ABSTRACT
Introduction and objectives: Ischemic postconditioning (iPost, coronary intermittent re-occlusion maneuvers immediately after PCI-mediated reperfusion) has been proposed to limit infarct size (IS). However, a few experimental and clinical contradictory results have been reported. We hypothesized that iPost cardioprotection is affected by the duration of ischemia. Our objective was to assess IS in the presence/absence of iPost in a pig model of myocardial infarction of variable ischemia duration.
Methods: Large white pigs (n = 38) underwent angioplasty balloon-induced coronary ischemia followed by reperfusion. Two set of experiments were carried out: intermediate (30 min) and prolonged (40 min) ischemia. In both, pigs were allocated on a 1:1 ratio to receive iPost (4 cycles of “1 min balloon inflation followed by 1 min deflation” upon reperfusion) or control. Animals underwent contrast-enhanced multiparametric cardiac magnetic resonance scan on day 7. Primary outcome measure was cardiac magnetic resonance-based IS (% of left ventricular mass). The interaction between treatment allocation and ischemia duration was assessed using a 2-way ANOVA test.
Results: iPost was not associated with smaller IS in any of the ischemia duration protocols (intermediate ischemia: 0.3% [0.0–3.9] vs 0.9% [0.0–2.6] in iPost and control, respectively; P = .378; long ischemia: 31.1% [27.3–32.8] vs 27.3% [25.1–27.5]; P = .248). When both ischemia-duration protocols were combined, iPost was not associated with smaller IS (3.9% [0.0–30.9] vs 4.6% [0.2–25.1]; P = .672). T1 relaxation times were longer in animals undergoing iPost compared to controls (1306.2 ms [1190.7–1492.7] vs 1240.7 ms [1167.1–1304.5]; P = .024).
Conclusions: In a pig model of reperfused myocardial infarction of variable ischemia duration, iPost failed to reduce IS. T1 relaxation times were longer in animals undergoing iPost indicative of the potential harm involved in this procedure.
Keywords: Reperfusion injury. Ischemic postconditioning. Cardioprotection. Myocardial infarction.
RESUMEN
Introducción y objetivos: Existen resultados contradictorios sobre la eficacia del poscondicionamiento isquémico local (iPost) como intervención para reducir el tamaño del infarto (TI). Pretendemos evaluar si el efecto del iPost se ve alterado por el tiempo de isquemia en un modelo porcino de infarto de miocardio.
Métodos: Se sometió a 38 cerdos Large-White a isquemia-reperfusión coronaria con balón de angioplastia. Se realizaron dos series de experimentos: isquemia de intermedia (30 minutos) y larga (40 minutos) duración. Los animales se asignaron 1:1 a iPost (4 ciclos de 1 minuto inflado/1 minuto desinflado, comenzando 1 minuto tras la reperfusión) o control. Se realizó una resonancia magnética 7 días después. El objetivo primario fue el TI medido por resonancia magnética (porcentaje de masa ventricular izquierda [%VI] con realce tardío de gadolinio). La interacción de la asignación al tratamiento y la duración de la isquemia se evaluó mediante un análisis de varianza de dos vías.
Resultados: El iPost no se asoció con un menor TI en ninguno de los grupos de duración de la isquemia (duración intermedia: 0,3% [0,0-3,9] frente a 0,9% [0,0-2,6] en iPost y control, respectivamente, p = 0,378; larga duración: 31,1% [27,3-32,8] frente a 27,3% [25,1-27,5] en iPost y control, respectivamente; p = 0,248). Al analizar conjuntamente todos los animales, el iPost tampoco se asoció con un TI más pequeño (3,9% [0,0-30,9] frente a 4,6% [0,2-25,1] en iPost y control, respectivamente, p = 0,672). Los tiempos de relajación en T1 fueron más largos en los animales sometidos a iPost (1.306,2 [1.190,7-1.492,7] ms frente a 1.240,7 [1.167,1–1.304,5] ms; p = 0,024).
Conclusiones: El iPost no logró reducir el TI en ninguna duración de la isquemia. Los tiempos de relajación T1 fueron más largos en los animales sometidos a iPost, lo que sugiere un daño potencial de esta intervención.
Palabras clave: Daño por reperfusión. Condicionamiento isquémico. Cardioprotección. Infarto de miocardio.
Abbreviations HF: heart failure; iPost: ischemic post-conditioning; IRI: ischemia - reperfusion injury; IS: infarct size; LGE: late gadolinium enhancement; MI: myocardial infarction.
INTRODUCTION
ST-segment elevation myocardial infarction (STEMI) is a life-threatening condition that affects more than 7 million people worldwide each year.1 Despite the improved short-term survival and reduced need for repeat revascularization achieved with primary percutaneous coronary intervention (PCI), long-term survival, and the rate of heart failure (HF) have barely improved over the last few years.2
Infarct size (IS), the extent of irreversible injury after MI, is a main contributor to long-term mortality and HF in STEMI survivors.3-5 Therefore, there is a strong need for identifying interventional (invasive) and/or pharmacological strategies than can limit the extent of MI. Upon coronary occlusion, there is a time-dependent progression of irreversible injury.6 Therefore, timely restoration of blood flow (reperfusion) in the ischemic region is of paramount importance to reduce IS and improve the left ventricular ejection fraction (LVEF).7 However, reperfusion per se causes additional damage to the myocardium and microcirculation that contributes to the final IS,8 the so-called ischemia/reperfusion injury (IRI).9
Ischemic postconditioning (iPost) is an interventional cardiology procedure that tested extensively in experimental10 and clinical trials.11-14 IPost is based on the idea that after index ischemia, gentle reperfusion results in less damage than abrupt straight reperfusion.14 This procedure has the great advantage of its easy implementation during primary percutaneous intervention (PCI). It consists of intermittent 1 min brief episodes of coronary flow reocclusion (ie, angioplasty balloon reinflation).
