients treated with TAVI have a high baseline risk of bleeding: peripheral vasculopathy, chronic kidney disease, acquired and reversible von Willebrand disease, and acquired thrombocytopenia30 increase the risk of bleeding events.31 Age has been associated with bleeding events after TAVI immediately after the procedure or later on,32 along with chronic kidney disease and comorbidities. Age and comorbidities are non-modifiable variables, which means that modifiable variables associated with bleeding like vascular access33 and antithrombotic therapy should be controlled. In this regard, intensive antithrombotic regimens with dual antiplatelet therapy with aspirin plus clopidogrel have been the standard antithrombotic therapy at discharge after TAVI.34 This could have a higher negative impact on nonagenarians since age is one of the major predictors of bleeding when on dual antiplatelet treatment.35 The recently published POPular TAVI trial36 showed that, among patients treated with TAVI without an indication for anticoagulation, aspirin alone was associated with fewer all bleeding and nonprocedural-related bleeding complications compared to aspirin and clopidogrel. The adoption of a single antiplatelet therapy regime after TAVI instead of dual antiplatelet therapy may reduce the number of major bleeding events in this high-risk population, thus improving the efficacy and long-term prognosis of TAVI in this population. Regarding the predictive ability of the surgical risk score, the STS-PROM is the only surgical risk score to accurately predict mortality risk in nonagenarians.20 Finally, although the presence of atrial fibrillation is less common among patients who died at the follow-up compared to those who survived, we think this correlation to be spurious given the results shown in other series of nonagenarian patients treated with TAVI that revealed the presence of atrial fibrillation associated with a worse prognosis.21
Study limitations
The main limitation of this study is its observational design, which implies an inherent selection bias. Also, it is difficult to capture and control all potential confounders such as the time trend of the technique used and the type of patients.15 Our data come from a voluntary registry whose data have not been externally audited and does not include all Spanish TAVI-capable centres. This limits the external validity of our results. In addition, the sample size may be lacking the statistical power to detect other statistically significant differences in the outcomes reported and does not allow us to develop a multivariate analysis to assess independent predictors of all-cause mortality in a cohort of nonagenarian patients. The lack of antithrombotic therapy at discharge does not allow us to determine its actual impact on this population. Also, we are lacking data on quality of life at the follow-up—a remarkable aspect in the elderly setting—and the prognosis of the procedure > 1-year follow-up. The inclusion of nonagenarians in large, well-designed, randomized clinical trials is needed to fully clarify the actual potential benefit of TAVI in this high-risk cohort of patients.
CONCLUSIONS
Nonagenarians are a growing very high-risk population with severe AS for whom TAVI may be a safe and effective option. Careful patient selection by the TAVI heart team is required to achieve maximum efficiency in this population where baseline kidney function and bleeding complications may determine the long-term prognosis after TAVI.
FUNDING
The Interventional Cardiology Association of the Spanish Society of Cardiology sponsored the maintenance and exploitation of the database.
AUTHORS’ CONTRIBUTIONS
P. L. Cepas-Guillén, X. Freira, and M. Sabaté designed the study. A. Regueiro, D. Sanmiguel Cervera, R. Blanco Mata, J. F. Oteo, I. Amat-Santos, F. Ten, J. M. Nogales, E. Fernández-Nofrerías, V. Mainar, G. Lasa-Larraya, L. Andraka, J. A. Baz-Alonso, M. Cruz Ferrer, E. Pinar, R. Romaguera, C. Cuellas Ramón, F. Alfonso, C. A. Urbano-Carrillo, S. García-Blas, A. Piñero, A. Albarrán, R. Ruíz-Salmerón, J. Moreu, Ó. Gil-Albarova, J. M. Melero, and T. Heredia-Cambra supervised the data mining, recruited the participating centers and patients, and managed the data. P. L. Cepas-Guillén, A. Regueiro, and M. Sabaté provided statistical counselling on the study design and analyzed the data. P. L. Cepas-Guillén, M. Sabaté, and X. Freira drafted the manuscript, and all authors contributed substantially to its revision. P. L. Cepas-Guillén, and M. Sabaté take full responsibility for this manuscript entirely. The authors submitting the manuscript accept full responsibility for its content as defined by the International Committee of Medical Journal Editors.
