RESUMEN
El estudio de la fisiología coronaria ha pasado de ser una técnica de investigación hace algunos años a convertirse en una herramienta necesaria para el abordaje óptimo de los pacientes con enfermedad coronaria epicárdica y para evaluar la microcirculación. La realización de estas técnicas requiere el uso de una guía de presión para la que hacen falta medios técnicos, tiempo y práctica en su ejecución, y es en parte por ello que su utilización es baja. Existe la necesidad de conocer la evidencia actualizada, las técnicas disponibles y la forma idónea de aplicarlas para ofrecer el mayor beneficio a los pacientes. Esta revisión ofrece un resumen práctico sobre el estado actual de los estudios de fisiología coronaria, con el fin de facilitar el mejor uso posible de esta herramienta diagnóstica esencial.
Palabras clave: Enfermedad coronaria. Fisiología coronaria. Angina microvascular.
Abstract
The study of coronary physiology has evolved from a research topic to a necessary component for the optimal management of patients with coronary artery disease when assessing both epicardial and microvascular coronary segments. The performance of these techniques requires the use of pressure wires with additional supporting systems, time, and practice, which explains the overall low rate of usage. It is essential to know the updated evidence, the techniques available, and how to perform them properly to offer the greatest possible benefit to our patients. This review provides a practical overview on coronary physiology, and it is ultimately aimed at improving the quality of care.
Keywords: Coronary artery disease. Coronary physiology. Microvascular angina.
Abbreviations FFR: fractional flow reserve. iFR: instantaneous wave-free ratio. IMR: index of microcirculatory resistance. Pd/Pa: distal to aortic coronary pressure. QFR: quantitative flow ratio.
INTRODUCTION
For decades angiography has been used as the reference procedure to diagnose coronary artery disease. However, this technique spares the physiological repercussion of epicardial coronary stenoses. Thus, by the end of the 20th century the functional characterization of coronary circulation thanks to the development of various tools both invasive (specific intracoronary guidewires) and non-invasive (angiography-derived indices) started gaining interest. The result was a change of paradigm in the diagnosis and management of coronary artery disease from an angiography to an ischemia-based strategy.1 This has become possible thanks to the abundant scientific evidence available supporting the use of physiological indices leading the ischemia-based strategy to the highest level of recommendation in the latest European guidelines on the management of myocardial revascularization.2 However, the recent publication of some clinical trials has put into question the impact of coronary physiology in certain clinical settings like multivessel disease and ST-segment elevation acute coronary syndrome (STEACS).3,4 On the other hand, these techniques take time, the use of coronary invasive instruments and, at times, the administration of vasodilators that are not always well tolerated by the patients. Also, a certain clinical experience is required. For all this, the adoption of physiology techniques to guide revascularization is still far from overall implantation.5
Over the following paragraphs we’ll be taking a practical approach on the physiological assessment of coronary stenosis and microcirculation using invasive and angiography-derived indices. Details of the physiological concepts behind every index will be left out or specific texts will be pointed out for that matter.
Physiological assessment of coronary stenoses
Invasive indices
Coronary fractional flow reserve (FFR) index is the ratio of maximum myocardial blood flow in the presence of a single stenosis with respect to the anticipated normal flow for the lack of stenosis; it is expressed as a fraction of its normal anticipated value. It is obtained by measuring intracoronary pressure with guidewires specifically designed for that matter. Determining FFR requires the vasodilation of microcirculation by using drugs, adenosine mainly—IV regadenoson and intracoronary nitroprusside have been used with similar results.6 Also, the measurement of minimal distal to aortic coronary pressure ratio (Pd/Pa) after the injection of intracoronary contrast (cFFR).7 Therefore, it is a hyperemic coronary physiological index. It is based on the fact that, in a situation of maximum hyperemia, a linear correlation between relative flow and relative intracoronary pressure is achieved since coronary resistance is both stable and minimum.8 Its result is independent of microcirculation, heart rate, arterial blood pressure, and other hemodynamic variables. The European guidelines on the management of chronic coronary syndrome give FFR an indication I, Level of Evidence A, for risk stratification in symptomatic patients who are unresponsive to medical therapy and asymptomatic patients in whom non-invasive tests show a high risk of events, and an indication type IIa when the results of non-invasive tests are inconclusive.1
A summary of the FAME trials (Fractional flow reserve vs angiography for multivessel evaluation) is shown on table 1.4,9-11 These results reinforce the need for studying the physiology field and individualizing the management of our patients inside the heart team.