There is controversy though with some studies showing strong cardioprotection through iPost,11 while others disagree.12 One potential explanation for these controversial results is that iPost is only protective in cases where previous ischemic time has not been very prolonged.
To address whether iPost has variable cardioprotective effects depending on ischemic time, we performed a controlled experimental study in which pigs undergoing different times of coronary ischemia are allocated to iPost or control. We used state-of-the-art technology (ie, cardiac magnetic resonance [CMR]) to accurately assess the effect of the procedure on cardioprotection.
METHODS
Studies were approved by Institutional and Regional Animal Research Committees.
Study design
The cardioprotection provided by iPost was tested in 2 different sets of experiment groups: intermediate ischemia duration (30 min) followed by reperfusion, and prolonged ischemia (40 min) followed by reperfusion. All animals underwent a multiparametric CMR scan 1 week after MI. This timing regarding the assessment of the effect of the intervention was chosen since it is the recommended one by international expert consensus.5 Selecting between the intermediate or prolonged ischemia time was based on our previous experiments where we saw that ischemia durations of < 30 min lead to very small IS (< 20% of the area at risk [AAR]) yielding no window for cardioprotection of established pharmacological interventions (eg, metoprolol).15 Similarly, in our experimental setting, ischemia durations > 40 min result in very large IS (> 80% of the AAR) thus reducing the window for cardioprotection.15
Following the 3Rs principle to reduce the use of animals, those on prolonged ischemia (40 min) with/without iPost (as well as the CMR data) were the ones already used in a former article with a different objective.16 In this study, the group of animals undergoing intermediate ischemia duration (30 min) with/without iPost (n = 28) were used ad hoc.
Apart from ischemia duration, the study protocol was equal for both ischemia–duration groups. For these experiments, we employed 3-month-old castrated male large white pigs weighing 30 to 40 Kg. Pigs were allocated to iPost or control before MI was induced. Myocardial AAR was assessed through multidetector computed tomography immediately after vessel occlusion following a previously published methodology.16 Seven days after the MI, all animals underwent a multiparametric CMR scan to asses IS (primary outcome measure), LVEF, and T2 and T1 relaxation times both in the AAR and in the remote area (figure 1).
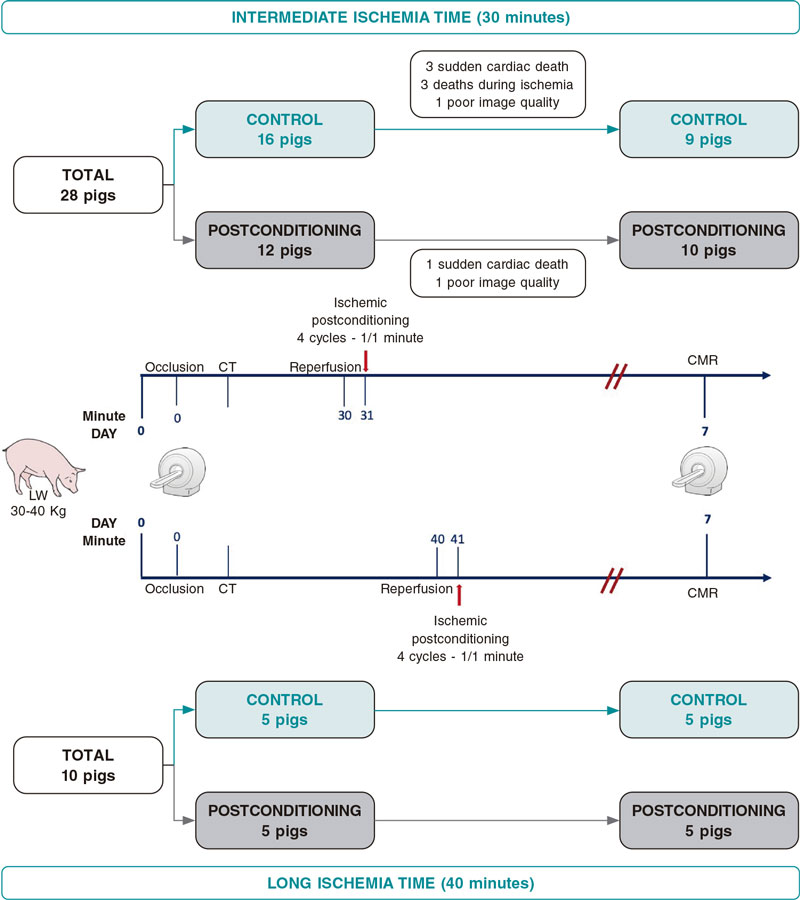
Figure 1. Central illustration. Study design and flowchart. CMR, cardiac magnetic resonance; CT, computed tomography.
Anesthesia and animal care protocol
Every test or experiment was performed under deep sedation. Sedation was induced through the intramuscular injection of ketamine (20 mg/Kg), xylazine (2 mg/Kg), and midazolam (0.5 mg/Kg); and maintained through the continuous infusion of ketamine (2 mg/Kg/h), xylazine (0.2 mg/Kg/h), and midazolam (0.2 mg/Kg/h). Buprenorphine (0.03 mg/Kg) was administered immediately before the MI experiment.
Animals were intubated and received mechanical ventilatory support with volume-control synchronized intermittent mandatory ventilation (fraction of inspired oxygen = 28%).