CONFLICTS OF INTEREST
M. Sabaté declared having received personal fees from Abbott Vascular, and Ivascular outside the setting of this manuscript.
ACKNOWLEDGMENTS
We wish to thank Dr. Pilar Jimenez-Quevedo, and Dr. María Jose Perez Vizcayno for their support during the preparation of this manuscript.
REFERENCES
1. Kapadia SR, Leon MB, Makkar RR, et al. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1):A randomised controlled trial. Lancet. 2015;385:2485-2491.
2. Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1):A randomised controlled trial. Lancet. 2015;385:2477-2484.
3. United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing 2019:Highlights (ST/ESA/SER.A/430). Available online https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf. Accessed 19 Mar 2021.
4. Coffey S, Cairns BJ, Iung B. The modern epidemiology of heart valve disease. Heart. 2016;102:75-85.
5. Alkhouli M, Alqahtani F, Ziada KM, Aljohani S, Holmes DR, Mathew V. Contemporary trends in themanagement of aortic stenosis in the USA. Eur Heart J. 2020;41:921-928.
6. SabatéM, Cánovas S, García E, et al. In-hospital and Mid-term Predictors of Mortality After Transcatheter Aortic Valve Implantation:Data From the TAVI National Registry 2010-2011. Rev Esp Cardiol. 2013;66:949-958.
7. Allende R, Webb JG, Munoz-Garcia AJ, et al. Advanced chronic kidney disease in patients undergoing transcatheter aortic valve implantation:Insights on clinical outcomes and prognostic markers froma large cohort of patients. Eur Heart J. 2014;35:2685-2696.
8. Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation:The Valve Academic Research Consortium-2 consensus document. Eur Heart J. 2012;33:2403-2418.
9. Leon MB, Piazza N, Nikolsky E, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials:A consensus report from the Valve Academic Research Consortium. Eur Heart J. 2011;32:205-217.
10. Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. N Engl J Med. 2011;364:2187-2198.
11. Makkar RR, Thourani VH, Mack MJ, et al. Five-Year Outcomes of Transcatheter or Surgical Aortic-Valve Replacement. N Engl J Med. 2020;382:799-809.
12. Taniguchi T, Morimoto T, Takeji Y, Kato T, Kimura T. Contemporary issues in severe aortic stenosis:Review of current and future strategies from the Contemporary Outcomes after Surgery and Medical Treatment in Patients with Severe Aortic Stenosis registry. Heart. 2020;106:802-809.
13. Foreman KJ, Marquez N, Dolgert A, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death:reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet. 2018;392:2052-2090.
14. Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M. Future life expectancy in 35 industrialised countries:projections with a Bayesian model ensemble. Lancet. 2017;389:1323-1335.
15. Jiménez-Quevedo P, Muñoz-García A, Trillo-Nouche R, et al. Evolución temporal en el tratamiento transcatéter de la estenosis aórtica:análisis del registro español de TAVI. REC Interv Cardiol. 2020;2:96-105.
16. OECD, Health at a Glance 2019:OECD Indicators, OECD Publishing, Paris, 2019. Available at https://doi.org/10.1787/4dd50c09-en. Accessed 19 Mar 2021.
17. Sorenson C, Drummond M, Khan BB. Medical technology as a key driver of rising health expenditure:Disentangling the relationship. Clin Outcomes Res. 2013;5:223-234.
18. Fontes-Carvalho R, Guerreiro C, Oliveira EI, Braga P. Present and future economic impact of transcatheter aortic valve replacement on the Portuguese national healthcare system. Rev Port Cardiol. 2020;39:479-488.
