ABSTRACT
Introduction and objectives: Information comparing left atrial appendage closure (LAAC) to direct oral anticoagulation (DOAC) therapy is scarce. Our aim is to compare the clinical outcomes between LAAC and DOACs on an elderly population (> 80 years of age).
Methods: We retrospectively collected 1144 octogenarian patients with atrial fibrillation from 3 different tertiary hospitals. A total of 970 patients received DOACs and 174 patients were treated with LAAC. At baseline, both groups had similar cardiovascular risk factors. The LAAC group had more history of bleeding, anemia or previous cancer. We conducted a propensity score matching study and obtained 2 different paired groups of 58 patients with similar baseline risk factors, comorbidities, and risk scores who received DOACs or were treated with LAAC. The outcomes of the therapeutic strategy used (DOACs or LAAC) were assessed using the Cox regression analysis.
Results: During a median follow-up of 2.0 years [range 0.9-3.5] no differences regarding the primary endpoint (a composite of death, major bleeding, and stroke) were found (HR, 1.05; 95%CI, 0.15-7.51). Bleeding events were similar in both groups with no statistically significant differences being reported (HR, 1.79; 95%CI, 0.73-4.41). Mortality rate was numerically higher in patients on DOACs (31.8%) vs LAAC (26.4%). However, this finding did not reach statistical significance (HR, 0.70; 95%CI, 0.33-1.47; P = .343).
Conclusions: Compared to DOACs, LAAC has not shown any differences regarding embolic events, bleeding, and mortality in a population of elderly patients > 80 years of age. In our population, LAAC is a strategy as safe and effective as DOACs, and is an alternative to be taken into consideration in real-world patients > 80 years.
Keywords: Atrial fibrillation. Left atrial appendage closure. Direct oral anticoagulants. Embolic risk. Bleeding risk.
RESUMEN
Introducción y objetivos: Existe poca información comparativa entre el cierre de la orejuela izquierda (COI) y los anticoagulantes orales de acción directa (ACOD). Nuestro objetivo fue comparar los resultados clínicos entre el COI y los ACOD en una población de pacientes mayores de 80 años.
Métodos: Se analizaron 1.144 pacientes octogenarios con fibrilación auricular provenientes de 3 hospitales terciarios. De ellos, 970 recibían ACOD y 174 fueron sometidos a COI. Ambos grupos presentaban similares factores de riesgo cardiovascular. El grupo de COI tenía mayor porcentaje de antecedentes de hemorragia, anemia y cáncer previo. Se llevó a cabo un análisis emparejado y se obtuvieron 2 grupos de 58 pacientes con similares factores de riesgo, comorbilidad y escalas de riesgo que fueron sometidos a COI o recibían tratamiento con ACOD. Los resultados de acuerdo con la estrategia terapéutica se obtuvieron mediante regresión de Cox.
Resultados: Durante una mediana de seguimiento de 2 años [rango: 0,9-3,5] no hubo diferencias en cuanto al evento combinado primario de muerte, hemorragia mayor o ictus (HR = 1,05; IC95%, 0,15-7,51). Las hemorragias fueron similares en ambos grupos, sin diferencias estadísticamente significativas (HR = 1,79; IC95%, 0,73-4,41). La mortalidad fue mayor en los pacientes con ACOD (31,8%) frente a aquellos con COI (26,4%), sin diferencias significativas (HR = 0,70; IC95%, 0,33-1,47).
Conclusiones: En comparación con los ACOD, el COI no ha mostrado diferencias en cuanto a eventos embólicos, hemorragias y mortalidad en una población de pacientes de edad avanzada. En nuestra cohorte, el COI es una alternativa que puede considerarse para los pacientes mayores de 80 años.
Palabras clave: Fibrilación auricular. Cierre de orejuela izquierda. Anticoagulantes orales directos. Riesgo embólico. Riesgo hemorrágico.
Abbreviations AF: atrial fibrillation. DOACs: direct oral anticoagulants. LAAC: left atrial appendage closure.
INTRODUCTION
Atrial fibrillation (AF) has emerged as a clinically relevant issue of public health since it is associated with significant mortality and morbidity rates.1 AF is known to be a powerful risk factor for stroke independently increasing up to 5-fold across all ages. A total of 23.5% of all strokes occurred at 80-89 years of age are due to AF.2 The prevalence of AF is predicted to rise within the next few decades because of the growing population of elderly patients. Unfortunately, these patients are not often given oral anticoagulants. Only 35% of the patients aged ≥ 85 years without any clear contraindications for anticoagulation therapy receive the prescription.3 The reasons could be the increased risk of bleedings, especially intracranial and fatal bleedings,4 and also the frailty status of these patients. Since 2011, direct oral anticoagulants (DOACs) have shown a better risk-benefit ratio in patients with AF confirmed by a lower rate of stroke, intracranial hemorrhage, and mortality compared to warfarin.5 Still, with an improved safety and efficacy profile DOACs still present several shortcomings. The rate of discontinuation, the persistent risk of bleeding in high-risk populations or the risk of stroke when prescribed at a lower than recommended dosage are a matter of concern.6
Left atrial appendage closure (LAAC) was developed as an alternative to warfarin therapy in patients with AF. Several randomized controlled trials and few large registries have addressed the safety and efficacy profile of this technique.7,8 Recently, the evidence provided by long-term follow-up registries confirm that efficacy has similar endpoint rates compared to randomized controlled trials, and lower rates of stroke compared to the rates expected in untreated patients of similar risk.9
However, to this date, information comparing LAAC to DOACs therapy is scarce,10 and no comparison between both alternatives has been conducted in the elderly population. The aim of our study was to compare the clinical outcomes between LAAC and DOACs of an elderly population (> 80 years of age) using a propensity score matching study.