To avoid coronary thrombosis following balloon-induced MI induction, animals received 150 mg of clopidogrel orally on the day of the procedure and 75 mg 24 and 48 hours later. All animals were euthanized immediately after the day-7 CMR scan.
Myocardial infarction and ischemic postconditioning protocol
All animals underwent the same closed-chest ischemia-reperfusion protocol16 consisting of 30-min or 40-minute left anterior descending coronary artery occlusion with a monorail angioplasty balloon inserted percutaneously through the femoral artery. Balloon was inflated at 8 atm immediately distal to the first diagonal branch. Both the location of the balloon and the status of inflation were monitored on the angiography. A single intra-arterial bolus of 300 IU/kg of unfractionated heparin was administered right before coronary occlusion. Furthermore, to reduce the rate of fatal ventricular arrhythmias, continuous infusion of amiodarone (300 mg/h, no bolus) was initiated immediately after coronary occlusion and maintained until catheters were removed.
Pigs were allocated on a 1:1 ratio to iPost or control before MI induction. After index ischemia duration (30 min or 40 min according to the protocol), animals allocated to control underwent straight chronic reperfusion (balloon deflation) while animals allocated to iPost underwent balloon deflation but 1 min after balloon was reinflated for 1 min. iPost was induced by repeating the 1 min inflation-1 min deflation cycle 4 times. Artery patency was assessed after every inflation/deflation cycle.
Arterial enhanced multidetector computed tomography protocol and analysis
Multidetector computed tomography (MDCT) arterial phase studies were performed during ongoing ischemia on a 64-slice computed tomography scanner (Brilliance CT 64; Philips Healthcare, Cleveland, OH, United States) after the IV administration of iodinated contrast media. Since the MDCT scan was performed during ongoing ischemia (ie, while the balloon was inflated), non-enhanced regions accurately represent the ischemic region (ie, the AAR). MDCT images were analyzed using dedicated software (Extended MR Workspace 2.6; Philips Healthcare, The Netherlands) by 2 observers who remained blind to group allocation. Short axes orientation was obtained from volumetric computed tomography images through multiplanar reconstruction. AAR and remote areas were visually identified based on contrast enhancement differences, manually delineated, and expressed as a percentage of left ventricular (LV) area.15,16
Cardiac magnetic resonance protocol
CMR scans were performed 7 days after the MI on a Philips 3-Tesla Achieva Tx whole-body scanner (Philips Healthcare, The Netherlands) equipped with a 32-element phased-array cardiac coil. The imaging protocol included a standard segmented cine imaging with a steady-state free-precession sequence to provide high-quality anatomic references and assessment of the left ventricular mass, wall thickness, and LVEF, a T1-mapping sequence (modified Look-Locker inversion recovery) to assess T1 native relaxation time, a T2 mapping based on gradient-spin-echo imaging to provide precise myocardial T2 relaxation time,17 and a T1-weighted inversion relaxation turbo field echo sequence acquired 10 min to 15 min after the administration of gadolinium contrast (late gadolinium enhancement, LGE) to assess IS. CMR images were analyzed using dedicated software (MR Extended Workspace 2.6; QMassMR 7.6; Medis, The Netherlands and IntelliSpace Portal, Philips Healthcare, The Netherlands) by 2 experienced observers in CMR analysis and blinded to group allocation.
Statistical analysis
Normal distribution of data was assessed using the Shapiro-Wilk test. Quantitative variables were expressed as median (interquartile range). Categorical variables were expressed as numbers and percentage and rounded to the nearest integer. A 2-way ANOVA test was run on the overall sample with CMR performed on day 7 (29 pigs) to examine the effect of ischemia time and iPost on primary and secondary outcomes (IS, % LV mass), IS indexed to the AAR, LVEF, T2 relaxation time, and native T1 relaxation time). Regarding variables where we found a significant interaction between the duration of ischemia and iPost, we performed a post hoc analysis (Tukey’s method) to confirm the differences seen. We estimated the sample size based on our previous experiments on cardioprotection with metoprolol.15 Lost animals were replaced to achieve the required sample size.
RESULTS
Study groups
Intermediate-ischemia protocol (30 min)
As shown on figure 1, 28 animals underwent MI induction after replacing the lost subjects. From the 16 pigs allocated to control, 3 (19%) died during ischemia induction, and 3 (19%) suddenly died before the day-7 CMR scan. One (10%) out of the 10 animals that completed the day-7 CMR protocol was excluded from the analysis due to poor image quality.
Zero out of the 12 pigs allocated to iPost died during MI induction (0%) while 1 (8%) suddenly died before the CMR scan. One (9%) out the 11 animals that completed the day-7 CMR was excluded from the analysis due to poor image quality.
Therefore, the final population available for outcome assessment was 19 (9 controls, and 10 iPost, figure 1).
Prolonged ischemia protocol (40 min)
As shown on figure 1, 10 animals that completed the protocol in a previously published study were included.
Baseline characteristics
Both control and iPost groups were similar in body weight and baseline CMR-based characteristics except for the indexed left ventricular mass that was larger in the control group of animals with 40 min ischemia times (table 1). A non-significant trend towards larger MDCT-based AAR (% left ventricle mass) was observed in the iPost group (table 1).