19. Puri R, Iung B, Cohen DJ, RodéS-Cabau J. TAVI or No TAVI:identifying patients unlikely to benefit from transcatheter aortic valve implantation. Eur Heart J. 2016;37:2217–2225.
20. Vlastra W, Chandrasekhar J, Vendrik J, et al. Transfemoral TAVR in Nonagenarians:From the CENTER Collaboration. JACC Cardiovasc Interv. 2019;12:911-920.
21. Deharo P, Bisson A, Herbert J, et al. Outcomes in nonagenarians undergoing transcatheter aortic valve implantation (TAVI):data from a nationwide analysis. EuroIntervention. 2020. http://dx.doi.org/10.4244/EIJ-D-19-00647.
22. Arsalan M, Szerlip M, Vemulapalli S, et al. Should Transcatheter Aortic Valve Replacement Be Performed in Nonagenarians?Insights from the STS/ACC TVT Registry. J Am Coll Cardiol. 2016;67:1387-1395.
23. Orvin K, Assali A, Vaknin-Assa H, et al. Efficacy and Safety of Transcatheter Aortic Valve Implantation in Aortic Stenosis Patients With Extreme Age. J Invasive Cardiol. 2015;27:475-480.
24. Kang D-H, Park S-J, Lee S-A, et al. Early Surgery or Conservative Care for Asymptomatic Aortic Stenosis. N Engl J Med. 2020;382:111-119.
25. Bing R, Everett RJ, Tuck C, et al. Rationale and design of the randomized, controlled Early Valve Replacement Guided by Biomarkers of Left Ventricular Decompensation in Asymptomatic Patients with Severe Aortic Stenosis (EVOLVED) trial. Am Heart J. 2019;212:91-100.
26. Lindman BR, Dweck MR, Lancellotti P, et al. Management of Asymptomatic Severe Aortic Stenosis:Evolving Concepts in Timing of Valve Replacement. JACC Cardiovasc Imaging. 2020;13:481-493.
27. Brown JM, O'Brien SM, Wu C, Sikora JAH, Griffith BP, Gammie JS. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years:Changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg. 2009;137:82-90.
28. Ferro CJ, Chue CD, de Belder MA, et al. Impact of renal function on survival after transcatheter aortic valve implantation (TAVI):an analysis of the UK TAVI registry. Heart. 2015;101:546-552.
29. Yamamoto M, Hayashida K, Mouillet G, et al. Prognostic value of chronic kidney disease after transcatheter aortic valve implantation. J Am Coll Cardiol. 2013;62:869-877.
30. Hernández-Enríquez M, Regueiro A, Romaguera R, et al. Thrombocytopenia after transcatheter aortic valve implantation. A comparison between balloon-expandable and self-expanding valves. Catheter Cardiovasc Interv. 2019;93:1344-1351.
31. Mangieri A, Montalto C, Poletti E, et al. Thrombotic Versus Bleeding Risk After Transcatheter Aortic Valve Replacement:JACC Review Topic of the Week. J Am Coll Cardiol. 2019;74:2088-2101.
32. Piccolo R, Pilgrim T, Franzone A, et al. Frequency, Timing, and Impact of Access-Site and Non–Access-Site Bleeding on Mortality Among Patients Undergoing Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2017;10:1436-1446.
33. Hernández-Enriquez M, Andrea R, Brugaletta S, et al. Puncture Versus Surgical Cutdown Complications of Transfemoral Aortic Valve Implantation (from the Spanish TAVI Registry). Am J Cardiol. 2016;118:578-584.
34. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease:A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70:252-289.
35. Costa F, van Klaveren D, James S, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score:a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389:1025-1034.
36. Brouwer J, Nijenhuis VJ, Delewi R, et al. Aspirin with or without Clopidogrel after Transcatheter Aortic-Valve Implantation. N Engl J Med. 2020;383:1447-1457.
* Corresponding author: Servicio de Cardiología, Hospital Clinic, Villarroel 170, 08036 Barcelona, Spain
E-mail address: masabate@ub.edu (M. Sabaté).