Table 1. Summary of results from the FAME trial
| Study | Year | N | Population | Comparison | Follow-up | Primary endpoint | Death | Myocardial infarction | New revascularization | Other results |
|---|---|---|---|---|---|---|---|---|---|---|
| FAME9 | 2009 | CCS: 677 UA: 328 | Stenosis ≥ 50% in 2 or more vessels, eligible for PCI | PCI with angiography-guided vs FFR-guided DES (≤ 0.80) | 1 year | Death, AMI, new revascularization: 13.2% vs 18.3%; HR, 0.72; 95%CI, 0.54-0.96 | 1.8% vs 3.0%; HR, 0.58; 95%CI, 0.26-1.32 | 5.7% vs 8.7%; HR, 0.66; 95%CI, 0.42-1.04 | 6.5% vs 9.5%; HR, 0.68; 95%CI, 0.45-1.05 | No differences in the events reported separately No differences in the rate of angina reported Less use of resources with FFR |
| FAME 210 | 2012 | CCS: 888 | ≥ 1 stenosis in 1 epicardial coronary artery with FFR ≤ 0.80 | PCI with second-generation stents and OMT vs OMT | 7 months (mean) | Death, AMI, emergency revascularization: 4.3% vs 12.7%; HR, 0.32; 95%CI, 0.19-0.53 | 0.2% vs 0.7%; HR, 0.33; 95%CI, 0.03-3.17 | 3.4% vs 3.2%; HR, 1.05; 95%CI, 0.51-2.19 | Emergency: 1.6% vs 11.1%; HR, 0.13; 95%CI, 0.06-0.30 Non-emergency: 1.6% vs 8.6%; HR, 0.17; 95%CI, 0.08-0.39 | No significant differences in the composite of death and AMI or cardiac death |
| FAME 2 – 5 years11 | 2018 | CCS: 888 | ≥ 1 stenosis in 1 epicardial coronary artery with FFR ≤0.80 | PCI with second-generation stents and OMT vs OMT | 5 years | Death, AMI, emergency revascularization: 13.9% vs 27.0%; HR, 0.46; 95%CI, 0.34-0.63 | 5.1% vs 5.2%; HR, 0.98; 95%CI, 0.55-1.75 | 8.1% vs 12.0%; HR, 0.66; 95%CI, 0.43-1.00 | Emergency: 6.3% vs 21.1%; HR, 0.25; 95%CI, 0.18-0.41 Non-emergency: 7.6% vs 35.1%; HR, 0.18; 95%CI, 0.12-0.26 | No significant differences regarding death and AMI The percentage of patients with angina is lower within the first 3 years being this difference non-significant at 5 years |
| FAME 34 | 2022 | CCS: 1500 | 3-vessel disease | Non-inferiority design: FFR-guided PCI (≤ 0.80) vs coronary revascularization surgery | 1 year | Death, AMI, stroke. new revascularization: 10.6% vs 6.9%; HR, 1.5; 95%CI, 1.1-2.2; P = .35 for non-inferiority | 1.6% vs 0.9%; HR, 1.7; 95%CI 0.7-4.3 | 5.2% vs 3.5%; HR, 1.5; 95%CI, 0.9-2.5 | 5.9% vs 3.9%; HR, 1.5; 95%CI, 0.9-2.3 | No significant differences in the composite endpoint of death, infarction, stroke Less major bleeding, kidney damage, AF, and rehospitalization at 30 days with PCI |
95%CI, 95% confidence interval; AF, atrial fibrillation; AMI, acute myocardial infarction; CCS, chronic coronary syndrome; DES, drug-eluting stent; FAME, fractional flow reserve vs angiography for multivessel evaluation; FFR, fractional flow reserve; HR, hazard ratio; OMT, optimal medical therapy; PCI, percutaneous coronary intervention; UA, unstable angina. | ||||||||||
Hyperemia, especially the one obtained with IV adenosine—needed to obtain FFR—takes time, is expensive, changes systemic hemodynamics, and can cause unpleasant side effects (conduction disorders, chest pain, nausea, dyspnea, dizziness, flashing, and headache). Therefore, after its arrival, resting indices—that do not require hyperemic drugs—soon gained popularity. Overall, these indices are phasic—unlike FFR that is rather based on mean pressures—and are measured in the middle or late portion of the diastolic period when there is a greater transstenotic flow naturally.6 Although the first description of a resting index was given by Grüntzig in his early publication of coronary angioplasty,12 its clinical use did not become popular until the appearance of the instantaneous wave-free ratio (iFR, Philips, The Netherlands). Several studies were conducted to compare the diagnostic concordance of iFR and FFR, as well as iFR, FFR, and other reference parameters of ischemia.13,14 Two multicenter randomized clinical trials—the DEFINE-FLAIR (Functional lesion assessment of intermediate stenosis to guide revascularization) and the iFR-SWEDEHEART (Evaluation of iFR vs FFR in stable angina or acute coronary syndrome)—randomized 4529 patients to receive FFR or iFR-guided percutaneous revascularization in both patients with ACS and chronic coronary syndrome.15,16 Both studies demonstrated the non-inferiority of the iFR compared to the FFR with low rates of events defined as all-cause mortality, acute myocardial infarction or unplanned revascularization at 1 year: iFR, 4.12% vs FFR, 4.05%; hazard ratio (HR), 1.13; 95% confidence interval (95%CI), 0.72-1.79; P = .60). Also, in the iFR groups, the number of functionally significant stenoses and rates of revascularization were lower, procedural time was shorter, and there were fewer patients with adverse symptoms associated with the administration of adenosine.15,16 Over the last few years, several resting indices have been developed based on the concept previously described: DFR (diastolic hyperemia-free ratio, Boston Scientific, United States),17 and the resting diastolic pressure ratio (dPR) (ACIST, United States).18,19 Except for the resting full-cycle ratio (RFR) (Abbott, United States),18 that is a non-hyperemic index to assess pressure along the entire cardiac cycle (table 2), all resting indices are highly reproducible, and identical to iFR both numerically and in their concordance with FFR.19 The prognostic capability of the Pd/Pa ratio is less robust compared to the FFR21 since its correlation with FFR in non-culprit lesions of patients who had an ACS is 80%;22 after the arrival of non-hyperemic indices, its clinical significance is scarce.
Table 2. Indices used to study epicardial coronary stenoses
| Vasodilation | Period of cycle | Cut-off value | Scientific evidence | |
|---|---|---|---|---|
| FFR | Hyperemic | – | ≤ 0.80 | RCTs: FAME, FAMEII, FAME III, DEFER, DANAMI-3-PRIMULTI, COMPARE ACUTE, FLOWER-MI, FUTURE |
| iFR | Non-hyperemic | Diastolic | ≤ 0.89 | RCTs: DEFINE-FLAIR, iFR-SWEDEHEART Observational: SYNTAX II |
| DFR | Non-hyperemic | Diastolic | ≤ 0.89 | Observational: Johnson et al.14 |
| dPR | Non-hyperemic | Diastolic | ≤ 0.89 | Observational: Lee et al.,15 Van’t Veer et al.16 |
| RFR | Non-hyperemic | The full cycle | ≤ 0.89 | Observational: Lee et al.15 |
| Pd/Pa | Non-hyperemic | The full cycle | 0.91-0.93 | Observational: Kobayashi et al.,20 Lee et al.15 |
DFR, diastolic hyperemia-free ratio; dPR, resting diastolic pressure ratio; FFR, fractional flow reserve; iFR, instantaneous wave-free ratio; Pd/Pa, distal to aortic coronary pressure ratio; RCT, randomized clinical trials; RFR, resting full-cycle ratio. | ||||
Supplementary data provides a detailed description on the practical management of invasive physiological indices.