METHODS
Study population
This retrospective multicenter study included a cohort of 1144 consecutive octogenarian patients with non-valvular AF treated with DOACs (N = 970) or LAAC (N = 174) from January 2014 through December 2018 at 3 Spanish and Canadian hospitals (Hospital Álvaro Cunqueiro, Vigo, Spain, Hospital Universitario, Salamanca, Spain, and Institut Universitaire de Cardiologie et Pneumologie de Quebec, Canada).
Authors defined non-valvular AF as AF unrelated to rheumatic mitral stenosis or prosthetic mechanical heart valves.11 Because the goal of the trial was to evaluate LAAC compared to DOACs in patients with non-valvular AF, patients treated with LAAC who received postoperative oral anticoagulation were not included in the study.
All the patients treated with LAAC were discussed and approved for LAAC by a multidisciplinary team. Regarding anticoagulated patients, the optimal dose of DOACs was based on the European recommendations.12 Electronic medical records were reviewed in all the patients to collect data regarding the baseline clinical variables, the therapeutic strategy, and the events occurred at the follow-up. The CHA2DS2-VASc and HAS-BLED scores were estimated for each patient.
The study was conducted in full compliance with the principles established in the Declaration of Helsinki and approved by the local ethics committee. Due to the retrospective nature of the study and its general interest, it was approved by each center local ethics committee without the need for informed consent.
Follow-up and outcomes
Primary endpoint was a composite of death, major bleeding, and stroke. Primary efficacy endpoints were all-cause mortality, and embolic events. Primary safety endpoint was the risk of major bleeding. Outcomes were censored at the last medical contact site in primary or secondary care, which was censored in November 2019 or until the end of anticoagulant therapy in the case of the DOAC group or the beginning of such therapy in the case of the LAAC group.
Embolic events were defined as a composite of any ischemic stroke, pulmonary embolism or peripheral embolism. Ischemic stroke was confirmed through concomitant imaging studies of the brain including computed tomography scan or magnetic resonance imaging. Major bleeding (MB) was defined using the definition established by the International Society on Thrombosis and Hemostasis.13 Bleeding was divided into intracranial hemorrhage (ICH) and non-ICH.
Statistical analyses
All statistical analyses were performed using IBM SPSS Statistics 25.0 and Stata 15.1 statistical software packages. Continuous variables were expressed as mean ± standard deviation and compared using the chi-square test. Categorical variables were expressed as percentages and compared using the Student t test.
A Cox analysis was performed to evaluate the unadjusted impact of LAAC vs DOAC on mortality, embolic and bleeding events. Due to the important differences reported in the baseline characteristics of patients treated with LAAC compared to those treated with DOACs we complemented our analysis with a propensity score matching (PSM) study. Patients were matched on a 1:1 ratio based on their nutritional status and on the propensity score using a < 0.2 caliper. Propensity score was estimated through logistic regression with the therapeutic group (LAAC or DOAC) as the dependent outcome with 21 baseline characteristics (table 1) as the independent variables. After PSM, we identified 58 patient-pairs with balanced baseline characteristics and no significant differences (table 2). Estimates were reported as hazard ratios (HR) with their 95% confidence intervals (95%CI). P values < .05 were considered statistically significant. Kaplan-Meier estimates were used to graphically evaluate the rate and timing of the events according to the therapeutic group (LAAC vs DOAC).