Table 1. Baseline characteristics
| Variable | Overall (N = 29) | Intermediate ischemia (30 min) (N = 19) | Prolonged ischemia (40 min) (N = 10) | |||
|---|---|---|---|---|---|---|
| Control (N = 14) | iPost (N = 15) | Control (N = 9) | iPost (N = 10) | Control (N = 5) | iPost (N = 5) | |
| Weight, kg | 36.2 (34.0-38.5) | 33.5 (31.0-40.0) | 36.0 (34.5-38.5) | 37.5 (31.0-41-0) | 36.5 (30.5-38) | 32.5 (32.5-33.5) |
| LVEF, % | 57.6 (55.2-63.0) | 55.9 (52.3-59.5) | 57.7 (55.9-61.3) | 55.8 (52.3-65.4) | 57.4 (55.2-63.0) | 56.0 (55.8-59.3) |
| iLVEDV, mL/m2 | 104.2 (93.5-105.8) | 105.9 (96.1-123.6) | 95.8 (87.5-105.3) | 97.7 (94.5-105.9) | 112.8 (105.8-123.5) | 127.4 (123.6-128.4) |
| iLVESV, mL/m2 | 43.0 (37.0-46.8) | 45.6 (38.0-54.5) | 40.7 (35.8-46.6) | 42.8 (37.3-45.7) | 46.1 (44.3-48.8) | 54.4 (51.7-61.4) |
| Area at risk (% LV) | 27.8 (26.2-27.2) | 31.7 (29.2-32.3) | 24.6 (23.3-27.6) | 27.3 (26.0-29.2) | 27.8 (26.2-27.8) | 31.7 (29.2-32.3) |
iLVEDV, indexed left ventricular end-diastolic volume; iLVESV, indexed left ventricular end-systolic volume; iPost, ischemic postconditioning; LV, left ventricle; LVEF, left ventricular ejection fraction. | ||||||
Cardiac magnetic resonance results
Effect of iPost in a model of intermediate ischemia protocol
In the intermediate-duration ischemia group, iPost did not have any effects on any of the CMR-based variables (table 2; figure 2, figure 3 and figure 4). Both iPost and control animals present small IS with no differences being reported between the intervention groups (0.3% of LV mass [0.0 – 3.9] vs 0.9% [0.0 – 2.6] %LV in iPost and control, respectively, P = .378). We did not find any differences regarding the indexed IS (IS/AAR) either (table 2).
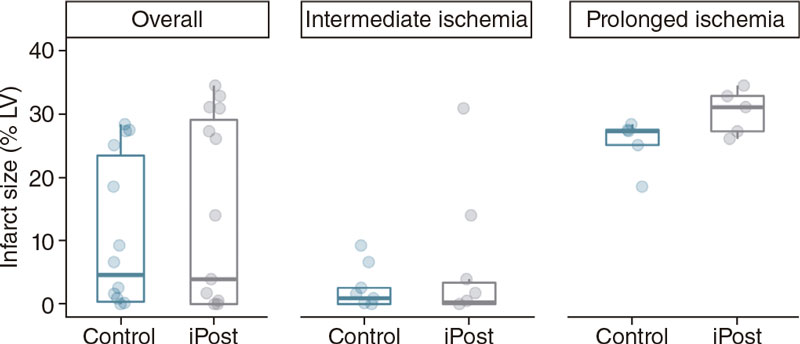
Figure 2. Differences in cardiac magnetic resonance-based infarct size (% of left ventricular mass) between control and iPost groups, in the overall population and based on ischemia duration.
Table 2. CMR-based parameters for intermediate ischemia group
| Variable | Intermediate ischemia duration (N = 19) | ||
|---|---|---|---|
| Control (N = 9) | iPost (N = 10) | P (post hoc analysis) | |
| IS, %LV mass | 0.9 (0.0-2.6) | 0.3 (0.0-3.9) | .378 |
| Indexed IS, IS/AAR (%) | 3.8 (0.0-8.5) | 0.9 (0.0-15.1) | .474 |
| LVEF, % | 54.0 (50.2-55.9) | 52.9 (47.1-56.0) | .521 |
| iLVEDV, mL/m2 | 98.7 (92.3-104.1) | 107.3 (90.0-118.6) | .438 |
| iLVESV, mL/m2 | 45.9 (40.4-52.4) | 48.5 (41.0-55.2) | .355 |
| T2 relaxation time AAR, ms | 51.3 (49.3-54.8) | 57.0 (53.2-58.5) | .583 |
| T2 relaxation time REM, ms | 45.6 (42.2-46.1) | 44.7 (43.7-47.4) | .881 |
| Native T1 relaxation time AAR, ms | 1179.2 (1167.1-1266.4) | 1225.9 (1170.2-1306.2) | .584 |
| Native T1 relaxation time REM, ms | 1087.8 (1075.1-1109.7) | 1078.4 (1051.2-1134.8) | .925 |
AAR, area at risk; iLVEDV, indexed left ventricular end-diastolic volume; iLVESV, indexed left ventricular end-systolic volume; iPost, ischemic postconditioning; IS, infarct size; LV, left ventricle; LVEF, left ventricular ejection fraction; REM, remote area. | |||
Effect of iPost on a model of prolonged ischemia protocol
The results of this experiment have already been published.16 In conclusion, iPost did not show any cardioprotective effects in terms of IS reduction (31.1% of LV mass [27.3–32.8] LV vs 27.3% [25.1– 27.5] in iPost and control respectively; P = .248). Regarding the previous group, we did not find any differences in indexed IS (IS/AAR). Differences in other CMR-based parameters were not observed except for a significantly longer AAR T1-relaxation time in the iPost group (1590.3 ms [1441.6 – 1591.4] vs 1309.7 ms [1248.1–1310.8] in iPost and control, respectively; P = .002) (table 3 and figure 2, figure 3 and figure 4).
