HOW WOULD I APPROACH IT?
Several cases have been published in the medical literature on peripartum spontaneous coronary artery dissection. Its pathophysiology largely remains unknown, although in a significant percentage of cases fibromuscular dysplasia seems to play some sort of role.1
We present the case of a 36-year-old woman with spontaneous coronary artery dissection of left main coronary artery (LMCA) and left anterior descending coronary artery (LAD) 2 months after delivery. The patient shows clinical signs of thoracic pain and dyspnea with 1-hour of evolution, T-wave inversion on the electrocardiogram, and elevated cardiac enzyme levels. She remains hemodynamically stable without compromise to the left ventricular function. The coronary angiography performed revealed a suspicious image of diffuse reduction of the blood lumen area on the LMCA and LAD. At an early moment, conservative treatment was administered.
In my opinion, the early treatment was appropriate since, according to the largest series published to this date—Canadian2—the in-hospital prognosis is often good and only 3.3% of the patients on conservative treatment required revascularization. This has been confirmed in a study that assessed the infarction size on a cardiac magnetic resonance imaging in patients with spontaneous coronary artery dissection.3 Our experience is consistent with this study and in patients with hemodynamic stability conservative treatment was prescribed.
Medical therapy has not been clearly established, but since the presence of intraluminal thrombi is scarce in patients who undergo an intravascular ultrasound (IVUS), treatment with acetylsalicylic acid seems to be enough. In the case studied I only miss an IVUS during the first angiography that would have confirmed the dissection and the wall hematoma. Also, to know the exact spread of the disease as the second angiography confirmed. In my opinion the IVUS technique is safer than the optical coherence tomography since the latter requires the intracoronary injection of contrast, which may aggravate the dissection.
The patient remained stable and 8 days after symptom onset a control coronary angiography is performed. According to our own experience, with the standard treatment, this period of time is too short to assess the sealing of the dissection and it is recommended to wait for, at least, 3 to 4 weeks.
A 6-Fr JL 3.5 catheter was used to perform the coronary angiography. It was not specified whether it was a diagnostic or guide catheter since we don’t know whether the idea was to perform an intracoronary imaging technique or not. The curve of the catheter guide selected seems appropriate to us because it is less aggressive than other curves like the AL2 or the extra back-up.
After the vessel catheterization reduced the distal LMCA and proximal LAD lumens reduced. The authors attribute this to a vasospasm. Unfortunately, the first angiographic image available shows the blood vessel after worsening luminal narrowing with the angioplasty guidewire inserted, which is why we cannot assess the first injection of contrast. In my opinion, it is unlikely that the worsening luminal narrowing is due to a vasospasm considering that the whole territory has a large intramural hematoma, which is why the contraction mechanism of the coronary artery media layer that causes the vasospasm would not be operative.
It is more likely that the catheterization of the coronary artery or the first injection of contrast increased the wall hematoma, therefore, narrowing the vessel lumen. This significant reduction of the LAD lumen compromises its blood flow. The IVUS shows a large intramural hematoma affecting almost the entire vessel lumen in some segments (figure 1), and a distal LMCA dissection (figure 2).
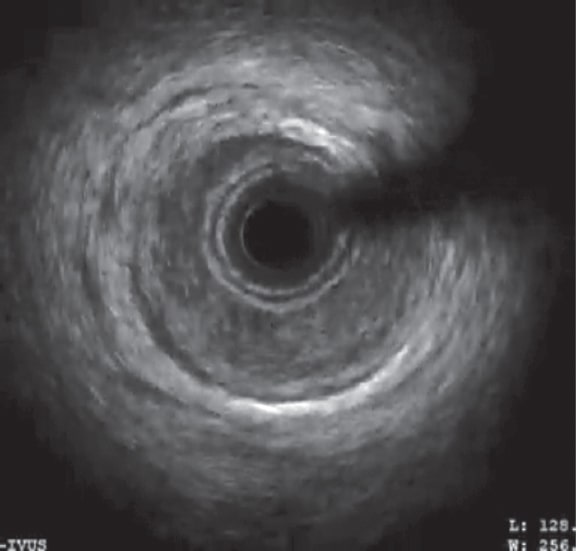
Figure 1. Intravascular ultrasound in the proximal anterior descending coronary artery. Image of a large intramural hematoma in the coronary artery compromising almost the entire vessel lumen.