Table 1. Comparison of baseline characteristics between patients treated with DOACs or LAAC
| Variables | DOACs (N = 970) | LAAC (N = 174) | P |
|---|---|---|---|
| Age (years) | 87.6 ± 3.6 | 83.6 ± 2.7 | < .001 |
| Female sex (%) | 67.3 | 41.4 | < .001 |
| Body mass index (kg/m2) | 29.1 ± 4.7 | 26.9 ± 3.7 | < .001 |
| Cardiovascular risk factors | |||
| Hypertension (%) | 70.3 | 90.2 | < .001 |
| Diabetes (%) | 20.3 | 37.4 | < .001 |
| Cardiovascular history | |||
| Peripheral arterial disease (%) | 12.2 | 29.9 | < .001 |
| Ischemic heart disease (%) | 14.4 | 35.1 | < .001 |
| Previous heart failure (%) | 25.8 | 42.0 | < .001 |
| Previous embolic events (%) | 26.4 | 17.5 | .006 |
| Comorbidities | |||
| Previous bleeding (%) | 10.1 | 57.5 | < .001 |
| Anemia (%) | 27.2 | 69.5 | < .001 |
| COPD (%) | 8.7 | 20.1 | < .001 |
| Dementia (%) | 5.1 | 7.5 | < .001 |
| Previous cancer (%) | 8.7 | 22.4 | < .001 |
| Laboratory data | |||
| Creatinine (mg/dL) | 1.0 ± 0.3 | 1.4 ± 0.9 | < .001 |
| Echocardiographic data | |||
| LVEF < 40% (%) | 5.3 | 9.2 | .042 |
| Severe aortic stenosis (%) | 3.8 | 6.3 | .129 |
| Concomitant therapy | |||
| Chronic use of NSAIDs (%) | 4.6 | 14.9 | < .001 |
| PPI (%) | 50.3 | 86.2 | < .001 |
| Risk scores | |||
| CHA2DS2-VASc (points) | 4.3 ± 1.3 | 5.2 ± 1.3 | < .001 |
| HAS-BLED (points) | 2.5 ± 0.9 | 3.5 ± 0.8 | < .001 |
COPD, chronic obstructive pulmonary disease; DOAC, direct oral anticoagulant; LAA, left atrial appendage; LVEF, left ventricular ejection fraction; NSAID, non-steroidal anti-inflammatory drug; PPI, proton pump inhibitor. | |||
RESULTS
Baseline characteristics
Out of a total cohort of 1144 patients with AF, 970 patients were treated with DOACs while 174 underwent successful LAAC. The baseline clinical characteristics of the 2 groups (unmatched population) are shown on table 1. Patients from the DOACs group were slightly older being women more predominant. Previous history of bleeding was more common in patients treated with LAAC and the same thing happened with anemia, previous cancer, and dementia. Both groups had similar cardiovascular risk factors. Among the patients from the LAAC group less than 30% received dual antiplatelet therapy (27.6%), and 75.9% single antiplatelet therapy.
Regarding thrombotic and bleeding risk, the CHA2DS2-VASc and the HAS-BLED scores were significantly higher in the LAAC group (5.2 ± 1.3 vs 4.3 ± 1.3 for CHA2DS2VASc, and 3.5 ± 0.8 vs 2.5 ± 0.9 for HAS-BLED).
Clinical outcomes
Entire population
The median follow-up was 2.0 years [range 0.9-3.5]. The events shown on table 3 section “before PSM” we collected and analyzed at the follow-up. Embolic events tend to be more frequent among patients on DOACs without statistical significance. Major bleeding events were statistically significant in patients treate with LAAC compared to DOAC (P < .001).
Based on the univariate analysis, LAAC was associated with a higher rate of death, major bleeding, and stroke compared to DOACs (HR, 1.54; 95%CI, 1.06-2.24; P = .024). Regarding the efficacy endpoint of all-cause mortality and embolic events no significant differences were observed between both groups (HR, 0.87; 95%CI, 0.53-1.44). The same thing happened with embolic events and stroke (HR, 0.59; 95%CI, 0.26-1.36, and HR, 0.82; 95%CI, 0.33-2.08, respectively). Major bleeding was significantly higher in the LAAC group (HR, 3.43; 95%CI, 2.05-5.76) based on the univariate analysis. ICH did not differ between LAAC and DOACs (HR, 1.49; 95%CI, 0.43-5.19). Based on the univariate analysis, the all-cause mortality rate was not statistically significance (HR, 1.09; 95%CI, 0.80-1.50).
Propensity score matching study
After PSM a total of 58 patients were obtained in each group. The 2 groups were uniform regarding age (85.8 ± 3.7 vs 85.6 ± 2.5 years, P = .758), cardiovascular risk factors (32.8% vs 29.3% diabetes, P = .688; 81.0% vs 87.9% hypertension, P = .305), previous heart failure (37.9% vs 43.1% P = .570), creatinine levels (1.2 ± 0.5 mg/dL vs 1.2 ± 0.6mg/dL P = .809), and ischemic and bleeding risk (CHA2DS2-VASc, 4.7 ± 1.5 vs 4.7 ± 1.1; P = .834, and HAS-BLED, 3.2 ± 1.0 vs 3.2 ± 0.7 P = .834) as shown on table 2.
