In 1995, my colleagues and I at the Washington Hospital Center (Washington, DC, United States) published an intravascular ultrasound (IVUS) vs angiographic assessment of calcium in 1155 lesions targeted for percutaneous coronary intervention (figure 1)1. Angiography detected calcium in 440 lesions (38%), but IVUS detected lesion calcium in 841 lesions (73%). Among these 1155 lesions, 27% had no IVUS calcium, 26% had 1-quadrant IVUS calcium, 21% had 2 quadrants, 15% had 3 quadrants, and 11% had 4-quadrant IVUS calcium. When present, target lesion calcium was only superficial in 48%, only deep in 28%, and both superficial and deep in 24%. Therefore, some superficial calcium was present in 72% of the 841 calcium-containing lesions (1-quadrant superficial calcium in 35%, 2 quadrants in 31%, 3 quadrants in 18%, and 4-quadrant superficial calcium in 18%). The diagnostic ability of angiography to detect calcium was primarily dependent on the arc and length of calcium, but also on whether calcium was or not superficial (figure 1). However, there was also a curious 10% rate of angiographic false positives attributed to the difficulty differentiating perivascular or reference segment calcium from intralesional calcium. However, it was never clear whether there was a systematic problem with angiographic calcium detection or whether it was because, in the early 1990s, angiography was primitive compared to today and would improve over time.
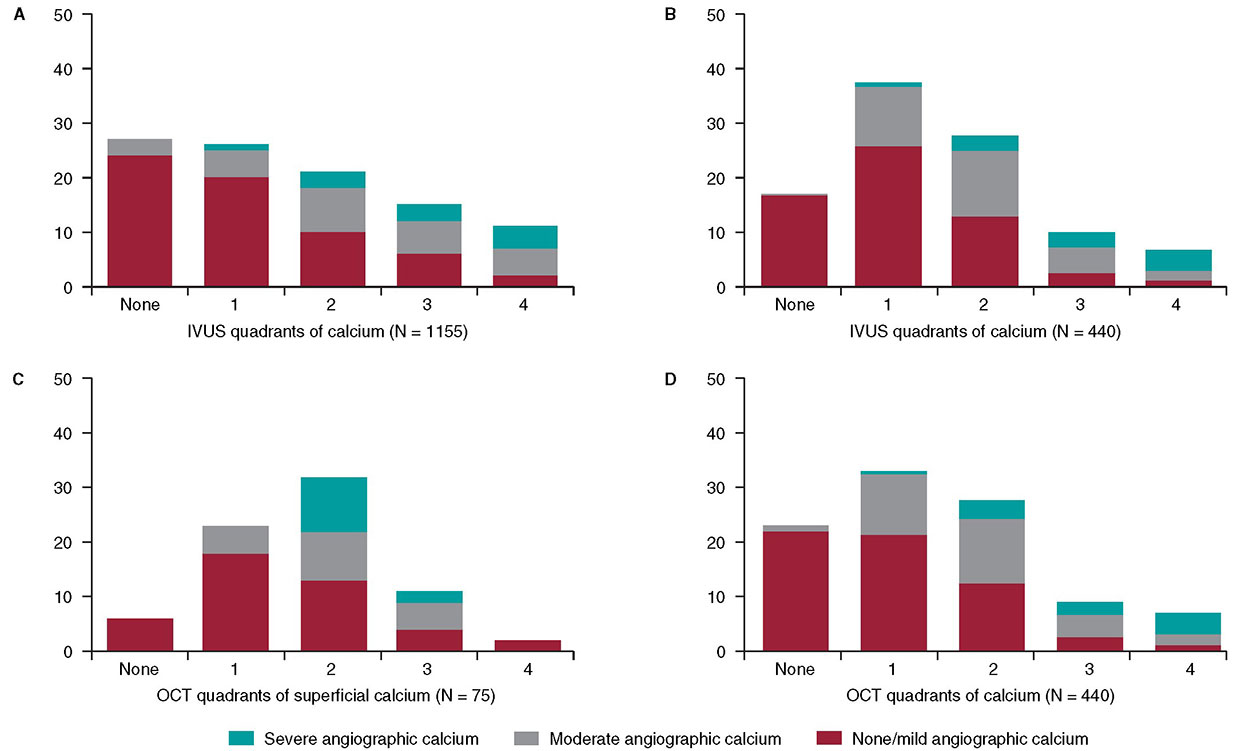
Figure 1. A total of 3 studies (clockwise starting in the upper left-hand corner) comparing intravascular imaging to the angiography detection of coronary lesion calcium. A: the study conducted by Mintz et al.1 from 1995. B and C: the study by Wang et al.2 from 2017. D: the study by McGuire et al.3 from 2021. IVUS, intravascular ultrasound; OCT, optical coherence tomography.