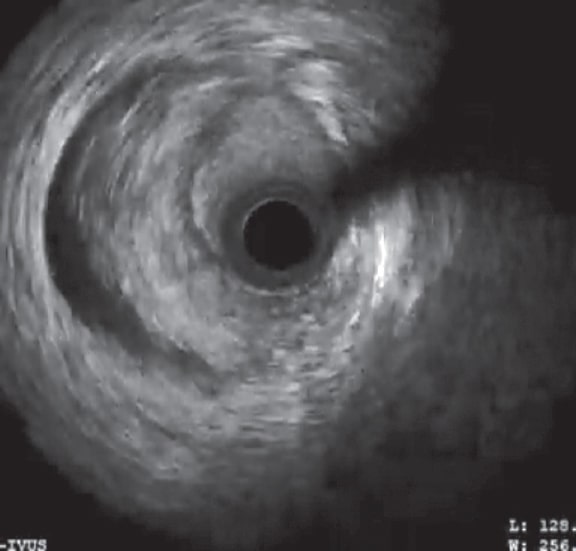
Figure 2. Image of coronary dissection in the distal portion of the left main coronary artery from 6 to 11 hours.
Consequently, the patient shows hemodynamic instability, thoracic pain, and ST-segment elevation on the electrocardiogram that improves partially with the administration of intracoronary nitrates.
To this point, due to the patient’s instability and compromised vessel lumen, in my opinion, an immediate percutaneous revascularization would have been required. Although, according to the angiography and the IVUS, the circumflex artery ostium is not compromised, another angioplasty guidewire should have been inserted to protect that vessel since stent implantation into the LMCA and proximal LAD can displace the hematoma towards the circumflex artery. As a matter of fact, in our own experience, stent implantation into spontaneous dissections often displaces hematoma and dissection towards other vessel segments being necessary to implant several stents into the different segments.
In this context, the best thing to do is to implant a single stent as opposed to several overlapping stents to cover the dissected area. Therefore, I would only implant 1 stent from the LMCA towards the LAD. The IVUS shows the most distal site of the dissection where stent implantation can start. In this case, the IVUS proved that there is a hematoma from the LMCA origin, which means that the vessel ostium should be covered by slightly protruding the stent towards the aorta. There is no doubt that the use of drug-eluting stents is advisable as well as the selection of stents with a scaffold with significant over-dilatation capabilities to adjust to the size of the vessel and into the LMCA. Checking the results on the IVUS is of paramount importance.
Regarding the circumflex branch that would be jailed by the stent, given the unstable clinical context, I wouldn’t prescribe any treatment unless flow or the vessel lumen were compromised. In a different procedure, treatment could be optimized by opening the stent mesh towards the circumflex branch through the simultaneous inflation of 2 kissing balloons.
FUNDING
No funding to declare.
CONFLICTS OF INTEREST
No conflicts of interest related to this work.
REFERENCES
1. Hinojal YC, Di Stefano S, Florez S, et al. Spontaneous coronary dissection during postpartum:Etiology and controversies in management. Ital Hear J. 2004;5:563-565.
2. Saw J, Humphries K, Aymong E, et al. Spontaneous Coronary Artery Dissection:Clinical Outcomes and Risk of Recurrence. J Am Coll Cardiol. 2017;70:1148-1158.
3. Al-Hussaini A, Abdelaty AMSEK, Gulsin GS, et al. Chronic infarct size after spontaneous coronary artery dissection:implications for pathophysiology and clinical management. Eur Heart J. 2020;41:2197-2205.
* Corresponding author: Unidad de Hemodinámica, Hospital Universitario Puerta de Hierro Majadahonda, Joaquín Rodrigo 1, 28222 Majadahonda, Madrid, Spain.
E-mail address: hemodinamic@gmail.com (J.A. Fernández Díaz).