Table 2. Comparison of baseline characteristics after propensity score matching between patients treated with DOACs or LAAC
| Variables | DOACs (N = 58) | LAAC (N = 58) | P | SMD |
|---|---|---|---|---|
| Age (years) | 85.8 ± 3.7 | 85.6 ± 2.5 | .758 | -0.068 |
| Female sex (%) | 46.6 | 44.8 | .852 | 0.035 |
| Body mass index (kg/m2) | 27.4 ± 4.2 | 27.8 ± 4.4 | .667 | 0.092 |
| Cardiovascular risk factors | ||||
| Hypertension (%) | 81.0 | 87.9 | .305 | 0.232 |
| Diabetes (%) | 32.8 | 29.3 | .688 | -0.071 |
| Cardiovascular history | ||||
| Peripheral arterial disease (%) | 13.8 | 13.8 | 1.000 | 0.000 |
| Ischemic heart disease (%) | 24.1 | 13.8 | .155 | -0.216 |
| Previous heart failure (%) | 37.9 | 34.5 | .699 | -0.070 |
| Previous embolic events (%) | 27.6 | 24.1 | .672 | -0.078 |
| Comorbidities | ||||
| Previous bleeding (%) | 37.9 | 43.1 | .570 | 0.104 |
| Anemia (%) | 70.7 | 62.1 | .326 | -0.187 |
| COPD (%) | 12.1 | 17.2 | .431 | 0.129 |
| Dementia (%) | 3.4 | 8.6 | .242 | 0.288 |
| Previous cancer (%) | 13.8 | 10.3 | .569 | -0.082 |
| Laboratory data | ||||
| Creatinine (mg/dL) | 1.2 ±0.5 | 1.2 ± 0.6 | .809 | -0.028 |
| Echocardiographic data | ||||
| LVEF < 40% (%) | 6.9 | 8.6 | .729 | 0.059 |
| Severe aortic stenosis (%) | 5.2 | 5.2 | 1.000 | 0.000 |
| Concomitant therapy | ||||
| Chronic use of NSAIDs (%) | 10.3 | 5.2 | .298 | -0.246 |
| PPI (%) | 79.3 | 77.6 | .821 | -0.050 |
| Risk scores | ||||
| CHA2DS2-VASc (points) | 4.7 ± 1.5 | 4.7 ± 1.1 | .834 | -0.040 |
| HAS-BLED (points) | 3.2 ± 1.0 | 3.2 ± 0.7 | .826 | -0.043 |
COPD, chronic obstructive pulmonary disease; DOAC, direct oral anticoagulant; LAA, left atrial appendage; LVEF, left ventricular ejection fraction; NSAID, non-steroidal anti-inflammatory drug; PPI, proton pump inhibitor; SMD, standardized mean difference. | ||||
At the follow-up, events were collected for both groups (see table 3 section “after PSM”). Patients on DOACs had more cardiovascular mortality compared to patients treated with LAAC (P = .555). Major bleeding was higher in patients treated with LAAC without statistical significance (P = .056).
Table 3. Clinical events in patients treated with DOAC and LAAC before and after propensity score matching between groups
| Before PSM | |||||
| Event | DOAC (N = 970) | LAAC (N = 174) | P | ||
| No. | Incidence rate (per 100 person/years) | No. | Incidence rate (per 100 person/years) | ||
| Mortality | 308 | 13.5 (12.1-15.1) | 46 | 14.7 (11.0-19.7) | .543 |
| CV mortality | 96 | 4.2 (3.5-5.2) | 9 | 2.9 (1.5-5.5) | .617 |
| Ischemic stroke | 47 | 2.1 (1.6-2.8) | 5 | 1.6 (0.7-3.9) | .248 |
| TIA | 26 | 1.2 (0.8-1.7) | 1 | 0.6 (0.1-4.3) | .460 |
| Peripheral embolism | 2 | 0.1 (0.0-0.3) | 0 | - | - |
| ICH | 14 | 0.6 (0.4-1.0) | 3 | 0.9 (0.3-2.9) | .905 |
| Major bleeding | 48 | 2.1 (1.6-2.8) | 21 | 7.5 (4.9-11.4) | < .001 |
| Minor bleeding | 54 | 2.5 (1.9-3.2) | 12 | 4.0 (2.3-7.0) | .318 |
| After PSM | |||||
| Event | DOAC (N = 58) | LAAC (N = 58) | P | ||
| No. | Incidence rate (per 100 person/years) | No. | Incidence rate (per 100 person/years) | ||
| Mortality | 20 | 15.2 (9.8-23.5) | 12 | 11.2 (6.4-19.7) | .343 |
| CV mortality | 6 | 4.5 (2.0-10.1) | 3 | 2.8 (0.9-8.7) | .555 |
| Ischemic stroke | 1 | 0.8 (0.1-5.5) | 2 | 1.9 (0.5-7.7) | .547 |
| TIA | 1 | 0.8 (0.1-5.5) | 0 | - | - |
| Peripheral embolism | 0 | - | 0 | - | - |
| ICH | 2 | 1.6 (0.4-6.2) | 1 | 0.9 (0.1-6.6) | - |
| Major bleeding | 4 | 3.0 (1.1-8.1) | 9 | 9.2 (4.8-17.7) | .056 |
| Minor bleeding | 6 | 4.6 (2.1-10.3) | 2 | 1.9 (0.5-7.5) | .292 |
CV, cardiovascular; DOAC, direct oral anticoagulant; ICH, intracranial hemorrhage; LAAC, left atrial appendage closure; PSM, propensity score matching; TIA, transient ischemic attack. | |||||
Regarding to primary endpoint (death, major bleeding, and stroke) after PSM, LAAC had a higher risk rate (HR, 1.62; 95%CI, 0.62-3.65) compared with DOACs. The primary efficacy endpoint (all-cause mortality, and embolic events) did not differ between both groups (HR, 0.83; 95%CI, 0.29-2.35).