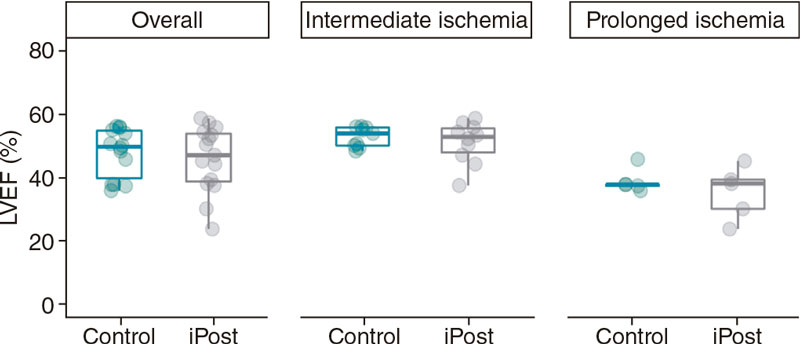
Figure 3. Differences in cardiac magnetic resonance-based LVEF (%) between control and iPost groups in the overall population and based on ischemia duration.
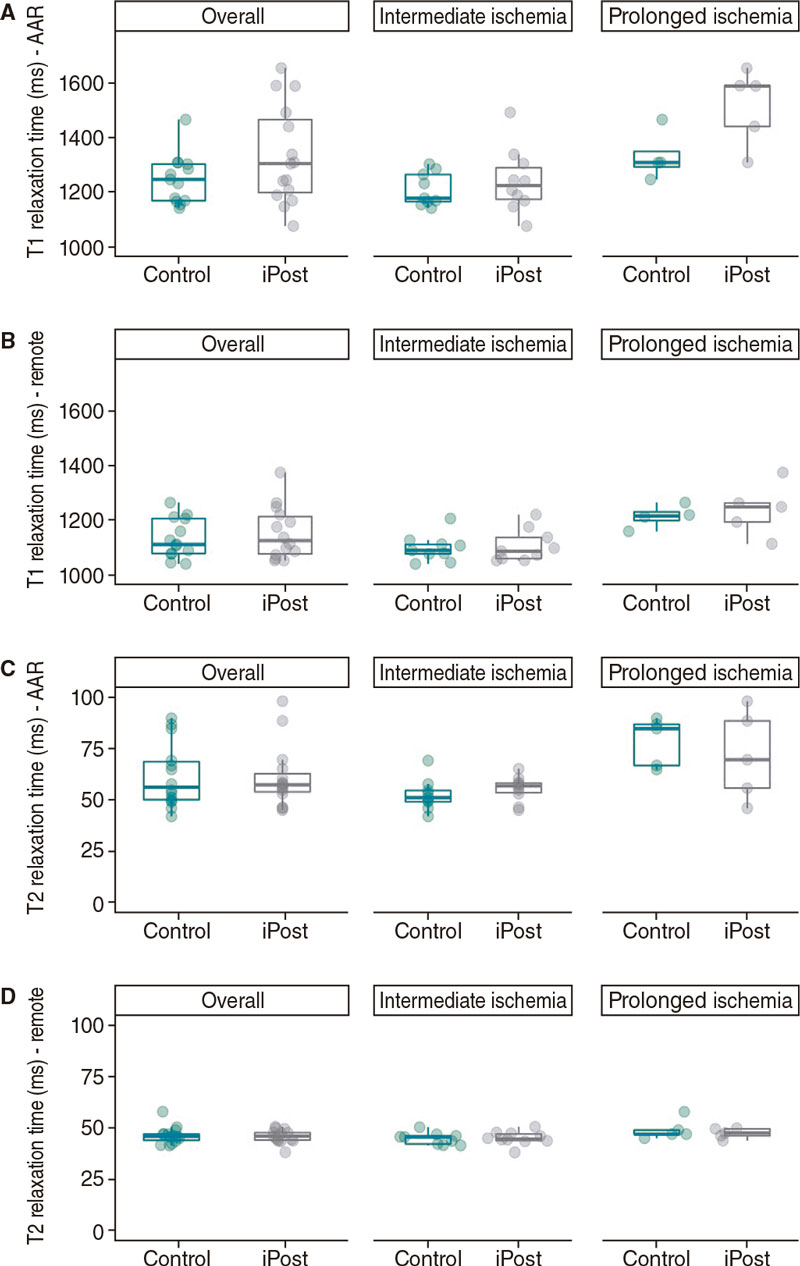
Figure 4. Differences in cardiac magnetic resonance native T1 and T2 relaxation times (ms) between control and iPost groups, in the overall population and based on ischemia duration. A: native T1 relaxation time at the area at risk. B: native T1 relaxation time at remote area. C: native T2 relaxation time at the area at risk. D: native T2 relaxation time at remote area. AAR, area at risk.