Procedure
Figure 1 shows the steps needed to measure resting indices and FFR. Supplementary data gives a step-by-step detailed description. Figure 2 shows the utility of pressure guidewires for the diagnosis and location of significant stenoses.
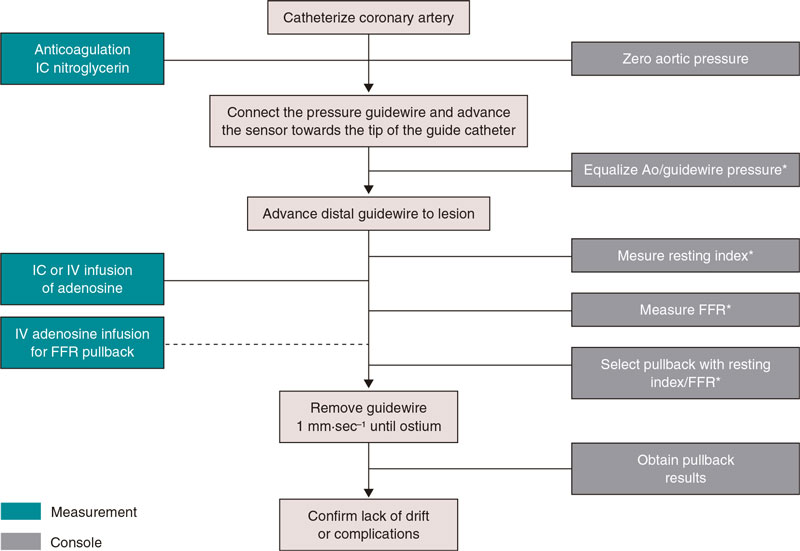
Figure 1. Steps to use intracoronary pressure guidewire to measure resting indices and fractional flow reserve. Ao, aorta, FFR, fractional flow reserve; IC, intracoronary; IV, intravenous. * Catheter purged with saline solution and no guidewire introducer sheath.
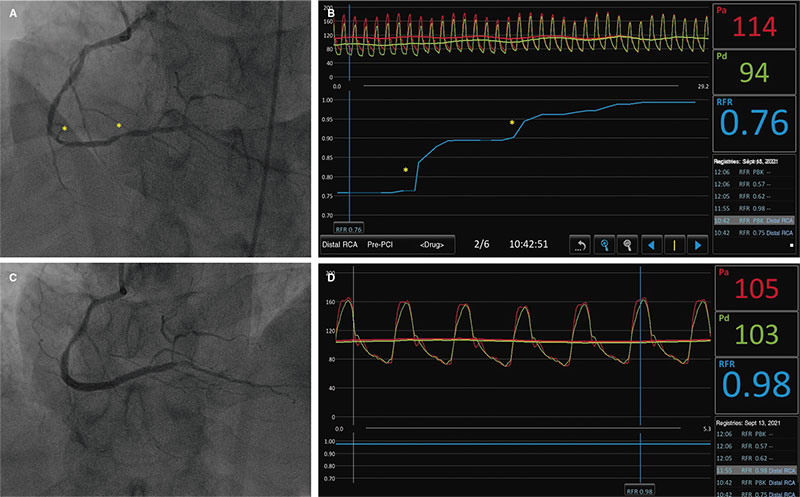
Figure 2. A: angiography of right coronary artery showing diffuse damage with more severe lesions at distal level (asterisks). B: pullback resting full-cycle ratio (RFR) measurement showing 2 focal jumps corresponding to the asterisks shown on A. C: final angiographic outcomes after implantation of 2 drug-eluting stents. D: final RFR with optimal result of 0.98.
Problems, causes, solutions, and specific settings
Table 3 shows some of the main problems that can be found when performing pressure guidewire studies, their causes, and possible solutions. Supplementary data provides a detailed description on how to deal with these problems, and a description on the use of pressure guidewire in different clinical settings (diffuse coronary artery disease, ostial lesions, aortic stenosis, ACS, post-angioplasty assessments).
Table 3. Pressure guidewire: main problems, causes, and possible solutions
| Problem | Cause | Recommendations |
|---|---|---|
| Aortic pressure damping | Catheter/vessel mismatch | Use a guide catheter of a smaller diameter |
| Falsely reduced aortic pressure | Loose connections of the guide catheter | Double check all connections before taking any measurements |
| Loss of drift | Need for multiple connections/disconnections | Repeat equalization and measurement |
| Spasm, pseudostenosis | Presence and manipulation of intracoronary guidewire | Always administer IC nitrates before the procedure |
| Scarce response to adenosine | Use of caffeine, theobromine (chocolate), theophylline | Talk to the patients and tell them that the use of coffee/chocolate/theophylline is ill-advised 24 hours before the procedure |
| Excessive measurement variability | Patient moving | Make sure that the patient is comfortable |
AF, atrial fibrillation; IC, intracoronary; IV, intravenous; PCI, percutaneous coronary intervention. | ||
Angiography-derived indices
The physiological study of epicardial stenoses is limited in the routine clinical practice due to the need for pressure guidewires and, in some cases, hyperemic agents that take up higher costs and possible side effects.23 Therefore, new angiography-derived indices like QFR, angio-FFR, CAAS-vFFR, and vFFR have been produced. These are based on 3D reconstructions of coronary tree through the angiography and then computational flow dynamics software or mathematical simplifications of it as a surrogate of coronary flow.