No differences regarding embolic events were apparent between the 2 matched groups (HR, 1.05; 95%CI, 0.15-7.51) (figure 1). No statistically significant differences were found regarding the ischemic stroke (HR, 2.12; 95%CI, 0.19-23.39).
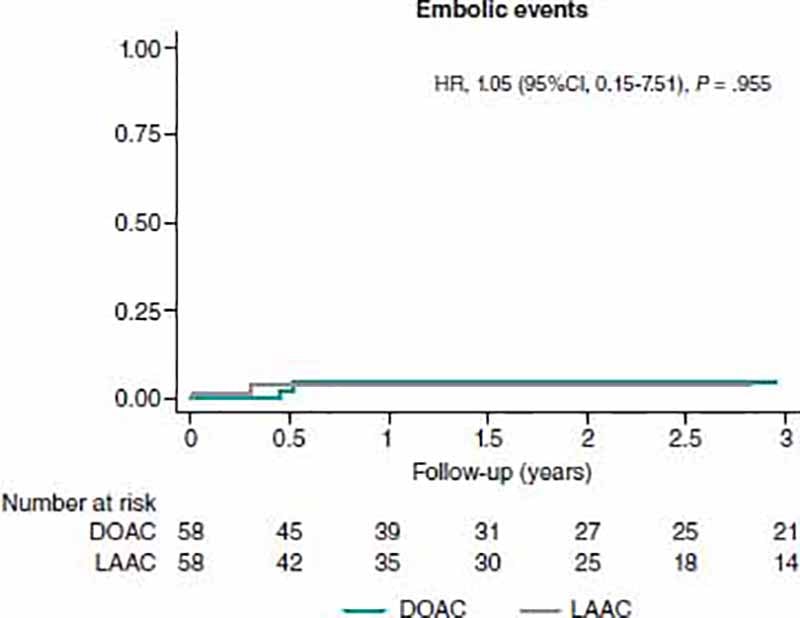
Figure 1. Analysis of embolic events at the follow-up between matched groups. 95%CI, 95% confidence interval; DOAC, direct oral anticoagulant; HR, hazard ratio; LAAC, left atrial appendage closure.
Safety endpoint (major bleeding) did not differ in either group (HR, 1.79; 95%CI, 0.73-4.41) (figure 2) after PSM.Also, ICH did not differ in either one of the 2 categories (HR, 0.61; 95%CI, 0.05-6.78).
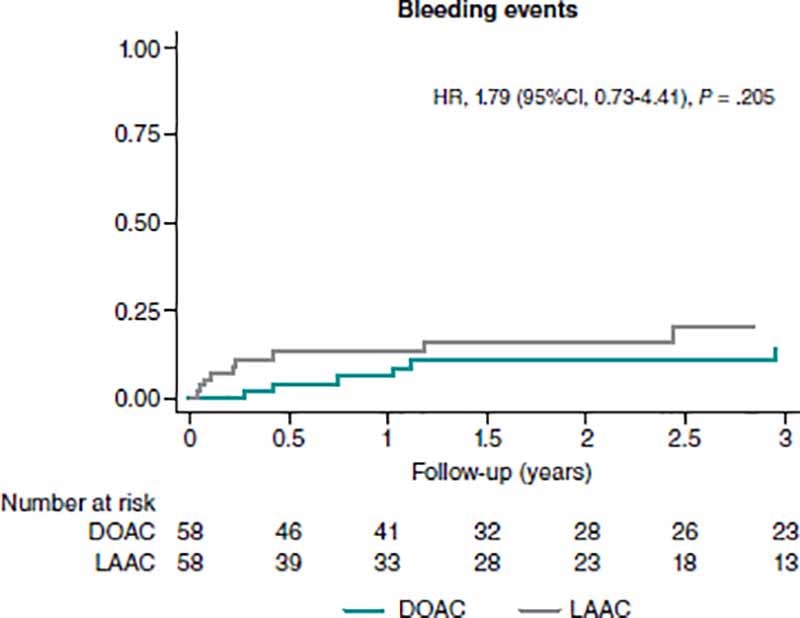
Figure 2. Analysis of major bleeding events at the follow-up between matched groups. 95%CI, 95% confidence interval; DOAC, direct oral anticoagulant; HR, hazard ratio; LAAC, left atrial appendage closure.
Mortality rate was numerically higher in patients on DOACs. After the PSM study, this finding did not reach statistical significance (HR, 0.70; 95%CI, 0.33-1.47) (figure 3).