Table 3. CMR-based parameters for the prolonged ischemia group
| Variable | Long ischemia duration (N = 10) | ||
|---|---|---|---|
| Control (N = 5) | iPost (N = 5) | P (post-hoc analysis) | |
| IS, %LV mass | 27.3 (25.1-27.5) | 31.1 (27.3-32.8) | .248 |
| Indexed IS, IS/AAR (%) | 98.2 (70.7-98.8) | 96.2 (93.4-100) | .640 |
| LVEF, % | 37.9 (37.4-38.0) | 38.2 (30.2-39.4) | .323 |
| iLVEDV, mL/m2 | 150.8 (150.4-153.1) | 150.9 (148.3-159.8) | .644 |
| iLVESV, mL/m2 | 95.1 (94.1-96.6) | 105.4 (88.8-109.8) | .234 |
| T2 relaxation time AAR, ms | 85.0 (67.0-87.0) | 69.8 (55.9-88.7) | .342 |
| T2 relaxation time REM, ms | 47.0 (47.0-49.0) | 47.6 (46.2-49.6) | .420 |
| Native T1 relaxation time AAR, ms | 1309.7 (1248.1-1310.8) | 1590.3 (1441.6-1591.4) | .002 |
| Native T1 relaxation time REM, ms | 1209.7 (1156.8-1217.3) | 1246.8 (1191.2-1260.9) | .101 |
AAR, area at risk; iLVEDV, indexed left ventricular end-diastolic volume; iLVESV, indexed left ventricular end-systolic volume; iPost, ischemic postconditioning; IS, infarct size; LV, left ventricle; LVEF, left ventricular ejection fraction; REM, remote area. | |||
Interaction between duration of ischemia and iPost benefits
We did not find a significant interaction between duration of ischemia time and the effect of iPost, CMR-based IS (3.9% of LV mass [0.0–30.9] vs 4.6% [0.2 – 25.1]) in iPost and control, respectively, F [1.25] = 0.18; P = .672). Therefore, iPost was not associated with smaller IS regardless of ischemia duration (table 4 and figure 2). We did not find any differences in indexed IS (IS/AAR), LVEF, LV end-diastolic or end-systolic volumes either (table 4, and figure 3).
Table 4. CMR-based parameters for the overall population including intermediate and prolonged ischemia times
| Variable | Overall population (N = 29) | ||
|---|---|---|---|
| Control (N = 14) | iPost (N= 15) | P (2-way ANOVA) | |
| IS, %LV mass | 4.6 (0.2-25.1) | 3.9 (0.0-30.9) | .672 |
| Indexed IS, IS/AAR (%) | 15.7 (0.7-70.7) | 15.1 (0.0-96.2) | .965 |
| LVEF, % | 49.8 (37.9-55.1) | 47.1 (38.2-54.4) | .738 |
| iLVEDV, mL/m2 | 104.9 (98.1-150.4) | 118.6 (90.3-148.3) | .751 |
| iLVESV, mL/m2 | 53.5 (45.4-94.1) | 55.2 (43.8-88.8) | .628 |
| T2 relaxation time AAR, ms | 56.4 (49.9-69.4) | 57.5 (53.2-65.4) | .531 |
| T2 relaxation time REM, ms | 45.9 (43.6-47.0) | 46.0 (43.8-47.8) | .892 |
| Native T1 relaxation time AAR, ms | 1240.7 (1167.1-1304.5) | 1306.2 (1190.7-1492.7) | .024 |
| Native T1 relaxation time REM, ms | 1107.5 (1075.1-1203.9) | 1111.4 (1057.8-1218.2) | .164 |
AAR, area at risk; iLVEDV, indexed left ventricular end-diastolic volume; iLVESV, indexed left ventricular end-systolic volume; iPost, ischemic postconditioning; IS, infarct size; LV, left ventricle; LVEF, left ventricular ejection fraction; REM, remote area. | |||
Conversely, animals treated with iPost presented longer native-T1 relaxation times in the AAR (1306.2ms [1190.7–1492.7] vs 1240.7ms [1167.1–1304.5] in iPost and control, respectively (F [1.25] = 5.79, P = .024) without any differences being reported in the remote area or in T2 relaxation time (figure 4).
DISCUSSION
In this study, we tested the potential cardioprotective effect of iPost in a large animal model of reperfused MI with intermediate (30 min) and prolonged (40 min) ischemia times.15 In our pig model of ischemia/reperfusion, iPost failed to reduce IS in any of the ischemia duration protocols as seen on the state-of-the-art CMR 7days after MI. A non-significant sign of damage (trend towards larger IS and lower LVEF, as well as significantly longer T1 relaxation times in the ischemic region) associated with iPost was observed in animals in the prolonged ischemia protocol. Our data do not support iPost as an intervention capable of improving outcomes in the IRI setting.
iPost is a very attractive intervention to reduce IRI since it can be applied in the cath lab at the time of reperfusion.2 Technically, it is a straightforward intervention that does not require any additional material to that already used during primary PCI.
Local ischemic preconditioning (repetitive cycles of brief coronary artery occlusion/blood flow restoration before prolonged ischemia) has consistently shown to be a very strong cardioprotective intervention18 to reduce IRI. In most (if not all) experimental settings this strategy is consistently associated with a massive reduction of IS. However, local ischemic preconditioning is not feasible to be applied at the centers where patients already have initiated coronary artery occlusion. To overcome this limitation, Vinten-Johansen´s group tested whether the same ischemic conditioning maneuver started right after reperfusion (iPost) could also be associated with smaller IS.10 This group reported, in the dog model of IRI (60 min ischemia followed by blood flow restoration), that 3 cycles of “30 sec re-occlusion/30 sec reperfusion” applied 1 min after reperfusion were associated with a significant reduction of IS.10 Due to its easiness of implementation, iPost was translated very fast to a pilot clinical trial. Ovize´s group reported that in a small group of patients with STEMI, iPost (in this case 4 cycles of 1 min occlusion/1 min reperfusion) started right after PCI-mediated reperfusion was associated with smaller IS.11 In another small trial of 79 patients with STEMI, Freixa et al. reported that iPost not only did not reduced IS, but was associated with significantly less myocardial salvage.19 Two larger clinical trials, POST20 (N = 700), and DANAMI-3–iPOST12 (N = 1234) failed to prove the benefits of iPost.