QFR (quantitative flow ratio; Qangio XA 3D, Medis Medical Imaging Systems, The Netherlands) uses a 3D reconstruction of the angiography. Afterwards, assuming a constant pressure and velocity of flow along a normal epicardial vessel, a proxy of the FFR value is estimated using different models: the fixed model (fQFR) uses information from a database from which FFR values and flow velocities have been previously obtained; the contrast-QFR model (cQFR) takes into account the flow velocity of the contrast injected into the epicardial artery by counting frames; and the QFR-adenosine model (aQFR) that studies it after inducing hyperemia through the administration of adenosine. The 3 models were tested against FFR, and the best diagnostic accuracy was obtained with the QFR-adenosine (87%) and cQFR (86%) models.24 Several studies conducted later have demonstrated the utility and high accuracy of this model for the functional diagnosis of epicardial stenosis,25,26 and how safe the revascularization decision is based on such model.27,28 The FAVOR III China trial of 3825 patients confirmed fewer major adverse events (HR, 0.65; 95%CI, 0.51-0.83; P = .0004) in patients with delayed revascularization based on QFRs ≤ 0.80 triggered by fewer myocardial infarctions and ischemia-guided revascularizations compared to angiography-guided revascularizations.28
Another index is the CAAS-VFFR (Cardiovascular angiographic analysis system for vessel FFR, CAAS-vFFR, Pie Medical, The Netherlands). It is based on a 3D reconstruction of the angiography acquired followed by an estimate of the pressure gradient through a lesion. Its validation study included patients with stable disease and non-ST-segment elevation acute coronary syndrome and showed a 93% accuracy for the diagnosis of lesions with FFR ≤ 0,80, and a 95% inter-observer correlation.29
Angio-FFR index (Cathworks, Israel) is also widely used. Unlike the former indices presented above, it uses, at least, 3 different angiographic views to sketch a 3D functional angiography mapping. Fearon et al.30 studied it in a large population and confirmed sensitivity, specificity, and diagnostic accuracy values of 94%, 91%, and 92%, respectively for FFR ≤ 0.80 with 96% high inter-observer consistency.
Other indices like the vFFR (virtual fractional flow reserve, VirtuHeart Medical Physics Group, United Kingdom) demonstrated, in its validation study, high diagnostic accuracy, sensitivity, and specificity of 97%, 86%, and 100%, respectively.31 This index, however, is still in the pipeline.
Recently, a meta-analysis conducted by Collet et al.32 demonstrated that angiography-based FFR measurements have an overall sensitivity and specificity of 89% and 90% compared to invasive FFR. However, there can be a relatively large gray area (0.75-0.86) where the invasive determination of FFR could be indicated.33 Assuming this gray area, the diagnostic accuracy of these methods could be > 95% like the FAVOR II China trial proved,25 preventing the need for an invasive study in 64% of the lesions.34
Despite the promising results obtained, these analyses have certain limitations. One of the main ones is to obtain proper angiographies for analysis without structure panning or overlapping.35 Another one is anatomy since the contouring of ostial or bifurcation lesion borders is more difficult to achieve meaning that its study may be biased. In a recent analysis on the population of the SYNTAX II trial, QFR assessment vs hybrid iFR/FFR assessment demonstrated a diagnostic accuracy of QFR close to 74% with a 8.3% rate of false positives and a 17.9% rate of false negatives being the main reasons for this mismatch the lesions found in marginal branches, small vessels or bifurcation regions.35 Also, the state of microcirculation is especially interesting since these techniques assume maximum vasodilation to estimate pressure from the flow obtained. However, the degree of response to hyperemia—whether due to contrast or pharmacological agents—is variable based on each patient’s state of microcirculation and, therefore, subject to error. Mejía-Rentería et al.36 reported on how the state of microcirculation impacts this type of non-invasive assessments of coronary flow reserve (CFR), and saw that the greatest source of mismatch came from an impaired microvascular function measured as an impaired value of the index of microcirculatory resistance (IMR) or situation of acute myocardial infarction. One could think that image processing time and its analysis can be longer compared to the physiological study using pressure guidewires. However, if trained, it has been confirmed that the study can be conducted faster compared to the traditional determination of FFR.37,38 Finally, a pending limitation that needs solving is observer-dependent variability (0.01 ± 0.08 match for repeat measurements), the quality of angiography, and the degree of FFR-based stenosis.39
Supplementary data provides a detailed description on the practical management of QFR, angio-FFR, and vFFR.
Physiological assessment of coronary microcirculation
Invasive indices
Although coronary artery disease is often associated with damage to epicardial arteries, up to 25% of the patients with typical angina do not show significant epicardial stenoses.1 Microvascular dysfunction is a contributing factor of angina and individualized treatment has proven to improve the patients’ quality of life,40 which is why a proper intracoronary diagnosis of microvascular disease in symptomatic patients without stenosis or with moderate coronary stenoses has a recommendation IIa in the European guidelines on the management of chronic coronary syndrome.1
Arterioles, the main component of coronary vascular resistance, plays a very dynamic role in coronary blood flow and are regulated by multiple metabolic, myogenic, endothelial, neural, and hormonal mechanisms.41,42 Impaired microcirculation can occur through any of these pathways and bring about unfavorable prognosis similar to that of obstructive epicardial disease.43 The size of these vessels complicates their angiographic assessment, and the use of other methods is essential. CFR measures the ratio of coronary flow in hyperemia compared to resting flow with normal values between 3 and 4 indicative that coronary flow increases by a factor of 3 or 4 with maximum hyperemia. CFR results represent the capacity to increase flow both of epicardial arteries and microvasculature. Reduced CFR is associated with a significant increase of mortality (HR, 3.78; 95%CI, 2.39-5.97), and major adverse cardiovascular events (HR, 3.42; 95%CI, 2.92-3.99) in multiple diseases including patients with ACS, microvascular dysfunction, heart transplant, and diabetes mellitus.44
Microcirculatory resistance can be measured through thermodilution or intravascular Doppler ultrasound in baseline conditions or in hyperemia.45 The IMR—reference index to study microcirculation—is based on measuring distal pressure and coronary flow through thermodilution as assessed by the inverse of the arrival (transit) time of a room temperature saline solution bolus to the artery distal segment during maximum hyperemia. High IMR > 25 is associated with poor cardiovascular prognosis; the combination of low CRF and high IMR is associated with worse prognosis.46,47 Recently, a new method based on thermodilution and a continuous flow of saline solution (RayFlow catheter, Hexacath, France) to estimate absolute coronary flow in hyperemic conditions and absolute microvascular resistance48,49 has been described. Its advantage is that it does not depend on baseline values, which lowers the significance of hemodynamic changes. It does not depend on the operator either. Its clinical utility still needs to be proven given the limitation interpreting absolute values.