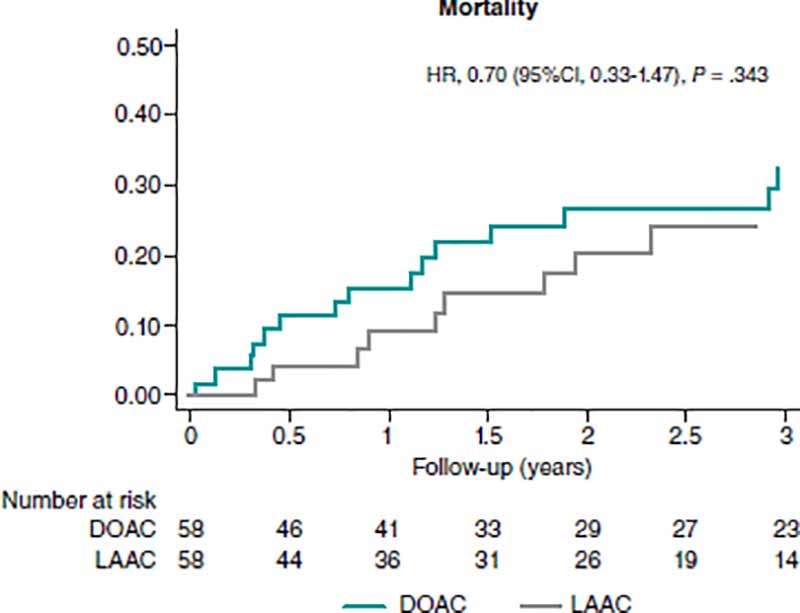
Figure 3. Analysis of mortality at the follow-up between matched groups. 95%CI, 95% confidence interval; DOAC, direct oral anticoagulant; HR, hazard ratio; LAAC, left atrial appendage closure.
DISCUSSION
This study has been designed with the intent to compare LAAC to DOACs in an elderly population (> 80 years old). The main finding of our study is that after PSM both DOACs and LAAC groups proved to have similar outcomes regarding the efficacy and safety profile.
As far as we are concerned this is the first study to compare both strategies in this population. We selected the cut-off value of 80 years not only because age is a known risk factor for stroke,2 but also because age is associated with bleeding events and fewer prescriptions of anticoagulants.14
Many studies have evaluated clinical outcomes with different antithrombotic strategies in elderly patients with AF. Two studies15,16 compared warfarin with aspirin supporting the use of anticoagulation in elderly and very elderly patients. Nonetheless, therapy with vitamin K antagonists is under-implemented in this population mostly due to the risk of falling (26.7%), poor prognosis (19.3%), bleeding history (17.1%), participant or family refusal (14.9%), older age (11.0%), and dementia (9.4%).17
As it has been discussed, DOACs provided an alternative to vitamin K antagonists. Dabigatran in both doses compared with warfarin—in patients aged ≥ 75 years—was associated with a similar or higher risk of major non-intracranial bleeding.18 Similarly, rivaroxaban described higher major gastrointestinal bleeding rates among the elderly population with no significant interaction between age and treatment efficacy.19 Apixaban proved beneficial compared to warfarin reducing the rates of stroke and major bleeding in our target population.20 Finally, edoxaban also proved beneficial in very elderly patients regarding major bleeding.21
Clinical trials21-24 comparing DOACs to warfarin led to the current guideline recommendation of DOACs as first-line therapy even in the elderly population.11,25 However, DOACs may present several limitations in this type of patients. We know from clinical registries that approximately 1 in 7 patients with AF receive reduced doses of DOACs even though they never met the criteria for reduced doses.26 Interestingly, this finding is more common among the elderly population. The rates of adverse events were higher in off-label dosed patients (HR for all-cause mortality, 2.18 [1.57-3.02]; HR for stroke, 1.50 [0.77-2.94]).27 In this sense, we did not evaluate the dose of DOACs in our patients, but it is known from previous registries that almost one third of the patients received inappropriate doses.28 Another important issue with DOACs is compliance. Recent data from studies conducted in the UK revealed poorer compliance with DOACs due to the lack of routine monitoring and, in some cases, the twice-daily dosing regime.29 Non-compliance in this group revealed adverse outcomes including mortality and stroke.30 Third, frailty is of major concern among the elderly population receiving anticoagulant drugs. A prospective study in hospitalized elderly patients showed that frailty is associated with a higher mortality rate at admission and a 2-fold increased risk of death at 1 year, particularly in anticoagulated patients.31 The risk of falling is an important parameter of frailty. In a recent study of older adults with a history of falls and AF, the risk of ICH at the follow-up was 1.9 times higher.32
LAAC may be a recommended therapeutic alternative in patients with AF ineligible for long-term oral anticoagulation who need stroke and embolism prevention according to the last EHRA/EACPI consensus statement.33 The PROTECT AF and PREVAIL 5-year outcome data were combined in a meta-analysis,34 and proved that LAAC with the Watchman device is equivalent to warfarin in stroke prevention and requires additional decreases of major bleeding and mortality. The safety and efficacy profile of the Amplatzer Cardiac Plug was examined in a multicenter study8 showing high procedural success rates and favorable outcomes preventing AF related thromboembolism.