There are some potential explanations for the divergent results. It has been speculated that there can be an interaction between the cardioprotection provided by iPost and the duration of preceding ischemia.21 However, this has not been tested in an ad hoc designed study. With this in mind, we conducted this study, where we did not find any cardioprotection provided by iPost regardless of the ischemia duration.
Although it seems that 30 min and 40 min of ischemia duration are not very different, we have previous reported in the pig model that occlusion times < 30 min are associated with a very small IS while occlusions > 40 min are associated with transmural infarction.15,22,23 Overall, in our study, 4 cycles of iPost (1 minute occlusion/1 minute reperfusion) did not have any cardioprotective effects regarding IS reduction neither expressed as % of LV mass nor as % of AAR (table 2). This was the case in both ischemia duration protocols. Although no formal interaction between ischemia duration and iPost effects on IS was found some findings suggest a possible deleterious effect of iPost in the longer ischemia duration protocol (trend towards a higher IS and a lower LVEF and, especially a significantly longer T1 relaxation time in the AAR). In addition, even in the absence of any significant differences in the intermediate ischemia group, when visualizing individual data (figure 2), asymmetry towards larger IS is seen in the iPost group including the 2 subjects with the highest IS of the entire 30 minute occlusion cohort. Furthermore, differences in secondary CMR-based outcomes suggest a potential deleterious effect of iPost in our ischemia-reperfusion model: pigs in the iPost group had significantly longer native T1 relaxation times, a surrogate marker of increased interstitial fibrosis.5 In addition, we found a non-significant trend towards poorer LVEF in animals undergoing iPost both in the intermediate and prolonged ischemia groups. Nevertheless, this finding can be due to the non-significant trend towards larger AAR in the iPost group (table 1).
One possible explanation for these results is the delayed start of the iPost protocol since animal experiments have shown that the cardioprotective effect of iPost is restricted to the first minute after reperfusion with no effect observed if the maneuver delays for another 60 seconds.24,25 In fact, in clinical trials in which iPost proved to be effective, the inflation/deflation protocol was started immediately after reperfusion.11
Study limitations
This study has some limitations that must be acknowledged. Despite being one of the most translatable, the present pig model has some differences with human IRI: tolerance to ischemia is species-dependent, and duration of ischemia in pigs is not equivalent to humans; similarly, the time-dependent progression of irreversible injury is much faster in pigs compared to humans as seen by the transmural progression of infarction between 30 min and 40 min of ischemia. Another limitation of the study is that animal allocation to iPost or control was not entirely random, but rather based on an alternative assignment; however, the person responsible for the CMR analysis validation was blind to the subjects’ allocation group. In addition, as previously presented, data on the prolonged ischemia group correspond to experiments previously conducted at our center by a different operator and published elsewhere. The use of animals of different breeds, different anesthesia protocols, different material or any other environmental factors could explain, at least partially, the great differences seen on IS between the intermediate and the prolonged ischemia groups. Nevertheless, the decision to use these previously reported data was based on the principle of reducing animal use in animal research.26,27
CONCLUSIONS
In a pig model of ischemia/reperfusion, iPost (4 cycles of 1 min balloon inflation/deflation) initiated immediately after reperfusion ineffectively reduced IS. The lack of benefits was consistent across different ischemia duration protocols, which ruled out an interaction between duration of coronary occlusion and iPost benefits. Overall, we observed a sign of harm due to iPost (significantly longer T1 relaxation times) mainly driven by an effect in the prolonged ischemia protocol.
FUNDING
This study was funded by the Spanish Ministry of Science and Innovation (“RETOS 2019” grant No PID2019-107332RB-I00 to B.I). B.I is funded by the European Commission (ERC-CoG grant No 819775, and H2020-HEALTH grant No 945118). J.N. is recipient of a predoctoral grant (Jordi Soler Soler) through CIBERCV. The CNIC is supported by the Instituto de Salud Carlos III (ISCIII), the Ministry of Science and Innovation and the Pro CNIC Foundation, and is a Severo Ochoa Center of Excellence (CEX2020-001041-S).
AUTHORS’ CONTRIBUTION
J. Nuche was responsible for conducting experiments and CMR analysis; also, for data analysis and manuscript drafting. C. Galán-Arriola was responsible for the blind CMR analysis and offered support for statistical analysis and figure design. R. Fernández-Jiménez was responsible for the experiments and analysis of the prolonged ischemia group. M. I. Higuero Verdejo, R. Vazirani, M. Anguita-Gámez, and A. Lanaspa gave support in the experimental setting. G. J. López Martín was responsible for CT and CMR acquisition. J. Sánchez-González designed the CMR protocol. B. Ibáñez was responsible for the project design, results supervision, and final approval of the manuscript draft.
CONFLICTS OF INTEREST
J. Sánchez-González is an employee of Philips Healthcare. The authors have no conflicts of interest to declare. All co-authors have seen and agreed on the contents of the manuscript and there is no financial interest to report.
ACKNOWLEDGEMENTS
We wish to thank Eugenio Fernández, Tamara Córdoba, Inés Sanz, Nuria Valladares, Antonio Benítez, Santiago Rodriguez-Colilla, and Rubén Mota for their technical and veterinary support at the CNIC animal facility and farm.
WHAT IS KNOWN ABOUT THE TOPIC?
- There is a strong need to identify interventional or pharmacological interventions that can reduce infarct size (IS), a main contributor to long-term heart failure in STEMI survivors.
- There is controversy surrounding the cardioprotection associated with local iPost in experimental models and clinical trials.
- A possible explanation to this controversy is the potential interaction between the duration of ischemia and the benefits of iPost.
WHAT DOES THIS STUDY ADD?
- It shows that iPost is not associated with IS reduction.