The Doppler guidewire estimates the CRF by dividing flow velocity in hyperemia by the baseline flow velocity. Cut-off values of ≤ 2.5 are consistent with a diagnosis of microvascular dysfunction in healthy epicardial arteries.50 The prognostic value of CRF measured invasively through Doppler in patients with angina is independent from the findings of non-invasive modalities with a 5-year HR of 2.97 (95%CI, 1.39-6.34) for major adverse cardiovascular events.51 Hyperemic microvascular resistance (HMR) can also be estimated by dividing intracoronary pressure by hyperemic flow velocity considering that HMR > 1.9 mmHg·cm–1·s–1 is diagnostic of microcirculatory dysfunction.50 However, it has been reported that HMR ≥ 2.5 mmHg·cm–1·s–1 has better sensitivity and specificity for the diagnosis of microvascular dysfunction.52
Practical approach
Thermodilution
Supplementary data provides a detailed description on the practical management of thermodilution both through boluses (figure 3) and continuous perfusion (figure 4), as well as physiological study using Doppler guidewires (figure 5).
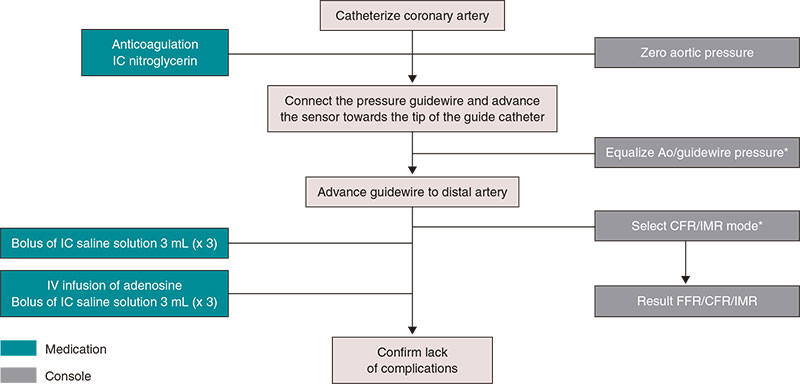
Figure 3. Steps for the study of microcirculation with bolus thermodilution. Ao, aorta; CFR, coronary flow reserve; FFR, fractional flow reserve; IC, intracoronary; IMR, index of microcirculatory resistance; IV, intravenous. * Catheter purged with saline solution and no guidewire introducer sheath.
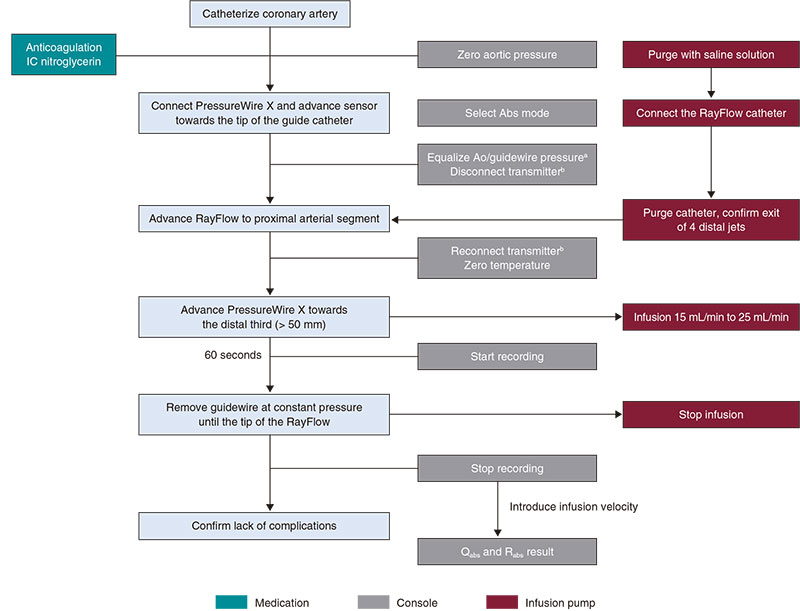
Figure 4. Steps for the study of microcirculation with continuous thermodilution. Ao, aorta, IC, intracoronary; IV, intravenous. a Catheter purged with saline solution and no guidewire introducer sheath. b Do not switch the transmitter of during the entire procedure.
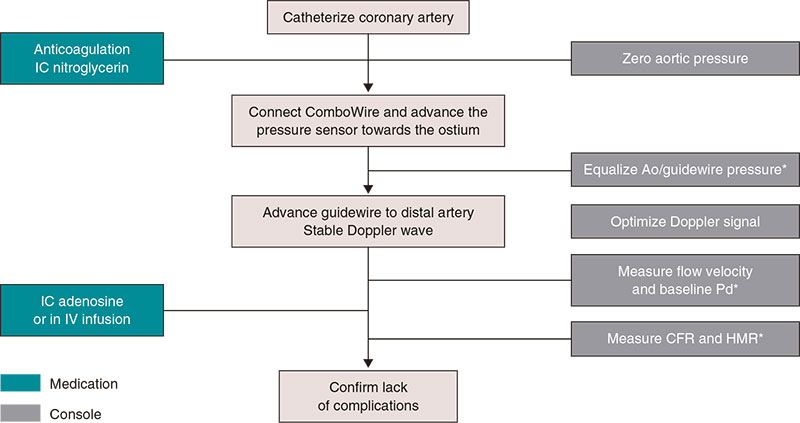
Figure 5. Steps for the study of microcirculation with intracoronary Doppler guidewire. Ao, aorta; CFR, coronary flow reserve; HMR, hyperemic microvascular resistance; IC, intracoronary; IV, intravenous; Pd, distal coronary pressure. * Catheter purged with saline solution and no guidewire introducer sheath.
Angiography-derived indices
Although assessing the state of microcirculation using the IMR has largely proven its clinical benefit,50,53 its study in the routine clinical practice is limited since pressure guidewires and hyperemic agents are needed. Therefore, recently, different alternatives have been developed to estimate the angiography-derived index of microvascular resistance (IMRangio) using computational fluid dynamics. Several formulae can be used.