A subanalysis of the EWOLUTION registry including patients aged ≥ 85 years showed that LAAC is a safe and effective procedure in these patients without any differences compared to younger patients regarding the annual stroke rates (2.0 vs 2.5 in ≥ 85 and < 85, respectively).35
Notwithstanding the above, the information available on this strategy compared to DOACs is scarce. To this date, only 2 studies have addressed this issue. The PRAGUE-17 was a prospective, multicenter, randomized non-inferiority trial conducted by Osmancik et al. that tried to compare LAAC with DOACs in high risk patients with AF (CHA2DS2-VASc ≥ 3, and HAS-BLED ≥ 2).36 Patients were younger compared to our cohort, mean age was 73.4 ± 6.7 in the LAAC group and 73.2 ± 7.2 in the DOACs group. They had similar CHA2DS2VASC scores (4.7 ± 1.5) in both groups, also similar to our cohort of patients. LAAC was non-inferior to DOAC therapy regarding the composite clinical and bleeding events through a median follow-up of 20.8 months. The rates of stroke and transient ischemic attack, cardiac death, clinically significant bleeding, and nonprocedural clinically significant bleeding did not differ between the study arms. These findings are consistent with the results obtained by Godino et al.10 Compared to our data, they selected a younger population (mean age 74.2 ± 7.7 in the LAAC group compared to 77.7 ± 6.9 in the DOACs group) with similar CHA2DS2VASc scores (4.3 ± 1.5 and 4.8 ± 1.5 in the LAAC and DOACs groups, respectively). They found similar outcomes between the 2 groups after PSM regarding thromboembolic events, ischemic stroke, transients ischemic attack, systemic embolism, and acute myocardial infarction, which is consistent with our own conclusions. Looking at the bleeding events, DOACs did not show an increased risk of major bleeding. In our population, results are consistent with previous findings even though we were dealing with older patients. Despite not being anticoagulated patients, the LAAC group did not have fewer bleeding events. Our hypothesis is that maybe many of them were treated with antiplatelet therapy.
Our observations are consistent with the previous studies mentioned, which supports the use of LAAC as an alternative to DOACs among elderly patients.
Study limitations
Our study has several limitations. First, its observational retrospective nature. Second, although rigorous matching was performed with 21 variables to neutralize the different clinical profile of patients, we cannot exclude the influence of other uncollected variables. Third, after PSM we achieved 2 well-balanced groups—though with a small sample size—that could lead to the underestimation of events at the follow-up. Also, we only selected patients with successful LAAC.
Despite all these limitations, we presented interesting data based on a multicenter study of consecutive octogenarian patients with non-valvular AF treated with DOAC vs LAAC.
CONCLUSIONS
This multicenter observational study proves the safety and efficacy profile after LAAC, with no differences regarding embolic and bleeding events, and mortality compared to DOACs in a propensity-matched population of real-world elderly patients > 80 years successful treated with LAAC without complications.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
All authors contributed to patient recruitment, data curation, and process of manuscript review. J. Rodés-Cabau, A. Íñiguez-Romo, S. Raposeiras-Roubín, and R. Estévez-Loureiro were responsible for the study design. S. Raposeiras-Roubín, and B. Caneiro-Queija conducted the statistical analysis. B. Caneiro-Queija, S. Raposeiras-Roubín, and R. Estévez-Loureiro were responsible for preparing the manuscript.
CONFLICTS OF INTEREST
R. Estévez-Loureiro is proctor for Watchman and has received honoraria from Boston Scientific. I. Cruz-González is proctor for Watchman and LifeTech and has received honoraria from Boston Scientific and Abbott Vascular. Rodés-Cabau has received a research grant from Boston Scientific. The remaining authors declared no other conflicts of interest.
WHAT IS KNOWN ABOUT THE TOPIC?
- Patients who are often treated with LAAC tend to be poor candidates for anticoagulation. As a matter of fact, older patients are excluded from randomized clinical trials and are more prone to receive reduced doses of DOACs. We know from previous trials about the noninferiority of LAAC compared to DOACs.
WHAT DOES THIS STUDY ADD?
- There was no current information on real-world older populations receiving DOACs compared to LAAC.
- Although our data come from a registry they reflect our routine clinical practice; in a comparable profile population of older patients, LAAC might be as safe and effective as DOACs.
REFERENCES
1. Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:213-220.
2. Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67-e492.
3. Go AS, Hylek EM, Borowsky LH, Phillips KA, Selby JV, Singer DE. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med. 1999;131:927-934.
4. Silverio A, Di Maio M, Prota C, et al. Safety and efficacy of non-vitamin K antagonist oral anticoagulants in elderly patients with atrial fibrillation: systematic review and meta-analysis of 22 studies and 440 281 patients. Eur Heart J Cardiovasc Pharmacother. 2021;7:f20-f29.
5. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
6. Kachroo S, Hamilton M, Liu X, et al. Oral anticoagulant discontinuation in patients with nonvalvular atrial fibrillation. Am J Manag Care. 2016;22:e1-8.
7. Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534-542.
8. Tzikas A, Shakir S, Gafoor S, et al. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: multicentre experience with the AMPLATZER Cardiac Plug. EuroIntervention. 2016;11:1170-1179.
9. Holmes DR, Jr., Reddy VY, Gordon NT, et al. Long-Term Safety and Efficacy in Continued Access Left Atrial Appendage Closure Registries. J Am Coll Cardiol. 2019;74:2878-2889.
10. Godino C, Melillo F, Bellini B, et al. Percutaneous left atrial appendage closure compared to non-vitamin K oral anticoagulants in patients with non-valvular atrial fibrillation and high bleeding risk.EuroIntervention. 2020;15:1548-1554.
11. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893-2962.
12. Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39:1330-1393.
13. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692-694.
14. Tulner LR, Van Campen JP, Kuper IM, et al. Reasons for undertreatment with oral anticoagulants in frail geriatric outpatients with atrial fibrillation: a prospective, descriptive study. Drugs Aging. 2010;27:39-50.
15. Mant J, Hobbs FD, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370:493-503.
16. Patti G, Lucerna M, Pecen L, et al. Thromboembolic Risk, Bleeding Outcomes and Effect of Different Antithrombotic Strategies in Very Elderly Patients With Atrial Fibrillation: A Sub-Analysis From the PREFER in AF (PREvention oF Thromboembolic Events-European Registry in Atrial Fibrillation). J Am Heart Assoc. 2017;6:e005657.
17. Cavallari I, Patti G. Efficacy and safety of oral anticoagulation in elderly patients with atrial fibrillation. Anatol J Cardiol. 2018;19:67-71.
18. Eikelboom JW, Wallentin L, Connolly SJ, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation. 2011;123:2363-2372.
19. Halperin JL, Hankey GJ, Wojdyla DM, et al. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Circulation. 2014;130:138-146.
20. Halvorsen S, Atar D, Yang H, et al. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: observations from the ARISTOTLE trial. Eur Heart J. 2014;35:1864-1872.
21. Kato ET, Giugliano RP, Ruff CT, et al. Efficacy and Safety of Edoxaban in Elderly Patients With Atrial Fibrillation in the ENGAGE AF-TIMI 48 Trial. J Am Heart Assoc. 2016;5:e003432.
22. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
23. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992.
24. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891.
25. January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140:e125-e151.
26. Steinberg BA, Shrader P, Pieper K, et al. Frequency and Outcomes of Reduced Dose Non-Vitamin K Antagonist Anticoagulants: Results From ORBIT-AF II (The Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II). J Am Heart Assoc. 2018;7:e007633.
27. Steinberg BA, Shrader P, Thomas L, et al. Off-Label Dosing of Non-Vitamin K Antagonist Oral Anticoagulants and Adverse Outcomes: The ORBIT-AF II Registry. J Am Coll Cardiol. 2016;68:2597-2604.
28. Ruiz Ortiz M, Esteve-Pastor MA, Roldan I, Muniz J, Marin F, Anguita M. Prognostic impact of inappropriate doses of direct oral anticoagulants in clinical practice. Rev Esp Cardiol. 2020;73:329-330.
29. Burn J, Pirmohamed M. Direct oral anticoagulants versus warfarin: is new always better than the old? Open Heart. 2018;5:e000712.
30. Borne RT, O’Donnell C, Turakhia MP, et al. Adherence and outcomes to direct oral anticoagulants among patients with atrial fibrillation: findings from the veterans health administration. BMC Cardiovasc Disord. 2017;17:236.
31. Gullon A, Formiga F, Diez-Manglano J, et al. Influence of frailty on anticoagulant prescription and clinical outcomes after 1-year follow-up in hospitalised older patients with atrial fibrillation. Intern Emerg Med. 2019;14:59-69.
32. Gage BF, Birman-Deych E, Kerzner R, Radford MJ, Nilasena DS, Rich MW. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med. 2005;118:612-617.
33. Glikson M, Wolff R, Hindricks G, et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion - an update. EuroIntervention. 2020;15:1133-1180.
34. Reddy VY, Doshi SK, Kar S, et al. 5-Year Outcomes After Left Atrial Appendage Closure: From the PREVAIL and PROTECT AF Trials. J Am Coll Cardiol. 2017;70:2964-2975.
35. Cruz-Gonzalez I, Ince H, Kische S, et al. Left atrial appendage occlusion in patients older than 85 years. Safety and efficacy in the EWOLUTION registry. Rev Esp Cardiol. 2020;73:21-27.
36. Osmancik P, Herman D, Neuzil P, et al; PRAGUE-17 Trial Investigators. Left Atrial Appendage Closure Versus Direct Oral Anticoagulants in High-Risk Patients With Atrial Fibrillation. J Am Coll Cardiol. 2020;75:3122-3135.
* Corresponding author.
E-mail address: raposeiras26@hotmail.com (S. Raposeiras-Roubín).