- The lack of benefits is consistent across different ischemia duration protocols, which rules out a possible interaction between duration of coronary occlusion and iPost benefits.
- iPost can be associated with damage especially when applied after long ischemia duration.
REFERENCES
1. Reed GW, Rossi JE, Cannon CP. Acute myocardial infarction. Lancet 2017;389:197-210.
2. De Maria GL, Garcia-Garcia HM, Scarsini R, et al. Novel device-based therapies to improve outcome in ST-segment elevation myocardial infarction. Eur Heart J Acute Cardiovasc Care 2021.
3. Stone GW, Selker HP, Thiele H, et al. Relationship Between Infarct Size and Outcomes Following Primary PCI: Patient-Level Analysis From 10 Randomized Trials. J Am Coll Cardiol 2016;67:1674-1683.
4. Ibáñez B, Heusch G, Ovize M, Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol 2015;65:1454-1471.
5. Ibanez B, Aletras AH, Arai AE, et al. Cardiac MRI Endpoints in Myocardial Infarction Experimental and Clinical Trials: JACC Scientific Expert Panel. J Am Coll Cardiol 2019;74:238-256.
6. Lorca R, Jiménez-Blanco M, García-Ruiz JM, et al. Coexistence of transmural and lateral wavefront progression of myocardial infarction in the human heart. Rev Esp Cardiol. 2021;74:870-877.
7. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119-177.
8. Heusch G, Gersh BJ. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur Heart J 2017;38:774-784.
9. Hausenloy DJ, Botker HE, Engstrom T, et al. Targeting reperfusion injury in patients with ST-segment elevation myocardial infarction: trials and tribulations. Eur Heart J 2017;38:935-941.
10. Zhao ZQ, Corvera JS, Halkos ME, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol 2003;285:H579-588.
11. Staat P, Rioufol G, Piot C, et al. Postconditioning the human heart. Circulation 2005;112:2143-2148.
12. Engstrøm T, Kelbæk H, Helqvist S, et al. Effect of Ischemic Postconditioning During Primary Percutaneous Coronary Intervention for Patients With ST-Segment Elevation Myocardial Infarction: A Randomized Clinical Trial. JAMA Cardiol 2017;2:490-497.
13. Eitel I, Stiermaier T, Rommel KP, et al. Cardioprotection by combined intrahospital remote ischaemic perconditioning and postconditioning in ST-elevation myocardial infarction: the randomized LIPSIA CONDITIONING trial. Eur Heart J 2015;36:3049-3057.
14. Heusch G, Rassaf T. Time to Give Up on Cardioprotection? A Critical Appraisal of Clinical Studies on Ischemic Pre-, Post-, and Remote Conditioning. Circ Res 2016;119:676-695.
15. Lobo-Gonzalez M, Galán-Arriola C, Rossello X, et al. Metoprolol blunts the time-dependent progression of infarct size. Basic Res Cardiol 2020;115:55.
16. Fernández-Jiménez R, Galán-Arriola C, Sánchez-González J, et al. Effect of Ischemia Duration and Protective Interventions on the Temporal Dynamics of Tissue Composition After Myocardial Infarction. Circ Res 2017;121:439-450.
17. Fernández-Jiménez R, Sánchez-González J, Aguero J, et al. Fast T2 gradient-spin-echo (T2-GraSE) mapping for myocardial edema quantification: first in vivo validation in a porcine model of ischemia/reperfusion. J Cardiovasc Magn Reson 2015;17:92.
18. Hausenloy DJ, Barrabes JA, Bøtker HE, et al. Ischaemic conditioning and targeting reperfusion injury: a 30 year voyage of discovery. Basic Res Cardiol 2016;111:70.
19. Freixa X, Bellera N, Ortiz-Pérez JT, et al. Ischaemic postconditioning revisited: lack of effects on infarct size following primary percutaneous coronary intervention. Eur Heart J. 2012;33:103-112.
20. Hahn JY, Song YB, Kim EK, et al. Ischemic postconditioning during primary percutaneous coronary intervention: the effects of postconditioning on myocardial reperfusion in patients with ST-segment elevation myocardial infarction (POST) randomized trial. Circulation. 2013;128:1889-1896.
21. Manintveld OC, Te Lintel Hekkert M, van den Bos EJ, et al Cardiac effects of postconditioning depend critically on the duration of index ischemia. Am J Physiol Heart Circ Physiol. 2007;292:H1551-1560.
22. Fernández-Jiménez R, García-Prieto J, Sánchez-González J, et al. Pathophysiology Underlying the Bimodal Edema Phenomenon After Myocardial Ischemia/Reperfusion. J Am Coll Cardiol. 2015;66:816-828.
23. García-Ruiz JM, Fernández-Jiménez R, García-Alvarez A, et al. Impact of the Timing of Metoprolol Administration During STEMI on Infarct Size and Ventricular Function. J Am Coll Cardiol. 2016;67:2093-2104.
24. Kin H, Zhao ZQ, Sun HY, et al. Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc Res. 2004;62:74-85.
25. Yang XM, Proctor JB, Cui L, Krieg T, Downey JM, Cohen MV. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J Am Coll Cardiol. 2004;44:1103-1110.
26. MacArthur Clark J. The 3Rs in research: a contemporary approach to replacement, reduction and refinement. Br J Nutr. 2018;120:S1-S7.
27. Bøtker HE, Hausenloy D, Andreadou I, et al. Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection. Basic Res Cardiol. 2018;113:39.
* Corresponding author.
E-mail address: bibanez@cnic.es (B. Ibáñez).