The first description was given by De Maria et al.,54 who saw the good diagnostic capabilities (92.4%) of IMRangio vs invasive IMR using a different QFR-adenosine formula in patients with myocardial infarction, and a high correlation between a high value on the IMRangio and the presence of microvascular obstruction as seen on the magnetic resonance imaging.
IMRangio has been studied in both stable patients and patients with ACS55 obtaining a good correlation between IMR and IMRangio and a high diagnostic accuracy of the latter when used with adenosine in patients with ACS and stable patients. However, it was seen that the correlation between IMR and cQFR-derived IMRAngio (NH-IMRangio) did not hold up in non-culprit arteries of the acute event or in cases of greater clinical stability only showing a good correlation in the infarction culprit arteries. Authors think that a possible explanation to this phenomenon would be the greatly impaired vasodilator capability of patients with ST-segment elevation acute myocardial infarction. Therefore, they proposed a hybrid algorithm by means of which it would only be necessary to use adenosine in cases with NH-IMRangio levels > 30 U and < 90 U, which would stop the use of adenosine in 38% of the cases. Also, in cases of ST-segment elevation acute myocardial infarction—where maybe the use of hyperemia may be more limited due to the clinical situation—this group showed that NH-IMRangio levels > 43 could detect IMR values > 40 very precisely and be predictor of long-term events56 without having to use adenosine.
Tebaldi et al.57 use a formula based on the cQFR value (NH-IMRangio) to assess the state of microcirculation in patients with stable angina finding a high correlation between IMRangio > 44.2 and invasive IMR > 25.
Parallel to this, another group used a different formula including the cQFR value58 to assess microvascular function in patients with chronic and acute coronary syndrome that confirmed a good overall diagnostic accuracy. Also, this group proved it could have an added value to reduce the rate of false positives of QFR since an impaired microvascular function can affect the accuracy of the QFR study.36 Recently, a meta-analysis of aggregate data demonstrated the good diagnostic performance of IMRangio compared to invasive IMR, with sensitivity, specificity, accuracy, positive predictive, and negative predictive values of 82%, 83%, 83%, 76%, and 85%.59
FlashAngio (Rainmed, China) is yet another software to determine non-invasive IMR,60,61 with similar diagnostic results. Added to its diagnostic value, Choi et al.61 proved the prognostic value of such index, since high IMRangio levels (< 40 U) were associated with cardiac death and rehospitalization due to long-term cardiovascular problems.
Supplementary data provides a detailed description on the practical management of IMRangio.
CONCLUSIONS
The study of coronary physiology is a tremendous breakthrough for the management of patients with coronary artery disease. Being able to fine tune the functional severity of epicardial lesions and how microcirculation impacts the symptomatology of patients allows us to personalize treatment to reduce symptoms and, in many cases, improve prognosis. Great advances have been made in this field achieving further physiological knowledge and greater diagnostic accuracy both with invasive and non-invasive tests. Although extensive, knowledge in this field still shows gaps that will still be solved with new studies. All this development requires specific and updated training so we can take advantage of knowledge and technology for the benefit of our patients.
FUNDING
None reported.
AUTHORS’ CONTRIBUTION
J.P. Vilchez-Tschischke, J. Sanz Sánchez, and E. Fernández Peregrina contributed to the design, drafting, and review process of the article, J.L. Díez Gil, M. Echevarría Pinto, and H.M. Garcia-Garcia contributed to both the drafting of the manuscript and the critical review of its intellectual content.
CONFLICTS OF INTEREST
J. Sanz Sánchez received conference fees from Cordis, and Terumo. H.M. Garcia-Garcia received conference fees from Biotronik, Abbot, Boston Scientific, Neovasc, Medtronic, Shockwave, Philips, and Corflow. The remaining authors declared no conflicts of interest whatsoever.
REFERENCES
1. Neumann FJ, Sechtem U, Banning AP, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407-477.
2. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. EuroIntervention. 2019;14:1435-1534.
3. Puymirat E, Cayla G, Simon T, et al. Multivessel PCI Guided by FFR or Angiography for Myocardial Infarction. N Engl J Med. 2021;385:297-308.
4. Fearon WF, Zimmermann FM, de Bruyne B, et al. Fractional Flow Reserve-Guided PCI as Compared with Coronary Bypass Surgery. N Engl J Med. 2022;386:128-137.
5. Dattilo PB, Prasad A, Honeycutt E, Wang TY, Messenger JC. Contemporary patterns of fractional flow reserve and intravascular ultrasound use among patients undergoing percutaneous coronary intervention in the United States: insights from the National Cardiovascular Data Registry. J Am Coll Cardiol. 2012;60:2337-2339.
6. Lee JZ, Singh N, Nyotowidjojo I, et al. Comparison of regadenoson and nitroprusside to adenosine for measurement of fractional flow reserve: A systematic review and meta-analysis. Cardiovasc Revasc Med. 2018;19:168-174.
7. Leone AM, Campo G, Gallo F, et al. Adenosine-Free Indexes vs. Fractional Flow Reserve for Functional Assessment of Coronary Stenoses: Systematic Review and Meta-Analysis. Int J Cardiol. 2020;299:93-99.
8. Warisawa T, Cook CM, Akashi YJ, Davies JE. Past, Present and Future of Coronary Physiology. Rev Esp Cardiol. 2018;71:656-667.
9. Tonino PAL, de Bruyne B, Pijls NHJ, et al. Fractional Flow Reserve versus Angiography for Guiding Percutaneous Coronary Intervention. N Engl J Med. 2009;360:213-224.
10. de Bruyne B, Pijls NHJ, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991-1001.
11. Xaplanteris P, Fournier S, Pijls NHJ, et al. Five-Year Outcomes with PCI Guided by Fractional Flow Reserve. N Engl J Med. 2018;379:250-259.
12. Grüntzig AR, Senning Å, Siegenthaler WE. Nonoperative Dilatation of Coronary-Artery Stenosis. N Engl J Med. 1979;301:61-68.