This experiment was repeated more than 20 years later by Wang et al. in a smaller cohort of 440 lesions using state-of-the-art angiographic equipment and both IVUS and optical coherence tomography (OCT) imaging (figure 1).2 Any amount of calcium was detected by coronary angiography in 40.2% (177 of 440) of the lesions, by IVUS in 82.7% (364 of 440) of the lesions, and by OCT in 76.8% (338 of 440) of the lesions. Notably and compared to the 1995 study, almost all calcium was superficial, fewer lesions had no calcium, and more lesions had 1- or 2-quadrant calcium (figure 1). In 13.2% of the lesions with IVUS-detected calcium, calcium was not visible by OCT mostly because of attenuation due to superficial lipid plaque accumulation. In a recent paper published in REC: Interventional Cardiology, McGuire et al.3 compared angiographic vs OCT calcium detection in 75 lesions. OCT detected calcium in 69 lesions vs 30 lesions by angiography with no angiographic false positives (figure 1).3 Compared to IVUS, OCT can measure the thickness, area, and volume that affect the angiographic detection of calcium in addition to its arc and length.2,3
Other than a reduced rate of false positives in the 2 contemporary studies, which could be attributed to the improved resolution of modern x-ray equipment, the lower x-ray doses being used today vs 1995, and the clinical recognition of the existence perivascular calcium, the results were remarkably similar to those of 1995. Thus, there appears to be a fundamental limitation to x-ray that cannot be improved by technological advances.
Why is calcium detection so important? The primary cause of in-stent restenosis is stent underexpansion, the primary cause of stent underexpansion is calcium, and the natural history of in-stent restenosis is not benign with an annual mortality rate of 5% to 7% (associated with treatment and at the follow-up).4-7 There are calcium scores for both OCT and IVUS that reliably predict calcium-related stent underexpansion (figure 2);8,9 and there are technologies and approaches that can be used to modify calcium to promote a better stent expansion.4,10
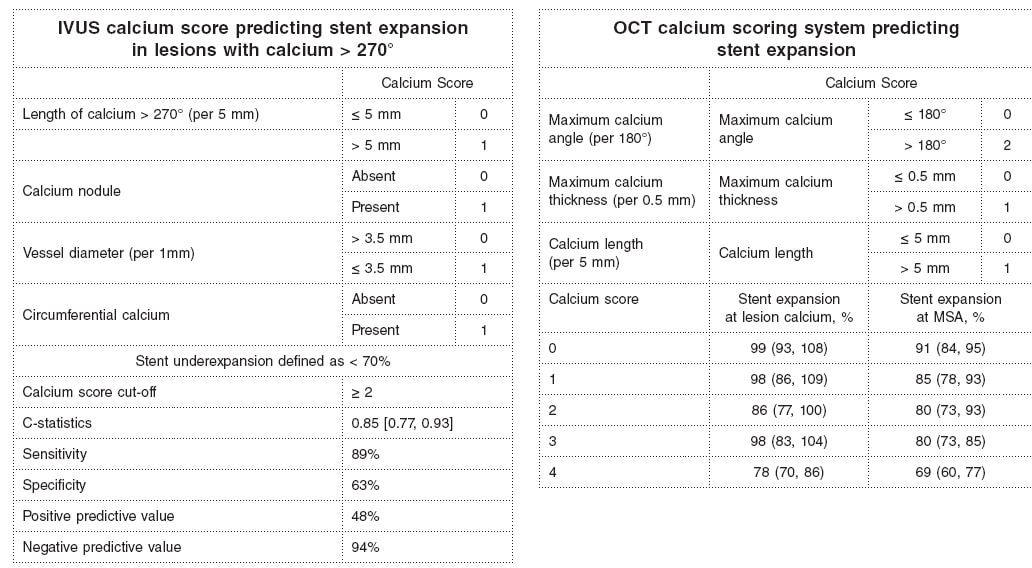
Figure 2. Intravascular ultrasound and optical coherence tomography calcium scores to predict stent underexpansion.8,9 IVUS, intravascular ultrasound; MSA, minimum stent area; OCT, optical coherence tomography.