13. Sen S, Asrress KN, Nijjer S, et al. Diagnostic classification of the instantaneous wave-free ratio is equivalent to fractional flow reserve and is not improved with adenosine administration. Results of CLARIFY (Classification Accuracy of Pressure-Only Ratios Against Indices Using Flow Study). J Am Coll Cardiol. 2013;61:1409-1420.
14. Petraco R, van de Hoef TP, Nijjer S, et al. Baseline instantaneous wave-free ratio as a pressure-only estimation of underlying coronary flow reserve: results of the JUSTIFY-CFR Study (Joined Coronary Pressure and Flow Analysis to Determine Diagnostic Characteristics of Basal and Hyperemic Indices of Functional Lesion Severity-Coronary Flow Reserve). Circ Cardiovasc Interv. 2014;7:492-502.
15. Davies JE, Sen S, Dehbi HM, et al. Use of the Instantaneous Wave-free Ratio or Fractional Flow Reserve in PCI. N Engl J Med. 2017;376:1824-1834.
16. Götberg M, Christiansen EH, Gudmundsdottir IJ, et al. Instantaneous Wave-free Ratio versus Fractional Flow Reserve to Guide PCI. N Engl J Med. 2017;376:1813-1823.
17. Johnson NP, Li W, Chen X, et al. Diastolic pressure ratio: new approach and validation vs. the instantaneous wave-free ratio. Eur Heart J. 2019;40:2585-2594.
18. Lee JM, Rhee TM, Choi KH, et al. Clinical Outcome of Lesions With Discordant Results Among Different Invasive Physiologic Indices - Resting Distal Coronary to Aortic Pressure Ratio, Resting Full-Cycle Ratio, Diastolic Pressure Ratio, Instantaneous Wave-Free Ratio, and Fractional Flow Reserve. Circ J. 2019;83:2210-2221.
19. Van’t Veer M, Pijls NHJ, Hennigan B, et al. Comparison of Different Diastolic Resting Indexes to iFR: Are They All Equal? J Am Coll Cardiol. 2017;70:3088-3096.
20. Kobayashi Y, Johnson NP, Berry C, et al. The Influence of Lesion Location on the Diagnostic Accuracy of Adenosine-Free Coronary Pressure Wire Measurements. JACC Cardiovasc Interv. 2016;9:2390-2399.
21. Ahn JM, Park DW, Kim SO, et al. Prognostic Value of Resting Distal-to-Aortic Coronary Pressure in Clinical Practice. Circ Cardiovasc Interv. 2020;13:e007868.
22. Piróth Z, Fülöp G, Boxma-de Klerk BM, et al. Correlation and Relative Prognostic Value of Fractional Flow Reserve and Pd/Pa of Nonculprit Lesions in ST-Segment-Elevation Myocardial Infarction. Circ Cardiovasc Interv. 2022;15:e010796.
23. Barauskas M, Ziubryte G, Barauskiene G, Unikaite R, Jodka N, Unikas R. Systemic analysis of diagnostic performance and agreement between fractional flow reserve and quantitative flow ratio. Cor Vasa. 2021;63:683-687.
24. Tu S, Westra J, Yang J, et al. Diagnostic Accuracy of Fast Computational Approaches to Derive Fractional Flow Reserve From Diagnostic Coronary Angiography The International Multicenter FAVOR Pilot Study. JACC Cardiovasc Interv. 2016;9:2024-2035.
25. Xu B, Tu S, Qiao S, et al. Diagnostic Accuracy of Angiography-Based Quantitative Flow Ratio Measurements for Online Assessment of Coronary Stenosis. J Am Coll Cardiol. 2017;70:3077-3087.
26. Westra J, Tu S, Campo G, et al. Diagnostic performance of quantitative flow ratio in prospectively enrolled patients: An individual patient-data meta-analysis. Catheter Cardiovasc Interv. 2019;94:693-701.
27. Spitaleri G, Tebaldi M, Biscaglia S, Westra J, Brugaletta S, Erriquez A. Quantitative Flow Ratio identifies nonculprit coronary lesions requiring revascularization in patients with ST segment elevation myocardial infarction and multivessel disease. Circ Cardiovasc Interv. 2018;11:e006023.
28. Xu B, Tu S, Song L, et al. Angiographic quantitative flow ratio-guided coronary intervention (FAVOR III China): a multicentre, randomised, sham-controlled trial. Lancet. 2021;398:2149-2159.
29. Masdjedi K, Tanaka N, Van Belle E, et al. Vessel fractional flow reserve (vFFR) for the assessment of stenosis severity: the FAST II study. EuroIntervention. 2022;17:1498-1505.
30. Fearon W, Achenbach S, Engstrom T, et al. Accuracy of fractional flow reserve derived from coronary angiography. Circulation. 2019;139:477-484.
31. Morris PD, Ryan D, Morton AC, et al. Virtual Fractional Flow Reserve From Coronary Angiography: Modeling the Significance of Coronary Lesions Results From the VIRTU-1 (VIRTUal Fractional Flow Reserve From Coronary Angiography) Study. JACC Cardiovasc Interv. 2013;6:149-157.
32. Collet C, Onuma Y, Sonck J, et al. Diagnostic performance of angiography-derived fractional flow reserve: A systematic review and Bayesian meta-analysis. Eur Heart J. 2018;39:3314-3321.
33. Yakazi K, Otsuka M, Kataoka S, Kahata, Kumagai A. Applicability of 3-Dimensional Quantitative Coronary Angiography-derived computed Fractional Flow Reserve for intermediate coronary stenosis. Circ J. 2017;81:988-992.
34. Collet C, Onuma Y, Sonck J, et al. Diagnostic performance of angiography-derived fractional flow reserve: A systematic review and Bayesian meta-analysis. Eur Heart J. 2018;39:3314-3321.
35. Asano T, Katagiri Y, Chang CC, et al. Angiography-Derived Fractional Flow Reserve in the SYNTAX II Trial: Feasibility, Diagnostic Performance of Quantitative Flow Ratio, and Clinical Prognostic Value of Functional SYNTAX Score Derived From Quantitative Flow Ratio in Patients With 3-Vessel Dis. JACC Cardiovasc Interv. 2019;12:259-270.
36. Mejía-Rentería H, Lee JM, Lauri F, et al. Influence of Microcirculatory Dysfunction on Angiography-Based Functional Assessment of Coronary Stenoses. JACC Cardiovasc Interv. 2018;11:741-753.
37. Westra J, Andersen BK, Campo G, et al. Diagnostic performance of in-procedure angiography-derived quantitative flow reserve compared to pressure-derived fractional flow reserve: The FAVOR II Europe-Japan study. J Am Heart Assoc. 2018;7:e009603.
38. Haley H, Ghobrial M, Morris P, Gosling R, Williams G, Mills M. Virtual (Computed) Fractional Flow Reserve: Future Role in Acute Coronary Syndromes. Front Cardiovasc Med. 2021;8:735008.
39. Westra J, Sejr-Hansen M, Koltowski L, et al. Reproducibility of quantitative flow ratio: The QREP study. EuroIntervention. 2022;17:1252-1259.
40. Ford TJ, Stanley B, Sidik N, et al. 1-Year Outcomes of Angina Management Guided by Invasive Coronary Function Testing (CorMicA). JACC Cardiovasc Interv. 2020;13:33-45.
41. Chilian WM, Eastham CL, Marcus ML. Microvascular distribution of coronary vascular resistance in beating left ventricle. Am J Physiol. 1986;251(4 Pt 2):H779-H788.
42. Goodwill AG, Dick GM, Kiel AM, Tune JD. Regulation of Coronary Blood Flow. Compr Physiol. 2017;7:321.
43. Broyd CJ, Echavarria-Pinto M, Cerrato E, Escaned J. Evaluation of Microvascular Disease and Clinical Outcomes. Interv Cardiol Clin. 2015;4:443-457.
44. Kelshiker MA, Seligman H, Howard JP, et al. Coronary flow reserve and cardiovascular outcomes: a systematic review and meta-analysis. Eur Heart J. 2022;43:1582-1593.
45. Taqueti VR, di Carli MF. Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72:2625-2641.
46. Lee JM, Jung JH, Hwang D, et al. Coronary Flow Reserve and Microcirculatory Resistance in Patients With Intermediate Coronary Stenosis. J Am Coll Cardiol. 2016;67:1158-1169.
47. Padro T, Manfrini O, Bugiardini R, et al. ESC Working Group on Coronary Pathophysiology and Microcirculation position paper on “coronary microvascular dysfunction in cardiovascular disease”. Cardiovasc Res. 2020;116:741-755.
48. Van’t Veer M, Adjedj J, Wijnbergen I, et al. Novel monorail infusion catheter for volumetric coronary blood flow measurement in humans: in vitro validation. EuroIntervention. 2016;12:701-707.
49. Xaplanteris P, Fournier S, Keulards DCJ, et al. Catheter-Based Measurements of Absolute Coronary Blood Flow and Microvascular Resistance: Feasibility, Safety, and Reproducibility in Humans. Circ Cardiovasc Interv. 2018;11:e006194.
50. Kunadian V, Chieffo A, Camici PG, et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International. Eur Heart J. 2020;41:3504-3520.
51. Lee SH, Shin D, Lee JM, et al. Clinical Relevance of Ischemia with Nonobstructive Coronary Arteries According to Coronary Microvascular Dysfunction. J Am Heart Assoc. 2022;11:e025171.
52. Williams RP, de Waard GA, de Silva K, et al. Doppler Versus Thermodilution-Derived Coronary Microvascular Resistance to Predict Coronary Microvascular Dysfunction in Patients With Acute Myocardial Infarction or Stable Angina Pectoris. Am J Cardiol. 2018;121:1-8.
53. Kobayashi Y, Fearon WF. Invasive coronary microcirculation assessment — Current status of index of microcirculatory resistance. Circ J. 2014;78:1021-1028.
54. De Maria GL, Scarsini R, Shanmuganathan M, et al. Angiography-derived index of microcirculatory resistance as a novel, pressure-wire-free tool to assess coronary microcirculation in ST elevation myocardial infarction. Int J Cardiovasc Imaging. 2020;36:1395-1406.
55. Scarsini R, Shanmuganathan M, Kotronias RA, et al. Angiography-derived index of microcirculatory resistance (IMRangio) as a novel pressure-wire-free tool to assess coronary microvascular dysfunction in acute coronary syndromes and stable coronary artery disease. Int J Cardiovasc Imaging. 2021;37:1801-1813.
56. Kotronias R, Terentes-Printzios D, Shanmuganathan M, et al. Long-Term Clinical Outcomes in Patients With an Acute ST-Segment-Elevation Myocardial Infarction Stratified by Angiography-Derived Index of Microcirculatory Resistance. Front Cardiovasc Med. 2021;1:717114.
57. Tebaldi M, Biscaglia S, Di Girolamo D, et al. Angio-based index of microcirculatory resistance for the assessment of the coronary resistance: A proof of concept study. J Interv Cardiol. 2020;2020:8887369.
58. Mejia-Renteria H, Lee JM, Choi KH, et al. Coronary microcirculation assessment using functional angiography: Development of a wire-free method applicable to conventional coronary angiograms. Catheter Cardiovasc Interv. 2021;98:1027-1037.
59. Fernández‐Peregrina E, Garcia‐Garcia HM, Sans‐Rosello J, et al. Angiography‐derived versus invasively‐determined index of microcirculatory resistance in the assessment of coronary microcirculation: A systema-tic review and meta‐analysis. Catheter Cardiovasc Interv. 2022;99:2018-2025.
60. Ai H, Feng Y, Gong Y, et al. Coronary Angiography-Derived Index of Microvascular Resistance. Front Physiol. 2020;11:605356.
61. Choi KH, Dai N, Li Y, et al. Functional Coronary Angiography–Derived Index of Microcirculatory Resistance in Patients With ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc Interv. 2021;14:1670-1684.
* Corresponding authors.
E-mail addresses: drmauroechavarria@gmail.com (M. Echevarria Pinto) and hect2701@gmail.com (H.M. Garcia-Garcia).