There are predictors of target lesion calcium at patient level (older age, non-insulin treated diabetes, stable angina rather than an acute coronary syndrome, chronic kidney disease—especially if a patient is on dialysis—, and calcium elsewhere in the coronary tree), and predictors at lesion level too (smaller vessels, more severe stenoses).11-15 However, for the most part, lesions behave independently with regards to calcium accumulation. Only intravascular imaging can reliably detect and quantify target lesion calcium and predict stent underexpansion in the severe target lesion calcium setting.
FUNDING
None.
CONFLICTS OF INTEREST
G.S. Mintz declares having received honoraria from BostonScientific, Philips, Medtronic, and Abiomed outside the present work.
REFERENCES
1. Mintz GS, Popma JJ, Pichard AD, et al. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation. 1995;91:1959-1965.
2. Wang Z, Matsumura M, Mintz GS, et al. In vivo calcium detection by comparing optical coherence tomography, intravascular ultrasound, and angiography. JACC Cardiovasc Imaging. 2017;10:869-879.
3. McGuire C, Shlofmitz E, Melaku GD, et al. Comparison of quantitative calcium parameters in optical coherence tomography and invasive coronary angiography. REC Interv Cardiol. 2022;4:6-11.
4. Mintz GS, Ali ZA, Maehara A. Use of intracoronary imaging to guide optimal percutaneous coronary intervention procedures and outcomes. Heart. 2021;107:755-764.
5. Wiebe J, Kuna C, Ibrahim T, et al. Long-term prognostic impact of restenosis of the unprotected left main coronary artery requiring repeat revascularization. JACC Cardiovasc Interv. 2020;13:2266-2274.
6. Tamez H, Secemsky EA, Valsdottir LR, et al. Long-term outcomes of percutaneous coronary intervention for in-stent restenosis among Medicare beneficiaries. EuroIntervention. 2021;17:e380-e387.
7. Shlofmitz E, Torguson R, Zhang C, et al. Impact of intravascular ultrasound on Outcomes following PErcutaneous coronary interventioN for In-stent Restenosis (iOPEN-ISR study). Int J Cardiol. 2021 https://doi.org/10.1016/j.ijcard.2021.08.003.
8. Fujino A, Mintz GS, Matsumura M, et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention. 2018;13:e2182-e2189.
9. Zhang M, Matsumura M, Usui E, et al. Intravascular ultrasound-derived calcium score to predict stent expansion in severely calcified lesions. Circ Cardiovasc Interv. 2021;14:e010296.
10. De Maria GL, Scarsini R, Banning AP. Management of calcific coronary artery lesions:Is it time to change our interventional therapeutic approach?JACC Cardiovasc Interv. 2019;12:1465-1478.
11. Mintz GS, Pichard AD, Popma JJ, et al. Determinants and correlates of target lesion calcium in coronary artery disease:a clinical, angiographic and intravascular ultrasound study. J Am Coll Cardiol. 1997;29:268-274.
12. Mintz GS, Pichard AD, Kent KM, Satler LF, Popma JJ, Leon MB. Interrelation of coronary angiographic reference lumen size and intravascular ultrasound target lesion calcium. Am J Cardiol. 1998;81:387-391.
13. Tuzcu EM, Berklap B, DeFranco AC, et al. The dilemma of diagnosing coronary calcification:angiography versus intravascular ultrasound. J Am Coll Cardiol. 1996;27:832-838.
14. Gruberg L, Rai P, Mintz GS, et al. Impact of renal function on coronary plaque morphology and morphometry in patients with chronic renal insufficiency as determined by intravascular ultrasound volumetric analysis. Am J Cardiol. 2005;96:892-896.
15. Chin CY, Matsumura M, Maehara A, et al. Coronary plaque characteristics in hemodialysis-dependent patients as assessed by optical coherence tomography. Am J Cardiol. 2017;119:1313-1319.











